Abstract
Background
As the copper ferrite gains more attention due to its unique electronic, thermal, and catalytic properties, the need to explore the mechanism of its formation is becoming a task to be studied.
Results
The mechanism of copper ferrite formation was studied by in situ XRD, and IR results showed comparison that the formation of Fe3O4 was formed by in situ heating.
Conclusions
These results shed light on the formation of ferrite first begun with the formation of Fe3O4, and then, incorporation of Cu2+ ion is done.
Similar content being viewed by others
Background
Spinel ferrites are showing increasing interest in the past few decades due to their unique electronic, magnetic, and catalytic properties. These properties make ferrites have their exclusive field of applications, among these applications are recording device, medical application, electronic devices, and catalytic application including an important contribution in CO2 decomposition (Gunther 1990; Ding et al. 1998; Mazaleyrat and Varga 2000; Riley et al. 2002; Tamaura and Tahata 1990). Among the most important ferrite spinel systems, the copper/iron/oxygen system is attracting more interest in many fields such as solid-state physics, ceramics, metallurgy, and mineralogy. Copper ferrites gather more interest because of its unique properties regarding its electrical conductivity in combination with semiconducting behavior. In addition to its thermal stability, copper ferrite showed a considerable catalytic activity for oxygen generation (McCurrie 1994; Stewart et al. 2004). The framework of copper ferrite is considered to be inverse spinel. Copper and iron ions could be positioned in different crystallographic positions surrounded by oxygen atoms in a cubic closed pack structure. Nominated as A and B sites, the ions could occupy either tetrahedral or octahedral positions. The formula of this type of allocations is represented as (Cux Fe1_x) A (Cu1_x Fe1 + x) BO4, where x is the inversion coefficient assigned to be zero or one describing the inverse or normal spinel (Agouriane et al. 2016). Copper ferrite can exist in two different spinel arrangements (Atia et al. 2016): cubic one exists at high temperature accompanied with lattice constant of 8.38 A° and the tetragonal one assigned with lattice constants of a = 8.216 A°, c = 8.709A°. The inverse model pattern composed of eight copper ions octahedrally positioned (B) and 16 iron ions in trivalent state distributed equally along the tetrahedral and octahedral positions (Jiang et al. 1999; Muthukumar et al. 2018; Schaefer et al. 1970; Goya et al. 1998). It is established that the production way plays an awfully significant function in the chemical and magnetic property of resulted ferrites, and one of the most important challenges is to explore the structure formation mechanism in order to manipulate and control formation condition to target certain structure to be applied in a specific and selective field of applications. In this paper, we would like to shed some light on how the Cu2+ enters in the spinel structure by using in situ XRD measurements.
Methods
Materials
The materials used are Cu(NO3).3H2O 99.5% (Merck), Fe(NO3)3.9H2O 99% (Merck), and NaOH 97% (Merck).
Techniques
X-ray diffraction spectroscopy (XRD) was measured using Bruker D8 Advance at 40 mA and 40 Kv, operated with temperature unit. Fourier transform infrared spectroscopy was collected using Bruker IR spectrophotometer.
Method of preparation
A solution of copper/iron with 2:1 M ratio was prepared in a 200-ml H2O. An equivalent amount of NaOH solution was added dropwisely till complete precipitation, and then, the precipitate was washed and dried at 100 °C overnight. Calcination was performed at 200 °C. In situ calcination at different temperatures was done in the temperature chamber of XRD instrument.
Results
XRD spectra
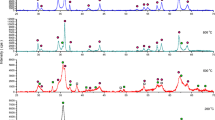
XRD diffractograms of in situ measurements of Cu–Fe precursors were showed in (Fig. 1).
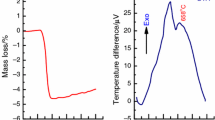
The XRD diffractograms showed that even at 600 °C, amorphous phase was observed. Moreover, as the calcination temperature increased up to 800 °C, a Fe3O4 phase started to appear being very clear at 1000 °C (Fig. 2).
Fourier transform infrared spectrophotometer (FTIR)
Figure 3 shows IR spectra of dried Cu:Fe sample, calcined at 200 °C and at 1000 °C.
Discussion
The mechanism of the formation of CuFeO4 could be assumed to consist of two steps depending on the fact that the aqueous NaOH solution acts as a reducing agent using the property of OH− group which could lose electron according to Scheme 1.
Four concurrent processes occur first, and second processes (Scheme 2) involve the reaction of Cu 2+ and Fe3+ producing the formation of iron(III) oxide and copper(II) oxide from their hydroxides as it is well known.
The heat involved in the conversion of iron(III) hydroxide into iron(III) oxide could be obtained from the solvation process of NaOH. Third concurrent process occurs due to the reducing property of the hydroxyl group which reduces Fe3+ to form Fe2+. Fe2+ reacts with OH− to form iron(II) hydroxide which in the second step produces iron(II) oxide (Scheme 3).
The final process is the reaction of the coexistence of different hydroxides (Scheme 4).
The above scheme could be complimentary to previous data studied by Kolta et al. (1981). Thus, they revealed a mechanism based on two steps and they suggest the last step at high temperature is the formation of ferrites. In our study, we declare that it could be explained by the formation of Fe3O4 in the first step followed by the insertion of copper ions in the last steps as could be seen from previous equations.
XRD spectra
XRD diffractograms of in situ measurements of Cu–Fe precursors were showed in (Fig. 1). The XRD diffractograms showed that even at 600 °C, amorphous phase was observed. Moreover, as the calcination temperature increased up to 800 °C, a Fe3O4 phase started to appear being very clear at 1000 °C. To confirm these results, we perform calcination of Cu:Fe precursors in a muffle furnace for 2 h. Although the same temperature of calcination, the prolonged heating in a muffle furnace showed only copper ferrite phase (Fig. 2). These results revealed that the Fe3O4 is being crystalized first, and then, incorporation of copper to form copper ferrite is being crystalized in a later stage. This shed some light on the mechanism of formation of copper ferrite which seemed to be formed only at high temperatures.
FTIR
Figure 3 shows IR spectra of dried Cu:Fe sample, calcined at 200 °C and at 1000 °C.
The FTIR spectra of Cu:Fe sample calcined at different temperatures are showed in Fig. 3. The spectra showed that the fingerprint region 375–400 is nearly the same for dried and 200 °C samples which reflect the vibration of separate Fe–O and Cu–O vibration modes. However, the sample calcined at 1000 °C showed some differentiated peaks especially at 457 °C which may be devoted to Cu–Fe–O vibration mode in spinel structure of copper ferrite.
Conclusions
The study of the formation of copper-iron binary oxide system by using in situ XRD measurements helped in exploring the mechanism of ferrite formation and the sequence of the binary system oxide formation. It was proved that the Fe3O4 is being formed firstly followed by the incorporation of Cu in the lattice reforming the structure into a spinel structure.
The application of copper ferrite formed could be used in many industrial applications depending on its magnetic and thermal properties.
References
Agouriane E, Rabi B, Essoumhi A, Razouk A, Sahlaoui M, Costa B, Sajieddine M (2016) Structural and magnetic properties of CuFe2O4 ferrite nanoparticles synthesized by co-precipitation. J Mater Environ Sci 7(11):4116–4120
Atia TA, Altimari P, Moscardini E, Pettiti I, Toro L, Pagnanelli F (2016) Synthesis and characterization of copper ferrite magnetic nanoparticles by hydrothermal route. Chem Eng Trans 47
Ding J, Miao W, McCormick P, Street R (1998) High-coercivity ferrite magnets prepared by mechanical alloying. J Alloys Compd 281(1):32–36
Goya G, Rechenberg H, Jiang J (1998) Structural and magnetic properties of ball milled copper ferrite. J Appl Phys 84(2):1101–1108
Gunther L (1990) Quantum tunnelling of magnetisation. Physics World 3(12):28
Jiang J, Goya G, Rechenberg H (1999) Magnetic properties of nanostructured CuFe2O4. J Phys Condens Matter 11(20):4063
Kolta GA, El-Tawil SZ, Ibrahim AA, Felix NS (1981) Kinetics and mechanism of copper ferrite formation. Thermochim Acta 43(3):279–287
Mazaleyrat F, Varga L (2000) Ferromagnetic nanocomposites. J Magn Magn Mater 215:253–259
McCurrie RA (1994) Ferromagnetic materials: structure and properties. London : Academic Press, cop
Muthukumar K, Lakshmi DS, Acharya SD, Natarajan S, Mukherjee A, Bajaj H (2018) Solvothermal synthesis of magnetic copper ferrite nano sheet and its antimicrobial studies. Mater Chem Phys 209:172–179
Riley M, Walmsley A, Speight J, Harris I (2002) Magnets in medicine. Mater Sci Technol 18(1):1–12
Schaefer S, Hundley G, Block F, McCune R, Mrazek R (1970) Phase equilibria and X-ray diffraction investigation of the system Cu−Fe−O. Metall Trans 1(9):2557
Stewart S, Tueros M, Cernicchiaro G, Scorzelli R (2004) Magnetic size growth in nanocrystalline copper ferrite. Solid State Commun 129(6):347–351
Tamaura Y, Tahata M (1990) Complete reduction of carbon dioxide to carbon using cation-excess magnetite. Nature 346(6281):255
Acknowledgements
All authors acknowledge the National Research Center Labs for making the analysis on it.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Author information
Authors and Affiliations
Contributions
All authors contribute in the work and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Manuscript does not contain studies involving human participants, human data, or human tissue.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hegazy, E.Z., Abd El-Maksod, I.H., Ibrahim, A.M. et al. New insights about the formation of copper ferrite: in situ X-ray diffraction study. Bull Natl Res Cent 42, 9 (2018). https://doi.org/10.1186/s42269-018-0010-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-018-0010-9