Abstract
Modulation of the nervous system by delivering electrical or pharmaceutical agents has contributed to the development of novel treatments to serious health disorders. Recent advances in multidisciplinary research has enabled the emergence of a new powerful therapeutic approach called bioelectronic medicine. Bioelectronic medicine exploits the fact that every organ in our bodies is neurally innervated and thus electrical interfacing with peripheral nerves can be a potential pathway for diagnosing or treating diseases such as diabetes. In this context, a plethora of studies have confirmed the important role of the nervous system in maintaining a tight regulation of glucose homeostasis. This has initiated new research exploring the opportunities of bioelectronic medicine for improving glucose control in people with diabetes, including regulation of gastric emptying, insulin sensitivity, and secretion of pancreatic hormones. Moreover, the development of novel closed-loop strategies aims to provide effective, specific and safe interfacing with the nervous system, and thereby targeting the organ of interest. This is especially valuable in the context of chronic diseases such as diabetes, where closed-loop bioelectronic medicine promises to provide real-time, autonomous and patient-specific therapies. In this article, we present an overview of the state-of-the-art for closed-loop neuromodulation systems in relation to diabetes and discuss future related opportunities for management of this chronic disease.
Similar content being viewed by others
Background
A key biological process of every living system is the maintenance of homeostasis to ensure stability via continuous and rapid self-adjustments of the physiological state. The nervous system has a major role in preserving homeostasis using closed-loop mechanisms called neural reflexes. These include sensing, integration and effector circuitries that modulate the organ function. Interfacing with the neural reflexes may allow for a direct and efficient access to the rapidly changing internal conditions of the organism.
In recent decades, modulation of the nervous system through delivery of electrical or pharmaceutical agents has allowed the development of effective treatments to several severe clinical conditions (Shamji et al. 2017). Recently, we have seen the introduction of bioelectronic medicine, an evolution of neuromodulation, which aims to provide real-time and patient-specific therapies by modulating the activity of specific peripheral nerves to improve or restore impaired biological function of specific organs (Vitale and Litt 2018; Medicine A for AB 2020).
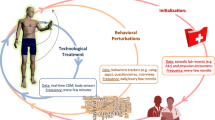
The therapeutic impact of bioelectronic medicine can be boosted by replicating the body’s closed-loop mechanisms: metabolic and neurophysiological biomarkers can be recorded and analyzed in real time to accordingly adjust the characteristics of the electrical stimulation delivered to the peripheral nerves or directly to the organs in order to modulate their function, as illustrated in Fig. 1 (Birmingham et al. 2014). Advances in bioelectronic medicine towards these closed-loop systems are supported by the development of new implantable technology and algorithms, which enable a safe, effective and minimally invasive interface with the nervous system (Rijnbeek et al. 2018; Zhou et al. 2018).
A depiction of future closed-loop neuromodulation systems for diabetes management. Metabolic biomarkers and neurophysiological recordings from a variety of peripheral nerves can be used to automatically control the stimulation dosage to be delivered back to the peripheral nervous system or directly to the organs to modulate their function
Initial research on bioelectronic medicine focused on the use of neuromodulation for treating inflammatory diseases by interfering with the neuro-immune reflex (Levine et al. 2014; Sundman and Olofsson 2014; Borovikova et al. 2000). Previous research confirmed that electrical stimulation of the vagus nerve modulates the production of inflammatory cytokines, and ultimately reduces the uncontrolled inflammatory activity associated with several disorders. This strategy has successfully improved the treatment of rheumatoid arthritis and colitis in clinical trials (Koopman et al. 2014), encouraging the application of bioelectronic medicine for the management of other chronic and severe diseases such as diabetes.
Diabetes is a chronic metabolic disease caused by an impairment of the hormone insulin which results in elevated blood glucose. This disorder currently affects 422 million people worldwide and is forecast to be the 7th leading cause of death in 2030 (World Health Organization, Organization WH 2016). Therapies involving insulin administration are indispensable for people with type 1 diabetes. For type 2, such therapies are sometimes used in early stages, and are critical at later stages of the disease. However, the costs associated with the insulin market are untenable, poised to reach $39.13 billion by 2020 based on the 2016 market research report published by MarketsandMarkets (MarketsandMarkets 2016). Moreover, the American Diabetes Association reported in March 2018 that the total costs of treating people diagnosed with diabetes has risen to $327 billion in 2017 from $245 billion in 2012 (American Diabetes Association AD 2018). These figures reflect the impact of diabetes on society and encourage the development of new treatments to improve the control of glucose. The application of bioelectronic medicine to treat diabetes has been previously criticized by some members of the scientific community due to the complexity of the underlying biological processes and the lack of legitimacy of preliminary simulations (Lowe 2016). However, recent work demonstrates the important role of the vagus nerve in the pathophysiology of diabetes and its comorbidities (e.g. cardiovascular diseases), and supports the potential benefits of modulating the peripheral nerve signals to improve the metabolic dysregulation (Steculorum et al. 2016; Zhang and van den Pol 2016).
State of the art
The majority of research related to glucose control and neuromodulation has occurred in the context of metabolic disorders, specifically in the control of appetite and food intake in managing obesity (Aelen et al. 2008; Ruiz-Tovar et al. 2014; Ruiz-Tovar and Llavero 2016; Sohbati and Toumazou 2015; Johnson and Wilson 2018). Direct stimulation of the gastric myoelectrical activity with gastric pacemakers, known as gastric pacing, is one of the most exploited techniques (Greenway and Zheng 2007; Favretti et al. 2004; Cha 2014). For example, delivery of high frequency and short pulse width vagal stimulation in human clinical studies with the Transcend gastric pacemaker (Transneuronix, Mt. Arlington, NJ), inhibited the efferent vagal activity to the stomach (Greenway and Zheng 2007; Favretti et al. 2004; Cigaina 2004)., which caused an increased gastric distension and slowed gastric emptying. These changes led to earlier satiety, and eventually a reduction of food intake. The study reported a 20 to 40% of weight loss, depending on the patient. These findings on gastric pacing support further research on direct modulation of vagal activity known as vagal pacing. In relation to this, a human study in patients using vagus nerve stimulation to treat epilepsy reported a variety of responses, with slightly more than 50% of the patients losing between 5 and 10% of body weight (Burneo et al. 2002). More advanced strategies automatically modulate vagal activity in response to stomach distension and/or presence of satiating hormones, among other markers (Apovian et al. 2017; Shikora et al. 2015; Sarr et al. 2012; de Lartigue 2016; Yao et al. 2018; Cork 2018). A recent study in rats successfully reported up to a 38% body weight reduction by delivering biphasic electric pulses based on the peristalsis measured on the stomach (Yao et al. 2018).
Conversely, research on bioelectronic medicine for direct control of diabetes has emerged recently, with two main areas of interest: i) recording from the peripheral nerves to extract metabolic information and ii) modulating their electrical activity to improve glycaemic fluctuations. Regarding the former, recent work aims to use the vagus nerve as a glucometer to identify hypoglycaemic events from neural readings (Bedows 2018; Masi et al. 2019). In fact, neural activity presents a negatively correlated response to glycemia (Masi et al. 2019). The complexity of the recorded data requires advanced strategies to identify and decode neural signals related to glycaemic levels. In particular, Masi et al. (2019) have developed a decoding algorithm that recreates blood glucose levels with high accuracy using regression models with regularization to avoid over-fitting (Masi et al. 2019). Preliminary results are promising and encourage application of their method for glucose monitoring and control in the near future, especially for type 1 diabetes.
Selective electrical activation or blockade of specific vagal fibers to specific organs has been shown to be a way to impact different metabolic processes resulting in different glycaemic outcomes. Vagal blocking of afferent/efferent fibers has been experimentally achieved by direct transection of the vagus nerve (Meyers et al. 2016), however this methodology cannot be translated into clinical research in humans. As a result, ongoing research is focused on finding a combination of parameters that can selectively block specific fiber types, or the afferent/efferent traffic (Johnson and Wilson 2018). One such technique uses high frequency stimulation targeting a variety of nerves and locations. For example, this strategy has shown to successfully block nerves conveying pain signals, making it an alternative efficient method to conventional spinal cord stimulation in the treatment of chronic pain (Kumar et al. 2018). As another example, Shikora et al. used intermittent vagal blocking at the level of the abdomen in people with type 2 diabetes, and reported a reduction in body weight and improvement of glycaemic control based on enhanced levels of HbA1c, which is a marker for diabetes development (Shikora et al. 2013). Further research has targeted the activity of the carotid sinus nerve (Sacramento et al. 2016; Sacramento et al. 2018; Conde and Guarino 2018). In this domain, Conde et al. successfully restored insulin sensitivity and glucose tolerance in people with type 2 diabetes by blocking the activity of the carotid sinus nerve using kilohertz frequency alternating current (KHFAC) modulation (Sacramento et al. 2018; Conde and Guarino 2018).
The impact of neurostimulation on type 1 diabetes has been less explored, but recent research from Guyot et al. (2019) suggests that stimulation of the pancreatic sympathetic nerves projecting to the pancreatic lymph nodes inhibits the progression of the disease, at least in mice (Guyot et al. 2019). Activation of these nerves with a stimulation frequency of 10 Hz and 450 μA amplitude resulted in a reduction of pro-inflammatory cytokines and of the proliferation of autoreactive T cells, therefore limiting the progression of the disease. Preliminary optimization of the stimulation parameters eliminated undesired effects such as blood flow alteration and axonal excitability exhaustion, hence allowing for therapeutic use.
It is also possible to directly stimulate the organs of interest to regulate their function. For example, acute electrical stimulation of the parenchyma tissue of the liver for 90 min resulted in a reduction of both fasting and postprandial glucose levels in the healthy state and type 1 and type 2 diabetic rats (Chen et al. 2010). Moreover, continuous stimulation for 8 h during 4 consecutive days not only resulted in decreased glucose levels in the three groups, but also delayed gastric empty and increased plasma glucagon-like peptide-1 (GLP-1) level. Adverse effects were not reported in either case, making it a feasible and safe approach for glycaemic control.
Finally, inflammation has been found to be related to obesity-induced insulin resistance in people with type 2 diabetes (Esser et al. 2015), as inflammatory cytokines such as TNF can directly induce insulin resistance (Chang et al. 2019; GKS et al. 1996). In addition, reduced vagal activity has been reported in these patients (Chang et al. 2019). As a result, modulation of the immune reflex arc using vagal activation might provide pathway to alleviate both the inflammatory and the metabolic dysregulation of these patients (Esser et al. 2015; Chang et al. 2019).
Future directions
The studies cited above are examples of current applications of bioelectronic medicine in diabetes. However, there is a plethora of opportunity to target the processes and organs involved in the glucose-response reflex through modulation of the nervous system (Güemes and Georgiou 2018). To begin with, recent research has given insight into neural control of the liver and its implication on the balance of hepatic glucose production and uptake (Yi et al. 2010; Van Den Hoek et al. 2008; Kalsbeek et al. 2004; Matsuhisa et al. 2000). As a result, modulating the neural signals to the liver would reduce the risk of hypoglycaemic events, especially for type 1 diabetes, by enhancing endogenous glucose production, which would rapidly increase glucose levels.
Secretion of hormones from the pancreas is also under the control of the nervous system (Meyers et al. 2016; Thorens 2011; Ahrén 2000; Ahren et al. 1987). These neural pathways could be modulated to regulate the secretion of insulin and glucagon in patients with type 2 diabetes and early stages of type 1 diabetes, where there are still a considerable number of β-cells intact. This can be particularly desirable in situations where glucose control in diabetic subjects is challenging, such as during exercise, which results in a decrease in glucose levels. Prompt inhibition of insulin secretion and a promotion of counterregulatory hormone secretion (such as glucagon) before starting exercise may also be used to improve control of glucose fluctuations and reduce the risk of hypoglycaemia.
Neuromodulation of pancreatic secretion could be further applied in patients with stem cell-derived β-cell transplants. This strategy has been found to improve glycaemic control in pre-clinical trials, but many challenges are yet to be resolved, including restoration of vascularization and neural innervation. Successful advances are being made in the former, but full restoration of the functionality of the transplanted islets is still far from optimal. To address this issue, Seicol et al. (2019) propose the development of biocircuit-augmented islet transplants containing mature, vascularized and neural innervated β-cells, where neurons will grow inside hydrogel-based micro tissue engineered neural network (micro-TENN) scaffolds (Seicol et al. 2019). We proposed bringing this idea one step further and using closed-loop neuromodulation of neural bio-circuits to restore the physiologic response to neural signalling that is present in healthy individuals (Güemes and Georgiou 2018).
Control of insulin sensitivity through neuromodulation, although generally relevant to type 2 diabetes, could be further extended for use in people with type 1 diabetes. These subjects require external injection of the insulin to lower glucose levels after a meal intake. Modulation of the neural pathways could increase the effect of this exogenous insulin in lowering blood glucose (i.e. insulin sensitivity) (Güemes et al. 2019).
The opportunities of closed-loop neuromodulation should not be restricted to treatment of early and late stage diabetes. Vagus nerve recordings, as previously demonstrated (Bedows 2018), can also be used as a monitoring tool of the glycaemic state for early detection of the disease and to control its progression (Medicine A for AB 2020).
Finally, it is crucial to consider the integration of this new strategy for glucose control with the systems that are currently implemented for diabetes management. In particular, closed-loop systems for insulin delivery, such as the artificial pancreas, are regarded as cutting-edge technology in glucose management for diabetes (Ramli et al. 2019; Taleb et al. 2019; Kovatchev 2018; Saunders et al. 2019; Herrero et al. 2019). An artificial pancreas comprises a sensor for continuous glucose monitoring, an insulin pump and an algorithm that replicates the endocrine function of a healthy pancreas and calculates the optimal dose to be delivered based on the real-time glucose readings from the sensor. One of the most employed artificial pancreas systems is the Medtronic 670G (Medtronic, Northridge, CA, USA), which is approved by the US Food and Drug for use in people with type 1 diabetes over 14 years of age (PMA P130013: FDA Summary of Safety and Effectiveness Data 2013), and is also CE Mark approved for use in people over 7 years of age within Europe. Despite the improved glycaemic control recently reported in clinical trials (Brown et al. 2019; Bailey et al. 2019), the system is still not fully autonomous and requires user input, especially during meal intake (Ramli et al. 2019; Gingras et al. 2018). Incorporating the opportunities that bioelectronic medicine offers for diabetes will undoubtedly enable improved computation of the insulin doses, increased autonomy, and personalized glucose control.
Challenges
In this article, current and future opportunities for applying bioelectronic medicine for improving diabetes have been presented, all of which would further benefit from the incorporation of a “closed-loop” control of both the immune and metabolic reflexes. That said, some important challenges and advances must be addressed in order to translate these strategies into real closed-loop therapeutic approaches.
Selective activation of the specific neural fibers that target the biological processes of interest is a major requirement to minimize the risk of undesired activation of other tissues, organs, and processes. This entails the development of novel stimulation strategies and devices to allow access and modulation of the activity of individual fibers. Moreover, stimulation selectivity relies on having a clear picture of the anatomy of the peripheral nervous system, and of the role of the individual fibers in healthy and disease conditions. This challenge is related with the issue of transferability, not only between species, but also between nerves and tissues in the same species, arises from the anatomical and conduction differences among nerve fibers in the peripheral nervous system, which can affect their sensitivity and response to electrical stimulation (Günter et al. 2019). For example, the pancreatic branch differs from the gastric-projecting neurons in that they have i) longer duration of action potentials, ii) longer after-hyperpolarization decay time, iii) smaller soma area and iv) larger diameter (Babic and Travagli 2016). More research is needed to overcome this limitation, as only hypothetical extrapolation of the results is possible due to a lack of studies providing a comprehensive view of the similarities/divergences of parameters between nerve fibers and species.
It is also necessary to acquire a better understanding of the electrode-nerve interface and the design of new electrode materials and fabrication strategies that ensure safety during stimulation, reliability during chronic recording, full biostability and biocompatibility with the surrounding tissue, and long term preservation of their functionality (Günter et al. 2019; Walker et al. 2009; Luan et al. 2014; Zanos 2019).
Improved neural decoding algorithms, coupled with advances in data analysis techniques, are allowing us to extract real-time information about the state of the nerve and the related organs (Vitale and Litt 2018; Zanos 2019). Combining the processing of these neural recordings with data acquired during continuous monitoring of other biomarkers, and capitalizing on machine learning algorithms, presents an ideal strategy to characterize the state of the biological processes of interest in great detail.
Current artificial pancreas systems also have several challenges that need to be addressed if they are to be used in conjunction with bioelectronic medicine. Among them, the delays in interstitial glucose sensing and hormonal subcutaneous absorption (Ramli et al. 2019; Taleb et al. 2019; Gingras et al. 2018; Herrero et al. 2017), failures in the insulin pump (Meneghetti et al. 2018), and glycaemic control during meals (Ramkissoon et al. 2018) and exercise (Ramkissoon et al. 2020) are the most critical. Despite these challenges, the use of the artificial pancreas is associated with high levels of satisfaction and quality of life, and reduced fear of hypoglycaemic events among the users (Ramli et al. 2019). Moreover, there are ongoing strategies for educating the users to understand and operate these closed-loop systems (Lewis 2018; Ehrmann et al. 2018; Smeets et al. 2010). These observations suggest that novel technology integrating closed-loop neuromodulation and metabolic systems will be efficiently implemented and successfully adopted by the diabetes community.
A final challenge involves integrating all the recordings and acquired information into patient-specific closed-loop systems that adaptively adjust stimulation parameters to selectively drive nerve fiber functionality to achieve the desired state that restores or maintains homeostasis. To achieve this ultimate goal, innovative solutions for data transmission and storage, and power supply, are needed.
Conclusion
Closed-loop neuromodulation of metabolic dysfunction is a promising new pathway to treat diabetes, as conceptualized in Fig. 1. To fully exploit the potential benefits of bioelectronic medicine as a new form of therapy, however, medicine and technology should work in synchrony. Advances in minimally invasive technology to reliably interface with the nervous system are needed to take us one step closer to using bioelectronic therapies for optimal metabolic control in daily life. Accordingly, it is crucial to fully understand the biological processes that underlie the glucose-response reflex to enable the creation of more efficient and safe systems. We believe that 1 day this will become a reality and provide personalized diagnostics and treatment for people with diabetes.
Availability of data and materials
Not applicable.
Abbreviations
- GLP-1:
-
Glucagon-like peptide-1
- KHFAC:
-
Kilohertz frequency alternating current
- TENN:
-
Tissue-engineered neural network
References
Aelen P, Neshev E, Cholette M, Crisanti K, Mitchell P, Debru E, et al. Manipulation of food intake and weight dynamics using retrograde neural gastric electrical stimulation in a chronic canine model. Neurogastroenterol Motil. 2008;20:358–68. https://doi.org/10.1111/j.1365-2982.2007.01027.x.
Ahrén B. Autonomic regulation of islet hormone secretion - implications for health and disease. Diabetologia. 2000;43:393–410. https://doi.org/10.1007/s001250051322.
Ahren B, Veith RC, Taborsky GJ. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1) effects on basal release of insulin and glucagon. Endocrinology. 1987;121:323–331. doi:https://doi.org/10.1210/endo-121-1-323.
American Diabetes Association AD. Economic Costs of Diabetes in the U.S. in 2017. Diabet Care. 2018;41:dci180007. https://doi.org/10.2337/dci18-0007.
Apovian CM, Shah SN, Wolfe BM, Ikramuddin S, Miller CJ, Tweden KS, et al. Two-year outcomes of vagal nerve blocking (vBloc) for the treatment of obesity in the ReCharge trial. Obes Surg. 2017;27:169–76. https://doi.org/10.1007/s11695-016-2325-7.
Babic T, Travagli RA. Neural control of the pancreas. Pancreapedia Exocrine Pancreas Knowl Base. 2016. https://doi.org/10.3998/panc.2016.27.
Bailey TS, Bode BW, Buckingham BA, Forlenza GP, Pinhas-Hamiel O, Kaiserman K, et al. 1059-P: Glycemic Outcomes from the MiniMed ™ 670G System Pivotal Trials in Patients 2–75 Years of Age. Diabetes. 2019;68(Supplement 1):1059.
Bedows A. When a nerve can be your glucometer. Substrate. 2018; https://medium.com/the-substrate/when-a-nerve-can-be-your-glucometer-3a198e4da4f3. Accessed 19 Nov 2018.
Birmingham K, Gradinaru V, Anikeeva P, Grill WM, Pikov V, McLaughlin B, et al. Bioelectronic medicines: a research roadmap. Nat Rev Drug Discov. 2014;13:399–400. https://doi.org/10.1038/nrd4351.
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458.
Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter tri al of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381:1707–17. https://doi.org/10.1056/NEJMoa1907863.
Burneo JG, Faught E, Knowlton R, Morawetz R, Kuzniecky R. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59:463–4.
Cha R. Updates on gastric electrical stimulation to treat obesity: systematic review and future perspectives. World J Gastrointest Endosc. 2014;6:419.
Chang EH, Chavan SS, Pavlov VA. Cholinergic Control of Inflammation, Metabolic Dysfunction, and Cognitive Impairment in Obesity-Associated Disorders: Mechanisms and Novel Therapeutic Opportunities. Front Neurosci. 2019;13:263. https://doi.org/10.3389/fnins.2019.00263.
Chen J, Pasricha PJ, Yin J, Lin L, Chen JDZ. Hepatic electrical stimulation reduces blood glucose in diabetic rats. Neurogastroenterol Motil. 2010;22:1109–e286. https://doi.org/10.1111/j.1365-2982.2010.01556.x.
Cigaina V. Long-term follow-up of gastric stimulation for obesity: the Mestre 8-year experience. Obes Surg. 2004;14(Suppl 1):S14–22. https://doi.org/10.1007/bf03342133.
Conde SV, Guarino MP. Targeting bioelectronically the carotid sinus nerve in type 2 diabetes: strengths, drawbacks and challenges for the future. Bioelectron Med. 2018;1:167–70. https://doi.org/10.2217/bem-2018-0007.
Cork SC. The role of the vagus nerve in appetite control: implications for the pathogenesis of obesity. J Neuroendocrinol. 2018;30:e12643. https://doi.org/10.1111/jne.12643.
de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594:5791–815. https://doi.org/10.1113/JP271538.
Ehrmann D, Kulzer B, Schipfer M, Lippmann-Grob B, Haak T, Hermanns N. Efficacy of an education program for people with diabetes and insulinpumptreatment(INPUT): results from a randomized controlled trial. Diabetes Care. 2018;41:2453–62.
Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24:283–307. https://doi.org/10.1517/13543784.2015.974804.
Favretti F, De Luca M, Segato G, Busetto L, Ceoloni A, Magon A, et al. Treatment of morbid obesity with the transcend® implantable gastric stimulator (IGS®): a prospective survey. Obes Surg. 2004;14:666–70.
Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes Metab. 2018;20:245–56. https://doi.org/10.1111/dom.13052.
GKS H, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-Mediated Inhibition of Insulin Receptor Tyrosine Kinase Activity in TNF-alpha- and Obesity-Induced Insulin Resistance. Science. 1996;271:665–70.
Greenway F, Zheng J. Electrical stimulation as treatment for obesity and diabetes. J Diabetes Sci Technol. 2007;1:251–9. https://doi.org/10.1177/193229680700100216.
Güemes A, Georgiou P. Review of the role of the nervous system in glucose homoeostasis and future perspectives towards the management of diabetes. Bioelectron Med. 2018;4:9. https://doi.org/10.1186/s42234-018-0009-4.
Güemes A, Herrero P, Georgiou P. A novel glucose controller using insulin sensitivity modulation for Management of Type 1 diabetes. Int Symp Circuits Syst. 2019;1–5. https://doi.org/10.1109/ISCAS.2019.8702535.
Günter C, Delbeke J, Ortiz-Catalan M. Safety of long-term electrical peripheral nerve stimulation: review of the state of the art. J Neuroeng Rehabil. 2019;16:13 http://www.ncbi.nlm.nih.gov/pubmed/30658656. Accessed 19 Aug 2019.
Guyot M, Simon T, Ceppo F, Panzolini C, Guyon A, Lavergne J, et al. Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nat Biotechnol. 2019. https://doi.org/10.1038/s41587-019-0295-8.
Herrero P, Bondia J, Adewuyi O, Pesl P, El-Sharkawy M, Reddy M, et al. Enhancing automatic closed-loop glucose control in type 1 diabetes with an adaptive meal bolus calculator – in silico evaluation under intra-day variability. Comput Methods Prog Biomed. 2017;146:125–31. https://doi.org/10.1016/J.CMPB.2017.05.010.
Herrero P, El-Sharkawy M, Daniels J, Jugnee N, Uduku CN, Reddy M, et al. The bio-inspired artificial pancreas for type 1 diabetes control in the home: system architecture and preliminary results. J Diabetes Sci Technol. 2019;13:1017–25. https://doi.org/10.1177/1932296819881456.
Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 2018;11:203–13. https://doi.org/10.2147/JIR.S163248.
Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004;24:7604–13.
Koopman FA, Schuurman PR, Vervoordeldonk MJ, Tak PP. Vagus nerve stimulation: a new bioelectronics approach to treat rheumatoid arthritis? Best Pract Res Clin Rheumatol. 2014;28:625–35. https://doi.org/10.1016/J.BERH.2014.10.015.
Kovatchev B. Automated closed-loop control of diabetes: the artificial pancreas. Bioelectron Med. 2018;4:14. https://doi.org/10.1186/s42234-018-0015-6.
Kumar V, Prusik J, Lin Y, Hwang R, Feustel P, Pilitsis JG. Efficacy of alternating conventional stimulation and high frequency stimulation in improving spinal cord stimulation outcomes: a pilot study. Neuromodulation. 2018;21:466–71.
Levine YA, Koopman FA, Faltys M, Caravaca A, Bendele A, Zitnik R, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One. 2014;9:e104530. https://doi.org/10.1371/journal.pone.0104530.
Lewis D. Setting expectations for successful artificial pancreas/hybrid closed loop/automated insulin delivery adoption. J Diabetes Sci Technol. 2018;12:533–4. https://doi.org/10.1177/1932296817730083.
Lowe D. Galvani: GSK Goes “bioelectronic” | in the pipeline. Sci Transl Med. 2016; http://blogs.sciencemag.org/pipeline/archives/2016/08/02/galvani-gsk-goes-bioelectronic. Accessed 19 Nov 2018.
Luan S, Williams I, Nikolic K, Constandinou TG. Neuromodulation: present and emerging methods. Front Neuroeng. 2014;7:27. https://doi.org/10.3389/fneng.2014.00027.
MarketsandMarkets. Human Insulin Market worth 39.13 Billion USD by 2020. 2016. https://www.marketsandmarkets.com/PressReleases/human-insulin.asp. Accessed 27 May 2018.
Masi EB, Levy T, Tsaava T, Bouton CE, Tracey KJ, Chavan SS, et al. Identification of hypoglycemia-specific neural signals by decoding murine vagus nerve activity. Bioelectron Med. 2019;5:9. https://doi.org/10.1186/s42234-019-0025-z.
Matsuhisa M, Yamasaki Y, Shiba Y, Nakahara I, Kuroda A, Tomita T, et al. Important role of the hepatic vagus nerve in glucose uptake and production by the liver. Metabolism. 2000;49:11–6.
Medicine A for AB. Building a bioelectronic medicine movement 2019: insights from leaders in industry, academia, and research. Bioelectron Med. 2020;6:1. https://doi.org/10.1186/s42234-020-0037-8.
Meneghetti L, Terzi M, Del Favero S, Susto GA, Cobelli C. Data-Driven Anomaly Recognition for Unsupervised Model-Free Fault Detection in Artificial Pancreas. IEEE Trans Control Syst Technol. 2018:1–15. https://doi.org/10.1109/TCST.2018.2885963.
Meyers EE, Kronemberger A, Lira V, Rahmouni K, Stauss HM. Contrasting effects of afferent and efferent vagal nerve stimulation on insulin secretion and blood glucose regulation. Physiol Rep. 2016;4:e12718.
FDA. PMA P130013: FDA Summary of Safety and Effectiveness Data. Summary Of Safety And Effectiveness Data (SSED). 2013. p. 1–48.
Ramkissoon CM, Bertachi A, Beneyto A, Bondia J, Vehi J. Detection and control of unannounced exercise in the artificial pancreas without additional physiological signals. IEEE J Biomed Heal Informatics. 2020;24:259–67.
Ramkissoon CM, Herrero P, Bondia J, Vehi J, et al. Sensors (Basel). 2018;18. https://doi.org/10.3390/s18030884.
Ramli R, Reddy M, Oliver N. Artificial pancreas: current Progress and future outlook in the treatment of type 1 diabetes. Drugs. 2019;79:1089–101.
Rijnbeek EH, Eleveld N, Olthuis W. Update on peripheral nerve electrodes for closed-loop Neuroprosthetics. Front Neurosci. 2018;12:350. https://doi.org/10.3389/fnins.2018.00350.
Ruiz-Tovar J, Llavero C. Long-term effect of percutaneous electrical Neurostimulation of dermatome T6 for appetite reduction and weight loss in obese patients. Surg Laparosc Endosc Percutan Tech. 2016;26:212–5. https://doi.org/10.1097/SLE.0000000000000271.
Ruiz-Tovar J, Oller I, Diez M, Zubiaga L, Arroyo A, Calpena R. Percutaneous electrical Neurostimulation of dermatome T6 for appetite reduction and weight loss in morbidly obese patients. Obes Surg. 2014;24:205–11. https://doi.org/10.1007/s11695-013-1091-z.
Sacramento J, Prieto-Lloret J, Melo B, Conde S. High fat diet increases basal carotid sinus nerve activity and the responsiveness to hypoxia; 2016.
Sacramento JF, Chew DJ, Melo BF, Donegá M, Dopson W, Guarino MP, et al. Bioelectronic modulation of carotid sinus nerve activity in the rat: a potential therapeutic approach for type 2 diabetes. Diabetologia. 2018;61:700–10. https://doi.org/10.1007/s00125-017-4533-7.
Sarr MG, Billington CJ, Brancatisano R, Brancatisano A, Toouli J, Kow L, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012;22:1771–82. https://doi.org/10.1007/s11695-012-0751-8.
Saunders A, Messer LH, Forlenza GP. MiniMed 670G hybrid closed loop artificial pancreas system for the treatment of type 1 diabetes mellitus: overview of its safety and efficacy. Expert Rev Med Devices. 2019;16:845–53. https://doi.org/10.1080/17434440.2019.1670639.
Seicol BJ, Bejarano S, Behnke N, Guo L. Neuromodulation of metabolic functions: from pharmaceuticals to bioelectronics to biocircuits. J Biol Eng. 2019;13:67. https://doi.org/10.1186/s13036-019-0194-z.
Shamji MF, De Vos C, Sharan A. The advancing role of Neuromodulation for the Management of Chronic Treatment-Refractory Pain. Neurosurgery. 2017;80:S108–13. https://doi.org/10.1093/neuros/nyw047.
Shikora S, Toouli J, Herrera MF, Kulseng B, Zulewski H, Brancatisano R, et al. Vagal blocking improves glycemic control and elevated blood pressure in obese subjects with type 2 diabetes mellitus. J Obes. 2013;2013:245683. https://doi.org/10.1155/2013/245683.
Shikora SA, Wolfe BM, Apovian CM, Anvari M, Sarwer DB, Gibbons RD, et al. Sustained weight loss with vagal nerve blockade but not with sham: 18-month results of the ReCharge trial. J Obes. 2015;2015:365604. https://doi.org/10.1155/2015/365604.
Smeets PA, Erkner A, De Graaf C. Cephalic phase responses and appetite. Nutr Rev. 2010;68:643–55. https://doi.org/10.1111/j.1753-4887.2010.00334.x.
Sohbati M, Toumazou C. Personalized microchips for health care. IEEE Potentials. 2015;34:33–7. https://doi.org/10.1109/MPOT.2014.2358695.
Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engström Ruud L, Timper K, et al. AgRP neurons control systemic insulin sensitivity via Myostatin expression in Brown adipose tissue. Cell. 2016;165:125–38. https://doi.org/10.1016/J.CELL.2016.02.044.
Sundman E, Olofsson PS. Neural control of the immune system. Adv Physiol Educ. 2014;38:135–9. https://doi.org/10.1152/advan.00094.2013.
Taleb N, Tagougui S, Rabasa-Lhoret R. Single-hormone artificial pancreas use in diabetes: clinical efficacy and remaining challenges. Diabetes Spectr. 2019;32:205–8.
Thorens B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab. 2011;13:82–8. https://doi.org/10.1111/j.1463-1326.2011.01453.x.
Van Den Hoek AM, van Heijningen C, der Elst JP, Ouwens DM, Havekes LM, Romijn JA, et al. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57:2304–10.
Vitale F, Litt B. Bioelectronics: the promise of leveraging the body’s circuitry to treat disease. Bioelectron Med. 2018;1:3–7. https://doi.org/10.2217/bem-2017-0010.
Walker GM, Ramsey JM, Cavin RK, Herr DJC. A Framework for BIOELECTRONICS Discovery and Innovation. 2009. https://pdfs.semanticscholar.org/24de/c3d126345928ad5d55df201dcd7949143916.pdf?_ga=2.135289016.841683399.1527701335-669496615.1527701335. Accessed 30 May 2018.
World Health Organization, Organization WH. Global report on diabetes 2016. Isbn. 2016;978:88. doi:ISBN 978 92 4 156525 7.
Yao G, Kang L, Li J, Long Y, Wei H, Ferreira CA, et al. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat Commun. 2018;9:5349. https://doi.org/10.1038/s41467-018-07764-z.
Yi C-X, La Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta (BBA)-Molecular Basis Dis. 2010;1802:416–31.
Zanos TP. Recording and Decoding of Vagal Neural Signals Related to Changes in Physiological Parameters and Biomarkers of Disease. Cold Spring Harb Perspect Med. 2019;9(12):a034157.
Zhang X, van den Pol AN. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat Neurosci. 2016;19:1341–7.
Zhou A, Johnson BC, Muller R. Toward true closed-loop neuromodulation: artifact-free recording during stimulation. Curr Opin Neurobiol. 2018;50:119–27. https://doi.org/10.1016/J.CONB.2018.01.012.
Acknowledgements
The authors would like to acknowledge the Rafael del Pino Foundation, the Broadcom Foundation and the EPSRC for supporting A. Güemes during this work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AGG carried out the literature research and was a major contributor in writing the manuscript. PG and REC contributed in writing and reviewing the manuscript. All authors read and approved the final manuscript.
Authors’ information
AGG received the B.S. degree in Biomedical Engineering from the Universidad Polit\’{e}cnica de Madrid (UPM), Madrid, Spain, in 2016, and the M.S. degree Biomedical Engineering from Imperial College London (ICL), London, UK, in 2017. She is currently a Ph.D. candidate in the Centre for Bio-Inspired Technology at Imperial College London. Her research interests lie in the fields of neurotechnology and diabetes treatment. In particular, she has worked in the design of physiological models of the pancreatic secretion and the development of glucose controllers for Type 1 diabetes management. Her PhD is focused on extending current models by including new insights into the neuro-pancreatic regulation through the application of bioelectronics medicine. PG received the M.Eng. degree in electrical and electronic engineering and the Ph.D. degree from Imperial College London (ICL), London, U.K., in 2004 and 2008, respectively. He is currently a Reader with the Department of Electrical and Electronic Engineering, ICL, where he is also the Head of the Bio- Inspired Metabolic Technology Laboratory, Centre for Bio-Inspired Technology. His research includes bio-inspired circuits and systems, CMOS based Lab-on-Chip technologies, and application of microelectronic technology to create novel medical devices. He has made significant contributions to integrated chemical-sensing systems in CMOS, conducting pioneering work on the development of ISFET sensors, which has enabled applications, such as point-of-care diagnostics and semiconductor genetic sequencing and has also developed the first bio-inspired artificial pancreas for treatment of type 1 diabetes using the silicon-beta cell. He received the IET Mike Sergeant Medal of Outstanding Contribution to Engineering in 2013. He is a member of the IET and serves on the BioCAS and Sensory Systems technical committees of the IEEE CAS Society. He is also the CAS representative on the IEEE Sensors council and the IEEE Distinguished Lecturer in Circuits and Systems.
REC received his B.Sc. in physics in 1988 from Lincoln University, Pennsylvania. He completed his M.S.E.E (‘91) and PhD (‘94) in electrical engineering at the University of Pennsylvania. Currently, Dr. Etienne-Cummings is a Professor and Chairman of Department of Electrical and Computer Engineering at Johns Hopkins University. He was the founding director of the Institute of Neuromorphic Engineering. He has served as Chairman of various IEEE Circuits and Systems (CAS) Technical Committees and was elected as a member of CAS Board of Governors. He also serves on numerous editorial boards and was recently appointed Deputy Editor in Chief for the IEEE Transactions on Biomedical Circuits and Systems. He is an IEEE Fellow and the recipient of the NSF’s Career and Office of Naval Research Young Investigator Program Award. He was a Visiting African Fellow at U. Cape Town, Fulbright Fellowship Grantee, Eminent Visiting Scholar at U. Western Sydney and has also won numerous publication awards, most recently the 2012 Most Outstanding Paper of the IEEE Transaction on Neural Systems and Rehabilitation Engineering. He was also recently recognized as a ScienceMaker by the HistoryMakers, an African-American history archive. He has published over 220 peer reviewed article, 11 books/chapters and holds 10 patents/applications on his work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Güemes Gonzalez, A., Etienne-Cummings, R. & Georgiou, P. Closed-loop bioelectronic medicine for diabetes management. Bioelectron Med 6, 11 (2020). https://doi.org/10.1186/s42234-020-00046-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42234-020-00046-4