Abstract
Background
Cytomegalovirus (CMV) is an opportunistic pathogen causing reactivation and disease in Systemic Lupus Erythematosus (SLE) patients. This study aims to systematically review the literature for risk factors associated with CMV disease in SLE patients, in order to identify those more susceptible to CMV infection during their treatment.
Methods
A systematic review was conducted on 4 different search engines and via hand search until May 2017. Studies were included after quality assessment via the Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields (HTA KMET).
Results
Two studies on CMV disease were included. Elevated CMV viral load, higher steroid doses, use of immunosuppressants and disease duration were the most commonly associated risk factors for CMV disease.
Conclusion
High CMV viral loads, longer SLE disease duration and higher steroid doses were associated with CMV disease. Further studies studying the risk of treatment drugs and role of interventions in the development of CMV infection are needed.
Similar content being viewed by others
Introduction
Human cytomegalovirus (CMV), under the class of beta-herpesvirus, stays latent in an infected host throughout its life and is rarely reactivated to cause clinical illness. However, it is an important opportunistic pathogen causing morbidity and mortality in immunocompromised patients [1]. Across the world, the overall prevalence of CMV ranges from 40 to 100% [2], and seropositivity for CMV is shown to be higher in SLE compared to the general population [3].
CMV can manifest in various ways. CMV may remain undetected as a latent infection in immunocompetent hosts, or persist as asymptomatic low level CMV viremia. CMV dissemination and disease may present subtly with fever and lethargy, or a myriad of atypical manifestations, most commonly with respiratory or gastrointestinal symptoms and SLE flare-like presentations [4]. CMV disease subsequently leads to damage of multiple organ systems such as lung, liver, gastrointestinal tract and retina [5]. Greater CMV viral loads has been shown to correlate with increased risk of developing CMV disease in Human-immunodeficiency Virus (HIV) and transplant patients [6, 7], but this has not been demonstrated conclusively in autoimmune disease patients.
Several studies and case reports have highlighted CMV reactivation, resulting in CMV antigenemia or disease, as a concern during treatment of rheumatic diseases, particularly SLE [8,9,10,11]. This occurs as the treatment of SLE and autoimmune disease often involves chronic use of immunosuppressive therapy [12], which is a potential risk factor for CMV infection and disease [8, 13,14,15]. This is of significant concern as aside from developing end-organ CMV disease as reported in aforementioned case reports, it can also lead to significant mortality. In a recently published retrospective 26-year review of death causes and pathogen analysis of SLE, infections, including cytomegalovirus, was amongst the top three most frequent causes of death in SLE patients [16].
While there are few studies which looked at the clinical characteristics of CMV infection in patients with rheumatic diseases, there is no systematic review till date which comprehensively studies the risk factors of CMV disease in the SLE population. Our study thus aims to systematically review the literature for risk factors associated with CMV disease in patients with SLE.
Methods
Study design
A systematic review was conducted using the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA).
Studies in our review included patients diagnosed with SLE using the 1997 or 1982 American College of Rheumatology criteria [17, 18]. CMV disease was defined as positive CMV antigenemia assay with associated CMV clinical syndromes, with or without biopsy-proven CMV in organ tissue samples, either via a positive CMV culture or immunohistochemical staining, or a bronchoalveolar lavage fluid specimen with a positive CMV immunohistochemical staining or detection of CMV DNA via PCR [19]. We included primary studies which fulfilled the above definition of CMV disease [20].
Identification and selection of studies
Literature search was conducted on PubMed®, Embase®, Cochrane Library and Web of Science®. The search was restricted to articles in English and in humans. The search strategy included MeSH terms and free text of related terms relating to risk factors (clinical characteristic, rate, association, burden), SLE (lupus, systemic lupus erythematosus), CMV (cytomegalovirus), disease and infection (reactivation, infection, transmission). Further hand searches were conducted using references of related articles. The start date of the search was unrestricted and end date was till May 2017. Two authors, Kwan YH and Cher WQ independently reviewed the articles and applied the inclusion criteria. Disagreements were resolved through discussion or by a third reviewer (Choo HMC) if necessary.
Inclusion and exclusion
This systematic review included peer-reviewed publications of both randomized controlled trials and observational data. Observational data included quantitative epidemiological studies and all forms of cohort, case control and cross-sectional studies.
We excluded studies not written in English, not concerned with human subjects, case reports, case series (N ≤ 10), systematic reviews and meta-analyses and grey literature such as opinion pieces, editorial letters, abstracts, comments, conference proceedings and reviews that were not systematic.
Data extraction
Evidence related to cytomegalovirus disease in SLE patients were included. Relevant data including author, year of publication, type of study, country, duration, study size, patient demographics, type of medications (steroids and immuno-suppressants), duration and severity of autoimmune disease were extracted.
Methodological data quality assessment
The quality of the studies included was appraised by two independent reviewers (Cher WQ, Choo HMC) using the criteria proposed by the HTA KMET (Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields) [21]. This tool was chosen because it captures the range in methodological quality and risk of bias across both qualitative and quantitative studies. The two reviewers applied a 14-item checklist for quantitative studies and a 10-item checklist for qualitative studies to assess study quality. The score helps to objectively quantify the quality of each study by taking the observed agreement score between two independent reviewers with maximum score of 1.0 (100%) with higher scores given to studies with better study design and accuracy of data. We did not exclude any studies from our review based on quality.
Results
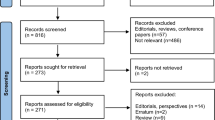
We reviewed 344 distinct titles and excluded 110 based on title screening. We assessed 234 potentially relevant abstracts and downloaded 126 full text articles. 2 studies were included in qualitative synthesis (Fig. 1). Reasons for exclusion of reviewed articles were included in Additional file 1: Table S1.
Among the total of 90 SLE patients across the primary studies, most were females (range from 77.7 to 81%), age ranging from 31.7 to 52.0 years. Both were cross-sectional studies. W. P Tsai (2012)‘s study included 38 SLE patients while Yu and Li et al. (2016)‘s study included patients with different rheumatological diseases, of which 52 out of 142 (37%) were SLE patients.
Reporting of study quality
Both primary studies had a quality score of more than 0.75 (Table 1). Inter-rater agreement for the primary studies were largely similar and discrepancies were discussed between the two reviewers until consensus was reached.
Predictors for CMV disease
The risk factors for CMV disease in rheumatic disease patients are reported in Table 2.
Lymphopenia
Yu and Li et al. (2016) found that 73 patients with CMV pneumonia had a lower lymphocyte count compared to 69 asymptomatic patients [median (range) 0.6 (0.1–4.0) × 109/L vs 1.2 (0.1–5.7) × 109/L respectively, p < 0.05] [22]. Receiver Operating Characteristic (ROC) curve analysis indicated that when CD4+ T cell count was < 0.39 × 109/L, patients with rheumatic diseases were at high risk for symptomatic CMV infection, with a sensitivity of 77.5% and specificity of 87.5%.
On the other hand, lymphocyte count was not significantly associated with CMV disease in W.P. Tsai et al. (2012)‘s study. Lymphocyte count was not statistically different between patients with CMV disease compared to those without CMV disease [mean (S.D.) 743 (615) mm3 vs 1062 (767) mm3, respectively, p = 0.175] [23].
Elevated CMV viral loads
Yu and Li et al. (2016) found that the mean (S.D.) CMV DNA value was higher in the patients with CMV pneumonia compared to asymptomatic patients [3.68 (1.90) × 104 copies/ml vs 0.60 (0.35) × 104 copies/ml, respectively, p < 0.01] [22]. ROC curve analysis showed that a level of 1.75 × 104 copies/ml was the optimal threshold for prediction of CMV-associated symptoms, with a sensitivity of 84.9% and specificity of 98.6% [22].
Treatment drugs
Both studies consistently reported higher corticosteroid doses being related with CMV disease. Yu and Li et al. (2016) noted that higher corticosteroid doses were correlated with CMV pneumonia [22]. The prednisolone dose was higher in the CMV pneumonia group compared to the asymptomatic group [median (range) 32(4–100) mg/day vs 20(1–50) mg/day, respectively, p < 0.010]. Similarly, the total prednisolone dose in the recent three months prior to diagnosis was higher in the CMV pneumonia group compared to the asymptomatic group [median (range) 2.8(0.1–9.0) g vs 1.8 (0.1–4.6) g, respectively, p < 0.01]. Likewise, in W.P. Tsai et al. (2012)‘s study of 38 SLE patients, the CMV disease group received a higher prednisolone dose than the non-CMV disease group [mean (S.D.) 25.9 (17.1) mg/day vs 9.0 (4.1) mg/day, respectively, p = 0.006] [23].
Apart from steroids, the use of other immunosuppressants were also associated with CMV disease. W.P. Tsai et al. (2012) noted a higher percentage of Azathioprine use 1 month prior to admission in the CMV disease group than the non-CMV disease group (7/20 cases vs 1/18, respectively, p = 0.045) [23].
Similarly, Yu and Li et al. (2016) found that use of immunosuppressants was more common in the CMV pneumonia group than the asymptomatic group (79% vs 58%, p < 0.010). For instance, MMF and Cyclosporine A were more frequently used in patients who developed CMV pneumonia than asymptomatic patients (p < 0.050) [22].
SLE disease characteristics
Yu and Li et al. (2016) found a positive correlation between SLE disease duration and CMV disease. Patients with CMV pneumonia had a longer SLE disease duration compared to patients without disease [median (range) 8 (0.03–360) months vs 3 (0.25–156) months, respectively, p < 0.05] [22].
Discussion
SLE is a chronic autoimmune disease which can be complicated by cytomegalovirus disease during its course but little attention has been given to investigate the factors that predispose SLE patients to this opportunistic infection. To the best of our knowledge, this is the first systematic review to explore the risk factors of developing CMV disease in SLE patients.
Elevated CMV viral loads has found to highly correlate with CMV disease. The presence of CMV antigen positive cells in bloodstream may reflect dissemination of CMV in host system, leading to potential CMV end-organ disease. In a study in bone marrow transplant patients, multivariate logistic regression analysis found that only elevated viral load remained a significant risk factor for CMV disease [24]. Similarly, in patients with Acquired-immune deficiency syndrome (AIDS), elevated levels of CMV antigenemia during follow-up was found to be associated with CMV disease [7]. In a cross-sectional study of 74 SLE patients, Takizawa Y. (2008) found that CMV viral load was higher in those with CMV disease (n = 117) compared to asymptomatic patients (n = 34) [median (range), 10.1 (0.0–2998.0)/105 PMNs vs 4.0 (1.3–1144.4)/105 PMNs, respectively, p = 0.001] [25]. A higher median (range) CMV antigenemia count of 5.6 (1.3–1144.4)/105 PMNs by ROC curve analysis predicted that patients have a higher risk of developing CMV disease with a higher mortality rate [25]. Future research with larger sample size can be useful to determine a cut-off CMV antigenemia level that predicts development of CMV disease in SLE patients and this can subsequently guide management on use of prophylactic therapy against CMV disease based on CMV antigenemia levels. This practice of pre-emptive therapy has been adopted as standard of care in patients following hematopoietic stem cell transplantation cases with CMV antigenemia or CMV seropositivity [26].
There are studies which have also looked at risk factors leading to elevated CMV viral load. In Fujimoto D. (2013)‘s cross-sectional study on patients with autoimmune diseases, a multi-variate analysis conducted found that lymphopenia < 700 mm3, (OR 34.44, 95% CI 7.82–151.66, p = 0.001), SLE (OR 6.71, 95% CI 1.23–36.49, p = 0.028) and Polymyositis/Dermatomyositis (OR 10.62, 95% CI 1.41–79.77, p = 0.022) were significantly associated with elevated CMV viral load [27].
Lymphocytes play a role in host cellular immune response against CMV, as seen in previous studies looking at human immunodeficiency virus (HIV) patients and stem cell transplant patients [28, 29]. Lymphopenia could thus suggest CMV reactivation, as CMV can lead to bone marrow suppression [19].
The mechanism of action of SLE treatment drugs can also explain the process of CMV disease in SLE patients [30]. Cyclophosphamide suppresses lymphocyte proliferation and function, thus increasing the risk of CMV reactivation. Cyclophosphamide use was found to be a risk factor for the reactivation of CMV in glomerulonephritis patients [31]. While the mechanism of action of these immunosuppressive drugs used in treatment of SLE makes development of CMV infection highly probable, other considerations such as the immunosuppression dose, duration of treatment and combination therapy with other immunosuppressive drugs may play a bigger role in the pathogenesis of CMV infection and disease in SLE patients. These factors were not sufficiently explored in the primary studies, thus it remains inconclusive.
Of note, two studies which looked into use of mycophenolate mofetil (MMF) as a risk factor for CMV antigenaemia consistently reported positive association [22, 32]. In a recent small case series, 3 out of the 4 SLE patients with disseminated CMV infection had prior treatment with 3 g of MMF daily in addition to concurrent use of other immunosuppressive drugs [9]. In another randomized controlled trial comparing Everolimus to MMF in renal transplant patients found that viral infections, particularly CMV infection increased in the MMF group (20% in MMF group compared to 6–7% in Everolimus group, p = 0.0001) [33]. This shows that use of MMF can be associated with higher incidence of CMV infection, and care should be taken to monitor for CMV disease when using MMF.
Our systematic review has its limitations due to the intrinsic nature of the primary studies. Firstly, there are only a small number of studies relevant to our research question. The two primary studies were conducted in Taiwan and China, thus limiting the scope to Asian patients. Secondly, the studies did not consistently analyze all of the same factors and stopped at univariable analysis and did not proceed with a multi-variable logistic regression. Thirdly, the baseline characteristics of SLE patients were variable across studies, and one of the two studies grouped SLE patients together with other rheumatic diseases patients in its analysis. This is a limitation as certain rheumatic diseases may be more predisposed to development of CMV disease than others. Lastly, treatment regimens and drugs doses were not standardized across studies, due to the heterogenous mix of different rheumatological disease patients, thus it was difficult to draw meaningful conclusions about treatment drugs and their implication on development of CMV disease. Both studies are also cross-sectional studies, thus it only captures data at one time-point and we are unable to know if the SLE subjects in the study subsequently developed CMV disease at a later time point.
Conclusion
This systematic review identified high CMV viral loads, lymphopenia and and higher corticosteroid doses to be associated with development of CMV disease in patients with SLE. Overall quality of studies ranged from 0.75 to 0.82. As reflected from our systemic review, there are few studies that have looked into the risk factors of developing CMV disease in SLE patients despite its prevalence in the SLE population. Studies on the risk of different treatment drugs and regimes on development of CMV disease are also lacking and is a potential for future research. These will be needed before guidelines on surveillance and pre-emptive treatment of CMV infection in SLE patients can be developed.
References
Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, Stevens-Ayers T, Flowers ME, Cunningham T, Corey L. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–14.
Krech U. Complement-fixing antibodies against cytomegalovirus in different parts of the world. Bull World Health Organ. 1973;49:103–6.
Barber C, Gold WL, Fortin PR. Infections in the lupus patient: perspectives on prevention. Curr Opin Rheumatol. 2011;23:358–65.
Ramos-Casals M, Cuadrado MJ, Alba P, Sanna G, Brito-Zeron P, Bertolaccini L, Babini A, Moreno A, D'Cruz D, Khamashta MA. Acute viral infections in patients with systemic lupus erythematosus: description of 23 cases and review of the literature. Medicine (Baltimore). 2008;87:311–8.
Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: a review. Infect Chemother. 2013;45(3):260–71.
Strippoli GF, Hodson EM, Jones CJ, Craig JC. Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2006. https://doi.org/10.1002/14651858.CD005133.pub3.
Chevret S, Scieux C, Garrait V, Dahel L, Morinet F, Modai J, Decazes JM, Molina JM. Usefulness of the cytomegalovirus (CMV) antigenemia assay for predicting the occurrence of CMV disease and death in patients with AIDS. Clin Infect Dis. 1999;28:758–63.
Mori T, Kameda H, Ogawa H, Iizuka A, Sekiguchi N, Takei H, Nagasawa H, Tokuhira M, Tanaka T, Saito Y, et al. Incidence of cytomegalovirus reactivation in patients with inflammatory connective tissue diseases who are under immunosuppressive therapy. J Rheumatol. 2004;31:1349–51.
Berman N, Belmont HM. Disseminated cytomegalovirus infection complicating active treatment of systemic lupus erythematosus: an emerging problem. Lupus. 2016;26. https://doi.org/10.1177/0961203316671817.
Ikura Y, Matsuo T, Fau - Ogami M, Ogami M, Fau - Yamazaki S, Yamazaki S, Fau - Okamura M, Okamura M, Fau - Yoshikawa J, Yoshikawa J, Fau - Ueda M, Ueda M. Cytomegalovirus associated pancreatitis in a patient with systemic lupus erythematosus. J Rheumatol. 2000;27:2715–7.
Rozenblyum EV, Allen UD, Silverman ED, Levy DM. Cytomegalovirus infection in childhood-onset systemic lupus erythematosus. Int J Clin Rheumatol. 2013;8(1):137–46.
Bertsias G. Ioannidis Jp Fau - Boletis J, Boletis J Fau - Bombardieri S, Bombardieri S Fau - Cervera R, Cervera R Fau - Dostal C, Dostal C Fau - font J, font J Fau - Gilboe IM, Gilboe Im Fau - Houssiau F, Houssiau F Fau - Huizinga T, Huizinga T Fau - Isenberg D, et al: EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics. Ann Rheum Dis. 2008;67:195–205.
Hanaoka R, Kurasawa K, Maezawa R, Kumano K, Arai S, Fukuda T. Reactivation of cytomegalovirus predicts poor prognosis in patients on intensive immunosuppressive treatment for collagen-vascular diseases. Mod Rheumatol. 2012;22:438–45.
Tamm M, Traenkle P, Grilli B, Soler M, Bolliger CT, Dalquen P, Cathomas G. Pulmonary cytomegalovirus infection in immunocompromised patients. Chest. 2001;119:838–43.
Yoon KH, Fong Ky Fau - Tambyah PA, Tambyah PA. Fatal cytomegalovirus infection in two patients with systemic lupus erythematosus undergoing intensive immunosuppressive therapy: role for cytomegalovirus vigilance and prophylaxis? JCR Journal of Clinical Rheumatology. 8:217–22. https://doi.org/10.1097/01.RHU.0000022543.49406.24.
Fei Y, Shi X, Gan F, Li X, Zhang W, Li M, Hou Y, Zhang X, Zhao Y, Zeng X, Zhang F. Death causes and pathogens analysis of systemic lupus erythematosus during the past 26 years. Clin Rheumatol. 2014;33:57–63.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism. 1982;25:1271–7.
Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, Pikis A, Razonable RR, Miller V, Griffiths PD. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91.
Francisci D, Tosti A, Fau - Baldelli F, Baldelli F, Fau - Stagni G, Stagni G, Fau - Pauluzzi S, Pauluzzi S. The pp65 antigenaemia test as a predictor of cytomegalovirus-induced end-organ disease in patients with AIDS. AIDS (London, England). 1997;11:1341–5. https://doi.org/10.1097/00002030-199711000-00007.
Leanne M. Kmet, Robert C. Lee, Linda S. Cook,: Standard Quality Assessment Criteria For Evaluating Primary Research Papers From A Variety Of Fields. Edmonton: Alberta Heritage Foundation for Medical Research (AHFMR) 2004. AHFMR - HTA Initiative #13.
Xue Y, Jiang L, Wan WG, Chen YM, Zhang J, Zhang ZC. Cytomegalovirus pneumonia in patients with rheumatic diseases after immunosuppressive therapy: a single center study in China. Chin Med J. 2016;129:267–73.
Tsai WP, Chen MH, Lee MH, Yu KH, Wu MW, Liou LB. Cytomegalovirus infection causes morbidity and mortality in patients with autoimmune diseases, particularly systemic lupus: in a Chinese population in Taiwan. Rheumatol Int. 2012;32:2901–8.
Gor D, Sabin C, Prentice HG, Vyas N, Man S, Griffiths PD, Emery VC. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant. 1998;21:597–605.
Takizawa Y, Inokuma S, Tanaka Y, Saito K, Atsumi T, Hirakata M, Kameda H, Hirohata S, Kondo H, Kumagai S, Tanaka Y. Clinical characteristics of cytomegalovirus infection in rheumatic diseases: multicentre survey in a large patient population. Rheumatology (Oxford). 2008;47:1373–8.
Chan ST, Logan AC. The clinical impact of cytomegalovirus infection following allogeneic hematopoietic cell transplantation: why the quest for meaningful prophylaxis still matters. Blood Rev. 2017;31:173–83.
Fujimoto D, Matsushima A, Nagao M, Takakura S, Ichiyama S. Risk factors associated with elevated blood cytomegalovirus pp65 antigen levels in patients with autoimmune diseases. Mod Rheumatol. 2013;23:345–50.
Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, Stevens-Ayers T, Flowers MED, Cunningham T, Corey L. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–14.
Salmon-Ceron D, Mazeron MC, Chaput S, Boukli N, Senechal B, Houhou N, Katlama C, Matheron S, Fillet AM, Gozlan J, et al. Plasma cytomegalovirus DNA, pp65 antigenaemia and a low CD4 cell count remain risk factors for cytomegalovirus disease in patients receiving highly active antiretroviral therapy. Aids. 2000;14:1041–9.
Ho M. Observations from transplantation contributing to the understanding of pathogenesis of CMV infection. Transplant Proc. 1991;23:104–8 discussion 108-109.
Lim CC, Tung YT, Tan BH, Lee PH, Mok IY, Oon L, Chan KP, Choo JC. Epidemiology and risk factors for cytomegalovirus infection in glomerular diseases treated with immunosuppressive therapy. Nephrology. 2018;23(7):676–81.
Su BY, Su CY, Yu SF, Chen CJ. Incidental discovery of high systemic lupus erythematosus disease activity associated with cytomegalovirus viral activity. Med Microbiol Immunol. 2007;196:165–70.
Vitko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, Oyen O, Viljoen HG, Filiptsev P, Sadek S, et al. Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2005;5:2521–30.
Acknowledgements
We would like to acknowledge Pang Jie Kie for her assistance in the formatting process of this manuscript. Both authors (Hui Min Charlotte CHOO and Wen Qi CHER) had equal contribution.
Funding
There was no external funding for this study and no authors declared conflict of interest related to the production of this manuscript.
Availability of data and materials
Materials used in this review were obtained from published research and papers.
Author information
Authors and Affiliations
Contributions
WF is the principal investigator of the study while WQC, CHMC and YHK are co-investigators. WQC and CHMC contributed equally to the study. They are responsible for the study. design, reviewing of articles and preparation of the manuscript. YHK and WF mentored the entire data collection, processing and manuscript preparation. All authors revised the draft critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was not deemed to be needed as this study uses data in the public domain and there is no interaction with patients nor animals.
Consent for publication
There were no individual’s person data requiring consent for publication.
Competing interests
There were no financial and non-financial competing interests in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Reasons for exclusion of reviewed articles. (DOCX 172 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Choo, H.M.C., Cher, W.Q., Kwan, Y.H. et al. Risk factors for cytomegalovirus disease in systemic lupus erythematosus (SLE): a systematic review. Adv Rheumatol 59, 12 (2019). https://doi.org/10.1186/s42358-019-0055-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-019-0055-y