Abstract
Background
Osteogenesis and angiogenesis are two closely correlated processes during bone growth, development, remodeling, and repair. Vascular endothelial growth factor (VEGF) is an essential mediator during the process of angiogenesis. The bone morphogenetic protein (BMP-2) family of growth factors plays critical roles in bone formation. VEGF has the potential to enhance BMPs-induced bone formation.
Purpose
This study attempted to assess VEGF and BMP-2 reflecting the effect of hybrid bio-composite scaffold on bone healing in dogs and evaluate the quality of the healing process radiologically.
Methods
This study was conducted on 12 adult mongrel dogs. All dogs were divided into four equal groups (n = 3 each): chitosan non-medicated (CH) (NM), chitosan medicated (CH) (M), chitosan bioglass non-medicated (CH.BG) (NM), and Chitosan Bioglass Medicated (CH.BG) (M). VEGF and BMP-2 were evaluated during fracture healing.
Results
Results have showed a non-significant decrease in serum VEGF activity in the (CH.BG) (M) group when compared to other groups during 2, 3 weeks, followed by gradual decrease, then increase at 12 weeks of interval period. There was highly significant increase from pre-surgery to 12 weeks in serum BMP-2 levels in the (CH.BG) (M) group when compared to other groups.
Conclusion
Biochemical parameters along with clinical and radiographical provide sound knowledge on the degree of bone healing with the use of chitosan bio-glass medicated by risedronate sodium drug. The statistical analysis will include the Fisher exact test and T test with significance level P < 0.05 (AU).
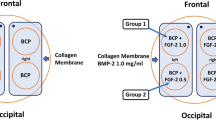
Graphical abstract

Similar content being viewed by others
Introduction
Bone repair or regeneration, like bone development, requires the concerted activity of several different cellular pathways. One such pathway, angiogenesis mediated by vascular endothelial growth factor (VEGF), is important for the coupling of cartilage resorption and mineralized bone formation during endochondral ossification in bone development (Huang et al., 2016; Brandi & Collin-Osdoby, 2006). In addition to interacting with certain humoral factors that regulate bone homeostasis, VEGF can interact synergistically with osteogenic proteins, such as BMP-2, to promote bone formation and bone healing by enhancing cell recruitment, prolonging cell survival, and increasing angiogenesis. These effects of VEGF lead to enhanced cartilage formation, accelerated resorption, and improved bone formation (Hu & Olsen, 2016).
Scaffolds alone do not usually completely heal the defects due to the lack of sufficient osteoinduction. The incorporation of osteoinductive factors into scaffolds is usually required to promote sufficient bone regeneration (Egri & Eczacioglu, 2017). Specific growth factors play important roles in the regeneration process of different tissues. Bone is a highly vascularized tissue in which blood vessels and bone cells interact to maintain skeletal integrity (Gemini et al., 2014). Therefore, angiogenesis and osteogenesis are both important for bone regeneration. Vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2 (BMP-2) are two key regulators of angiogenesis and osteogenesis and promote the endothelial and osteogenic differentiation of stem cells respectively. These two factors synergistically enhance bone regeneration (Tatullo et al., 2019; Xiao et al., 2011; Tarassoli et al., 2013).
Expression of particular growth factors such as vascular endothelial growth factor (VEGF) and bone morphogenetic proteins (BMPs) during the healing suggests a possible role for these secreted factors in bone repair. In fact, VEGF has been shown to stimulate bone healing in animal models. Although VEGF can control hypertrophic cartilage structure and vascularity within the developing growth plate (Gittens & Uludage, 2001), BMP-2 pro-protein contains four glycosylation sites. Among which, one glycosylation site is present in the polypeptide chain of the mature BMP-2 (Katagiri & Watabe, 2016). The aim of this study is to investigate the osteoconductive property and efficacy of bioactive glass combined with risedronate sodium drug in repairing surgically created bony defects in the mandible. Assess of VEGF and BMP-2 reflecting the effect of hybrid bioactive chitosan—glass graft on bone remodeling in experimental animal models.
Materials and methods
Biomaterials synthesis
The scaffolds containing chitosan (CH) and polyvinyl alcohol (PVA) as mixtures of both types of polymers named CH/PVA were prepared by freeze-drying method. The scaffolds were prepared as follows: CH was dissolved in 1% acetic acid solution for 2-3 h till formation of a clear solution. Then, risedronate (10 mg/g) was added to the CH solution and stirred for another 1 h at room temperature. And then, PVA was gradually added to the above solution at 60 ± 5 °C with continuous stirring for an additional hour to dissolve PVA. After complete dissolution, the prepared solution was sonicated (Digital sonicator, MTI, USA) for 30 mins to eliminate air bubbles and get homogeneous solutions. The prepared solutions were poured in molds (diameter of 15 mm) to create disks and layers and frozen overnight at −80 °C before freeze-drying process. Finally, the frozen scaffolds were freeze dried in a freeze dryer (Christ freeze dryer, ALPHA 2-4 LD plus, Germany) for 24 h to obtain dried scaffolds. For CH/PVA scaffolds, 10% w/w of CH, PVA was used in weight ratios; 30:70 blank non-medicated scaffold containing no drug were prepared using the same procedure. Each scaffolds contained 10% w/w risedronate. The solid content in the CH/PVA scaffold forming solutions was 10% w/v. Furthermore, the glass was added in percentage 10% in the CH/PVA 3:7 scaffolds (Oudadesse et al., 2013; Bui et al., 2010; Sun et al., 2013).
Narrative description of accomplishments
Selection of the most reliable anti-osteoporotic drug
For the management of osteoporosis, risedronate sodium was selected as one of the most distinguished anti-osteoporotic drugs. Risedronate sodium belongs to the bisphosphonate category that exerts its effect in the treatment of osteoporosis through binding to hydroxyapatite crystals at the bone matrix and through inhibiting of bone resorption by inhibiting osteoclast activity. Risedronate has a relative potency 2000 times higher than that for the reference bisphosphonate and etidronate. On the other hand, risedronate renal clearance is about 87% of total clearance, indicating that only a small proportion of a systematically available dose is associated with the bone.
Characterization of the prepared scaffolds has been undertaken through porosity measurements
The scaffold porosity was measured using a pycnometer (Ultrapyc 1200e Quantachrome pycnometer, USA). All the prepared scaffolds had porosity values higher than 75%. Such high percentages of porous scaffold structure with interconnected pores are required to enhance bone formation. Furthermore, such a scaffold structure would also facilitate cell migration, adhesion, and proliferation. Addition of the drug lowers the percentage porosity to some extent.
Experimental studies
In vivo animal studies were designed to evaluate the osteogenic potential of different bone scaffolds on the healing of experimentally induced critical-sized mandibular bone defect in dogs.
-
Study I (effect of bio-glass)—was designed to evaluate the osteogenic potential of different concentrations (10% and 30%) of bio-glass (BG) combined with chitosan-polyvinyl acid (CH/PVA) as an osteoconductive scaffold for the healing of experimentally induced critical-sized mandibular bone defect in dogs.
-
Study II (effect of risedronate drug)—these groups were designed to evaluate the osteogenic potential of risedronate (M) combined with chitosan-polyvinyl acid (CH/PVA) with or without bio-glass (BG) osteoconductive scaffold for the healing of experimentally induced critical-sized mandibular bone defect in dogs.
Scaffold drug-loaded CH/PVA-BG and physico-chemical characterization. Newly developed scaffold has been prepared from a mixture of two types of polymers without/with bioactive glass and the selected antiosteoporosis drug (risedronate).
Animals
The present study was conducted on 12 skeletally mature mongrel dogs (Canis lupus familiaris). Before enrollment in the study, each dog was given a complete clinical, physical and radiographic examination to exclude the evidence of systemic, orthopedic, and neurologic disease. Dogs included in the study were with a 15-kg body weight and aged 20 months. The animals were kept under normal laboratory conditions and given free access of food and water. All study procedures were done in accordance to and approved by the “Institutional Animal Care and Use Committee (IUCUC)” of Faculty of Veterinary Medicine- Cairo University.
All dogs were divided into four equal groups (n = 3 each):
-
Group 1: CH.PVA with no BG. (NM)
-
Group 2: CH.PVA with no BG. (M)
-
Group 3: CH.PVA with BG. (NM)
-
Group 4: CH.PVA with BG. (M)
Dogs were housed individually in separate cages at the Department of Veterinary Surgery, Anesthesiology & Radiology; Faculty of Veterinary Medicine, Cairo University. Before enrolment in the study, dogs were quarantined for 2 weeks, kennels were sprayed with 6/1000 ml Neocidal diazinone (Diaenone® 60 EC; Ciba-Geigy, Switzerland), dogs were bathed in 1/1000 Neocidal diazinone, and Ivermectine (Ivomec® ; merc, Sharp and Dome, USA) was injected in a dose of 0.1 mg/kg body weight subcutaneously to guard against ecto-parasitic and endoparasitic infestation.
Surgery
After initial preparation, surgery was performed. A straight approximately 5 cm wide incision was performed on the right side of the mandible. Dissection and division were performed by planes up to the periosteum. With the surgical field exposed, monocortical bone cavities were prepared using 5 mm trephine burs 12 mm in diameter under thorough irrigation with saline. A small chisel and mallet were used to facilitate the removal of the cortical disk with great care was taken to avoid injury of the inferior alveolar canal. The surgical procedure was performed unilaterally (Fig. 1a). The defect of the right side was obliterated by the sterile graft particles, the material was mixed with blood (Fig. 1b), and the obliterated bone defect was covered with resorbable tissue guiding membrane (Biocollagen membrane). The operated wound was closed in a routine manner (Fig. 1c).
Radiological measurements
The mandibular defect was radiographed using X-ray potentiality of 45 KV, 15 MA, and 1/10 s.
Laboratory measurements
Blood samples were collected from all animals before and after surgery, weekly intervals for 3 months, using sterile disposable syringes. Part of the blood sample will be left to clot and centrifuged at 3000 rpm for 15 min and then stored at −70 °C until further processing, finally sera were processed for estimation of the following:
-
VEGF and BMP-2 were purchased from KORAIN BIOTECH CO., LTD using Sandwich technique kits.
Statistical analysis
Data were collected and analyzed by computer program SPSS “version 17” ((The Statistical Package for the Social Science Program), Chicago, USA). All data were expressed as mean ± SD and percentages. Unpaired t test was used to compare a quantitative variable between two independent groups in parametric data. Mann Whitney test was used to compare quantitative variables between two independent groups when data were nonparametric (SD > 25% of mean).
Results
Serum VEGF (ng/L) level among the studied groups
After 12 weeks of (CH.BG) (NM) implantation, VEGF level in the (CH.BG) (NM) group shows a significant increase when compared to the control group (CH) (NM) (P value < 0.05). Also, there was a highly significant increase during 2 weeks in serum VEGF level in the (CH) (M) group (P value < 0.001), followed by gradual decrease then increase at 12 weeks of interval period. And also, there was a non-significant decrease during 2, 3 weeks in serum VEGF activity in the (CH.BG) (M) group (P value > 0.05), followed by the gradual increase in the value at 4, 5 weeks (P value < 0.05) and then decrease at 6, 7 weeks, finally reaching increase at different time changes when compared to control group (CH) (NM) (P value < 0.001) (Table 1 and Fig. 2 a,b and c) respectively.
Serum BMP-2 (ng/L) level among the studied groups
There was a highly significant increase in serum BMP-2 activity in the (CH.BG) (M), (CH.BG) (NM) groups when compared to control group (CH) (NM) (P value < 0.001). Also, there was highly significant increase during 2 weeks in serum BMP-2 activity in the (CH) (M) group (P value < 0.001), followed by gradual decrease in the value at 3 weeks (P value > 0.05) and finally reaching increase at different time changes (P value < 0.001) (Table 2 and Fig. 3a,b, and c) respectively.
Discussion
The combination of VEGF and BMP-2 has been documented for its effects on angiogenesis and osteogenesis (Kanczler et al., 2010; Kempen et al., 2009). VEGF stimulates the formation of supportive vascular networks, which enhance the bone formation effects of BMP-2 (Lee et al., 2008). In addition, VEGF can also serve as a mobilization cytokine for endothelial progenitor cells that promote bone regeneration (Hutchings et al., 2003). VEGF and BMP-2 released from scaffolds can stimulate endothelial cell and osteoblast migration from neighboring tissue (Behr et al., 2011). VEGF and BMP-2 can also promote MSC homing and induce these stem cells to differentiate into endothelial and osteogenic cells. In addition, VEGF and BMP-2 also have cross-coupling effects on osteogenic differentiation and angiogenesis, respectively. VEGF promotes bone regeneration by not only promoting angiogenesis but also directly inducing the osteogenic differentiation of MSCs (Jebahi et al., 2012). Bioglass/chitosan composite graft has a great osteogenic potential because the new bone was formed inside the defect in the form of multiple connected sites or islands away from the peripheral margins of the defect. However, this type of bone got connected with the margins when mature remodeled bone examined at later periods of the experimental study. These results were in accordance and compatible with those studies of Jebahi et al. (Ichigatani et al., 2001).
In the present study, there was a significant increase from presurgery to 12 weeks in serum VEGF level in the (CH.BG) (NM) group when compared to the control group (CH) (NM) (P value < 0.05). This study agrees with that reported by Ichigatani et al. (Horner et al., 1999) found that VEGF, expressed in cartilage in mouse femoral head, was related to the regulation of vascular formation in the elongating femur, and also, this study agrees with Horner et al. (Street et al., 2000) who reported that VEGF regulated angiogenesis in human neonatal growth plate cartilage. In addition, we found that there was a highly significant increase during 2 weeks in serum VEGF level in the (CH) (M) group (P value < 0.001), followed by a gradual decrease in the value at 3 weeks then increase at 12 weeks of interval period. That finding was in agreement with that reported by Street et al. (Betz et al., 2007) demonstrated a vascularity at 7 days after fracture indicate the importance of VEGF in early angiogenesis. In the early stages of bone repair, large amounts of active VEGF are found in the fracture hematoma, a VEGF source that is not present in developing bones.
In the present work, there was a non-significant decrease during 2, 3 week in serum VEGF activity in the (CH.BG) (M) group, followed by a gradual increase in the value at 4, 5 weeks and then decrease at 6, 7 weeks, finally reaching increase at different time changes when compared to control group (CH) (NM) (P value < 0.001).
There was a highly significant increase in serum BMP-2 activity in the (CH.BG) (M) and (CH.BG) (NM) groups when compared to the control group (CH) (NM). This result was in accordance with Betz et al. (Becker et al., 2012) who found that BMP-2 at different time points after initial implantation of hydroxy-apatite matrices revealed a less effective ectopic ossification compared to the simultaneous application of BMP and the matrices (Mochizuki et al., 2010). The implantation of the matrix 4 weeks before BMP application resulted in the weakest ossification and indicated that tissue already formed around the implant might have reduced the ability of applied BMP-2 to recruit mesenchymal progenitor cells from the surrounding to stimulate bone formation. A less delayed application (1 week) revealed no significant difference compared to simultaneous application.
The current study showed a highly significant increase during 2 weeks in serum BMP-2 activity in the (CH) (M) group, followed by a gradual decrease in the value at 3 weeks then increase at different time changes. This study agrees with that reported by Asamura et al. [26], who described an enhanced formation of new bone and improvement in defect healing after the usage of the slow release construct. A direct comparison of drug release kinetics on bone healing was carried out using BMP-2 absorbed to deproteinized bone (fast release) or by deproteinized bone bearing a coating-incorporated depot of BMP-2 (slow release).
Conclusion
In conclusion, it has been found that VEGF and BMP-2 act as homing molecules to stimulate MSC homing and subsequently induce the differentiation of MSCs into endothelial and osteogenic cells. Porous silk scaffolds served as suitable matrix vehicles to release VEGF and BMP-2 and bone defects were repaired by promoting both angiogenesis and new bone formation. These findings suggest that combined treatment with VEGF and BMP-2 could be a promising strategy for clinical bone regeneration.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Asamura S, Mochizuki Y, Yamamoto M, Tabata Y, Isogai N (2010) Bone regeneration using a bone morphogenetic protein-2 saturated slow-release gelatin hydrogel sheet: evaluation in a canine orbital floor fracture model. Ann Plast Surg 64:496–502
Becker ST, Bolte H, Schunemann K, Seitz H, Bara JJ, Beck-Broichsitter BE, Russo PA, Wiltfang J, Warnke PH (2012) Endocultivation: the influence of delayed vs simultaneous application of BMP-2 onto individually formed hydroxyapatite matrices for heterotopic bone induction. Int J Oral Maxillofac Surg 41:1153–1160
Behr B, Tang C, Germann G, Longaker MT, Quarto N (2011) Locally applied vascular endothelial growth factor a increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells 29:286–296
Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, Vrahas MS, Bouxsein ML, Evans CH (2007) Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther 14:1039–1044
Brandi ML, Collin-Osdoby P (2006) Vascular biology and the skeleton. J Bone Miner Res 21(2):183–192
Bui XV, Oudadesse H, Le Gal Y, Mostafa A, Pellen P, Cathelineau G (2010) Chemical reactivity of biocomposite glass-zoledronate. J Australian Ceramic Society 46(2):24–28
Egri S, Eczacioglu N (2017) Sequential VEGF and BMP-2 releasing PLA-PEG-PLA scaffolds for bone tissue engineering: I. design and in vitro tests. Artif Cells Nanomed Biotechnol 45(2):321–329
Gemini SU, Piperni S, Chatterjee S (2014) Arch Biochem Biophys 561:109–117
Gittens SA, Uludage H (2001) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis. Drug Targeting 9:407–429
Horner A, Bishop NJ, Bord S, Beeton C, Kelsall AW, Colleman N, Compston JE (1999) Immunolocalisation of vascular endothelial growth factor (VEGF) in human neonatal growth plate cartilage. J Anat 194(4):519–524
Hu K, Olsen BR (2016) Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Investig 126(2):509–526
Huang B, Wang W, Li Q, Wang Z, Yan B, Zhang Z, Wang L, Huang M, Jia C, Lu J, Liu S, Chen H, Li M, Cai D, Jiang Y, Jin D, Bai X (2016) Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nat Commun 7:13885
Hutchings H, Ortega N, Plouët J (2003) Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J 17:1520–1522
Ichigatani M, Yamaki K, Saga T, Yoshizuka M (2001) Appearance of vascular endothelial growth factor (VEGF) in femoral head in the growing rat. Histol Histopathol 16(2):463–468
Jebahi S, Oudadesse H, Bui XV, Keskes H, Rebai T (2012) Repair of bone defect using bioglass-chitosan as a pharmaceutical drug: An experimental study in an ovariectomised rat model. Afr J Pharm Pharmaco 6:1276–1287
Kanczler JM, Ginty PJ, White L, Clarke NM, Howdle SM, Shakesheff KM, Oreffo RO (2010) The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 31:1242–1250
Katagiri T, Watabe T (2016) Bone morphogenetic proteins. Cold Spring Harb Perspect Biol 8:a021899
Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJ (2009) Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 30:2816–2825
Lee DY, Cho TJ, Kim JA, Lee HR, Yoo WJ, Chung CY, Choi IH (2008) Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone. 42:932–941
Oudadesse H, Wers E, Bui XV, Roiland C, Bureau B, Akhiyat I, Mostafa A, Chaair H, Benhayoune H, Fauré J, Pellen-Mussi P (2013) Chitosan effects on glass matrices evaluated by biomaterial. MAS-NMR and biological investigations. Korean J Chem Eng 30(9):1775–1783
Street J., Winter D. Wang J.H., Wakai A., McGuinness A. and Redmond H.P. (2000): Is human fracture hematoma inherently angiogenic?. Clinical Orthopaedics and Related Research. 224–237.
Sun X, Kang Y, Bao J, Zhang Y, Yang Y, Zhou X (2013) Modeling vascularized bone regeneration within a porous biodegradable CaP scaffold loaded with growth factors. Biomaterials. 34(21):4971–4981
Tarassoli P, Khan WS, Hughes A, Heidari N (2013) A review of techniques for gene therapy in bone healing. Curr Stem Cell Res Ther 8:201–209
Tatullo M, Spagnuolo G, Codispoti B, Zamparini F, Zhang A, Esposti MD (2019) PLA-based mineral-doped scaffolds seeded with human periapical cyst-derived MSCs: a promising tool for regenerative healing in dentistry. Materials 12:E597. https://doi.org/10.3390/ma12040597
Xiao C, Zhou H, Liu G, Zhang P, Fu Y, Gu P, Hou H, Tang T, Fan X (2011) Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomed Mater 6:015013
Acknowledgements
I would first like to thank my thesis advisor staff members and my colleagues at the Biochemistry Department in the Faculty of Science at Ain Shams University. I owe special thanks to Dr. Amany A. Mostafa, Prof. of Nanomedicine & Tissue Engineering Laboratory, for the suggestion of the point of research and his kind supervision, his tremendous effort, and creative guidance. His intelligent remarks motivated me a lot to finish this work.
Consent to participate
Not applicable.
Funding
The authors acknowledge the financial support of STDF project number 5024.
Author information
Authors and Affiliations
Contributions
MA participated in the surgical part of the thesis and revision of my research. SM participated in the statistical part of the current research. MG. and SA., professor of biochemistry, helped in the part of the current research. AA, a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The authors follow the rules of ethics in using of animals, Institutional Animal Care and Use Committee (IACUC) has approved the above referenced Animal Use Protocol (AUP) with VetCU06202019050.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rady, A.A.M., Hamdy, S.M., Abdel-Hamid, M.A. et al. The role of VEGF and BMP-2 in stimulation of bone healing with using hybrid bio-composite scaffolds coated implants in animal model. Bull Natl Res Cent 44, 131 (2020). https://doi.org/10.1186/s42269-020-00369-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-020-00369-x