Abstract
Background
One of the most common problems in parturients receiving regional anesthesia during cesarean section is shivering. It usually interferes with the readings of the oxygen plethysmography (SpO2) and electrocardiogram (ECG). It expands the needs for oxygen and increases creation of carbon dioxide about four folds.
The aim of this work is to compare the efficacy of dexamethasone and dexmedetomidine in prevention of perioperative shivering when added to hyperbaric bupivacaine intrathecally in cesarean sections (CS) and their effect on the intraoperative hemodynamics, intensity of the block, sedation, and postoperative analgesic requirement.
Results
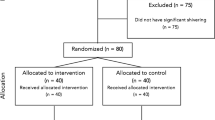
Study included 60 obstetric patients who fulfilled all the inclusion criteria and were randomized into 2 equal groups, each consisting of 30 patients, namely group A (dexmedetomidine group) and B (dexamethasone group).
-
Group A patients received 5 μg dexmedetomidine with 12.5 mg hyperbaric bupivacaine 0.5% intrathecally.
-
Group B patients received 8 mg dexamethasone then 12.5 mg hyperbaric bupivacaine 0.5% intrathecally.
The comparison included assessment of intra- and postoperative hemodynamics, duration of surgery, assessment of sensory and motor block, assessment for shivering and sedation, and assessment of adverse events.
This study showed that there were a small number of patients complaining of shivering (five patients in group A and seven patients in group B) with no statistical difference between both groups in the incidence and intensity of shivering. Time to two segment regression (minutes) was longer in group B compared to group A, and also, time to first analgesic rescue was longer in group B compared to group A. For sedation intensity, there was statistical difference between both groups as all patients in group A were sedated compared to six patients only in group B. There was no statistical difference between both groups as regards incidence of adverse effects.
Conclusion
We concluded that both drugs can be added safely to bupivacaine, and both dexmedetomidine and dexamethasone decreased the incidence and the intensity of shivering. Dexamethasone was found to prolong the duration of sensory block and delay opioid requirements post-operatively, while dexmedetomidine is more effective in sedating the patients intra- and postoperatively.
Similar content being viewed by others
Background
One of the most common problems in parturients receiving regional anesthesia during caesarean section is shivering. It usually interferes with the readings of the oxygen plethysmography (SpO2) and electrocardiogram (ECG). It can likewise expand the needs for oxygen and increase creation of carbon dioxide about four folds; it also increases the intensity of the pain arising from the wound; it can delay the healing of the wound and can also increase the length of stay in the post anesthesia care unit (PACU) (Nasseri et al., 2017).
The thermoregulatory system is usually disrupted by spinal anesthesia (SA) through inhibiting of vasoconstriction which has an important role in the regulation of the temperature. The spinal anesthesia also redistributes the central core heat to the tissues in the periphery. Those two effects lead to hypothermia and shivering (Yanshuai & Shuang, 2017).
Dexmedetomidine is a highly selective α2-adrenoreceptor agonist that binds to a transmembrane G protein-binding receptor. The studies done showed that it can be added to local anesthetics in the spinal anesthesia to decrease time needed for the start of the block, decrease the pain severity postoperatively, increase the time of the block, and decrease the analgesic use postoperatively. It decreases the incidence of shivering through increasing vasodilatation and inhibition of central thermoregulation (Zhang et al., 2017).
Dexamethasone is known to modify the inflammatory reaction of the body and reduce the gradient between central and peripheral tissue temperatures that is why it has been used as an intravenous medication to decrease shivering. It has been also used to be injected in the cerebrospinal fluid safely and can be given as an adjuvant to local anesthetics to enhance the efficacy of regional anesthesia, so we considered that dexamethasone can be used as an adjuvant to local anesthetics intrathecally to decrease the intensity of shivering (Solphour et al., 2016).
The aim of this work is to compare the efficacy of dexamethasone and dexmedetomidine in prevention of perioperative shivering when added to hyperbaric bupivacaine intrathecally in cesarean sections (CS) and their effect on the intraoperative hemodynamics, intensity of the block, sedation, and postoperative analgesic requirement.
Methods
Randomized prospective double blinded comparative study was carried out at a gynecology and obstetrics hospital from June 2018 to January 2020 after obtaining approval from ethical committee number FMASU M D 46/2018, and written informed consent from all participants will be obtained.
Inclusion criteria
American Society of Anesthesiology physical status (ASA-PS) ІІ female patients, gestational age > 37 weeks, and age from 20 to 40 years who are scheduled for cesarean section under spinal anesthesia
Exclusion criteria
Patient refusal, age < 20 or > 40, known allergy to the study medication, patients with coagulopathies or on anticoagulant medications, cerebrovascular insufficiency, neuromuscular diseases, severe preeclampsia, cardiopulmonary disease, hyperthyroidism, diabetic neuropathy, peripheral vascular diseases, renal and hepatic disorders, and patients with psychiatric disorders were excluded from the study.
The study included 60 obstetric patients who fulfill all the points in the inclusion and exclusion criteria and were randomized into 2 equal groups, each consisting of 30 patients, namely group A (dexmedetomidine group) and B (dexamethasone group).
-
Group A patients will receive 5 μg dexmedetomidine with 12.5 mg hyperbaric bupivacaine 0.5% intrathecally.
-
Group B patients will receive 8 mg dexamethasone then 12.5 mg hyperbaric bupivacaine 0.5% intrathecally.
Routine preoperative assessment was done for each patient including routine history taking, clinical examination, and laboratory investigations (complete blood picture, kidney function tests, liver function tests, prothrombin time, partial thromboplastin time).
Patients did not receive any premedication. All the patients were preloaded with 10 ml/kg of lactated Ringer’s solution through 18 gauge intravenous cannula and monitored with five leads electrocardiography, pulse oximetry, and non-invasive blood pressure (NIBP) (which records systolic, diastolic, and mean blood pressure every 5 min intraoperatively and every 15 min in the recovery room, heart rate was recorded in the same intervals).
Under complete aseptic technique, local anesthetic in the form of 3 ml of lidocaine 2% was given at the site of spinal injection. Subarachnoid block was administered in the sitting position midline approach with 27 gauge (Quincke needle) at L3–L4/L4–L5 space. For group A, the preservative-free dexmedetomidine 100 μg/ml was loaded into a 100 unit insulin syringe (1 μg/unit) and 5 units was given, after that patients received 2.5 ml (12. 5 mg) of 0.5% hyperbaric bupivacaine. For group B, dexamethasone 8 mg/2 ml was given intrathecally, after that 2.5 ml (12.5 mg) of 0.5% hyperbaric bupivacaine was given separately. Patients were immediately placed in the supine position after completing the spinal block.
The assessment of sensory blockade was performed by pin prick method using a 25 gauge needle at every 2 min until the highest level was achieved, and then every 15 min interval until two segment regression of the block occurs. Motor blockade was assessed at the same time intervals using a modified Bromage scale (Moeen & Moeen, 2017) (0 = no paralysis, 1 = unable to raise extended legs, 2 = unable to flex knee, 3 = unable to flex ankle). Peak sensory level and time to reach this level, time to two segment regression, duration of sensory block, motor block onset (time to reach Bromage 4), duration of motor blockade, and time to first analgesic rescue (pethidine 0.5 mg/kg) were recorded.
Shivering intensity was assessed with a five-point scale validated by Crossley and Mahajan, where 0 = no shivering, 1 = piloerection or peripheral vasoconstriction but no visible shivering, 2 = muscular activity in only one muscle group, 3 = muscular activity in more than one muscle group, and 4 = whole body shivering (Nasseri et al., 2017). Shivering incidence and intensity was registered every 15 min during surgery and in the recovery room.
Sedation was checked every 15 min during surgery and in the recovery room and quantified as 0 = fully awake; 1 = somnolent; 2 = closed eyes, opens to call; 3 = closed eyes, opens to physical stimuli; and 4 = closed eyes, non-responsive to painful stimuli (Nasseri et al., 2017).
Hypotension is defined as fall in blood pressure of more than 30% of baseline value and was treated with incremental doses of 5 mg IV injection of ephedrine and IV infusion of 250 ml lactated Ringer. Bradycardia is defined as heart rate less than 60 beats per minute and treated with injection of bolus of 0.01–0.02 mg/kg of atropine; duration of surgery was noted; nausea/vomiting was treated with injection ondansetron 4 mg IV.
Statistical analysis
The collected data will be revised, coded, tabulated, and introduced to a PC using Statistical Package for Social Sciences (SPSS 15.0.1 for windows; SPSS Inc, Chicago, IL, 2001). Data will be presented as mean and standard deviation (± SD) for quantitative parametric data and median and interquartile range for quantitative non-parametric data. Frequency and percentage will be used for presenting qualitative data. Suitable analysis will be done according to the type of data obtained. Student’s t test or Mann–Whitney test will be used to analyze quantitative data while Chi square test and Fisher extract test will be used to analyze qualitative data. P value < 0.05 will be considered statistically significant.
Sample size
Using PASS program, setting alpha error 5% and power 80%. Result from previous study (Qi et al., 2016a) showed that 33% of cases receiving dexmedetomidine had shivers compared to 6% among cases receiving dexamethasone (Moeen & Moeen, 2017). Based on this, the needed sample is 30 cases per group (total 60), and the estimated effect size according to Cohen’s d = (0.066666 − 0.333333)/0.377123 = 0.707109.
Results
Sixty obstetric patients who fulfilled all the inclusion criteria were randomized into two equal groups, each consists of 30 patients namely group A (dexmedetomidine group) and group B (dexamethasone group). All the patients completed the study.
Demographic data
Age, weight, height, and gestational age were recorded for each patient at the beginning of the study and were compared statistically as shown in Table 1.
There was no statistical difference between both groups as regards demographic data.
Assessment for shivering and sedation
There were a small number of patients complaining of shivering (five patients in group A and seven patients in group B) with no statistical difference between both groups in the incidence and intensity of shivering as shown in Table 2.
For sedation intensity, there was statistical difference between both groups as all patients in group A were sedated while six patients only in group B as shown in Table 3.
Assessment of intra- and postoperative hemodynamics
Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), and heart rate (HR) were recorded intraoperatively every 5 min and postoperatively every 15 min, and there was no statistical difference between both groups (Tables 4, 5, 6, and 7).
Duration of surgery and assessment of sensory and motor block
As shown in Table 8, there was no statistical difference between both groups as regards duration of surgery.
For peak sensory level and the time to reach this level and also for motor blockade onset and motor block duration, there was no statistical difference between the two groups as shown in Table 8.
As regards time to two segment regression (minutes), there was statistical significance as it was longer time in group B (96.32 ± 9.8) compared to (76.24 ± 8.34) group A as shown in Table 8; also, duration of sensory block was longer in group B (161.83 ± 7.00) compared to (124.50 ± 6.72) group A.
Also time to first analgesic rescue was statistically significant as it was longer time in group B (198.21 ± 21.22) compared to (174.44 ± 16.3) as shown in Table 8.
Assessment of adverse events
Most of the patients in both groups complaining of hypotension (blood pressure < 30% of the pre induction value) especially at the first 15 min after spinal anesthesia were treated with incremental doses of 5 mg IV ephedrine sulfate with no statistical difference between both groups as shown in Table 9.
The same for bradycardia, three cases in group A and two cases in group B were treated with atropine sulfate 0.01–0.02 mg/kg with no statistical difference as shown in Table 9.
Only two cases complaining of nausea and vomiting in group A and three cases in group B were treated with ondansetron 4 mg IV with no statistical difference as shown in Table 9.
Discussion
Spinal anesthesia is long established technique used in cesarean section as it supplies competent anesthesia and analgesia while avoiding the complication with general anesthesia. Shivering is a frequent dreadful complication which causes increased needs for oxygen and increased release of carbon dioxide and metabolic rate. Shivering causes disruption of the reading of the electrocardiogram, blood pressure, and oximetry. Moreover, it is an unpleasant experience for the pregnant woman (Varshney et al., 2019).
A lot of attempts have been tried to prevent shivering whether pharmacological or non-pharmacological, but the most appropriate method is not yet settled. Dexmedetomidine has highly specific alpha 2 adrenergic receptor-agonist, affecting mainly the central nervous system without respiratory depression. Dexmedetomidine was proved to decrease post anesthetic shivering when given intravenously in a dose 0.5 to 1 μg/kg. But it is inappropriate to be used intravenously due to its possible side effects. In contrast to intravenous dexmedetomidine, the effect of intrathecal dexmedetomidine on shivering is not well-known (Rai & Bhutia, 2017); this study hypothesized that intrathecal dexmedetomidine could decrease the incidence and intensity of shivering.
Dexamethasone also was used to reduce postoperative shivering and known to be effective; there was no significant difference between the three groups of dexamethasone, pethidine, and normal saline receivers regarding the reduction of shivering in the study done to compare the dexamethasone with pethidine in the control of perioperative shivering during transurethral prostatectomy (Moeen & Moeen, 2017).
The aim of this study is to evaluate the efficacy of both dexamethasone and dexmedetomidine in prevention of perioperative shivering when added to hyperbaric bupivacaine intrathecally in cesarean sections and their effect on the intraoperative hemodynamics, intensity of the block, sedation, postoperative pain, and analgesic requirement.
Randomized prospective comparative study was carried out at a gynecology and obstetrics hospital after obtaining approval from ethical committee. The study population comprised of 60 patients with ASA physical status II, scheduled for elective caesarean section.
As regards results from the current study, hemodynamic data including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), and heart rate (HR) were statistically insignificant between both groups.
As regards the duration of surgery, peak sensory level, time to reach peak sensory level, motor blockade onset, and motor blockade duration, there was no statistical significance between the 2 groups. But as regards the time of 2 segment regression (minutes), it was longer in dexamethasone group (96.71 ± 3.77) compared to (76.68 ± 4.62) in dexmedetomidine group.
This results agreed well with the study which revealed that the addition of intrathecal dexamethasone to bupivacaine for spinal anesthesia in orthopedic surgeries significantly prolong the duration of sensory block and decrease opioid requirements in post-operative management (Bani-Hashem et al., 2011).
Another study which studied the effect of lignocaine plus dexamethasone 4 mg compared to lignocaine plus 100 μg epinephrine intrathecally in patients undergoing cesarean sections and revealed that intrathecal dexamethasone increased the sensory block duration and prolonged the duration of analgesia more than epinephrine (Naziri et al., 2010).
The same results agreed with the results of the study in which they added dexamethasone (4 mg) to levobupivacaine in parturients receiving combined spinal epidural analgesia for vaginal delivery and concluded that it prolonged the duration of analgesia (Wahdan et al., 2017).
Also, this study results agreed with the results of the study in which dexamethasone 5 mg was added to 0.5% hyperbaric bupivacaine in spinal anesthesia for patients undergoing lower abdominal urological and lower limb orthopedic surgeries and revealed that the addition of dexamethasone to bupivacaine in spinal anesthesia significantly improve the duration of sensory block/surgical analgesia as well as postoperative analgesia/pain-free period without any complications (Haque et al., 2018).
When the peripheral tissues are subjected to acute painful stimulus, this leads to the release of substances as glutamate and aspartate which causes stimulation of the dorsal horn neurons of the spinal cord. Activation of N-methyl D–aspartate (NMDA) receptors by those two amino acids leads to activation of phospholipase A2 through activation of calcium channels and increase calcium ion influx. The phospholipase A2 is responsible for the activation of arachidonic acid from membrane phospholipase. Corticosteroids can produce calcium dependent phospholipid binding protein called annexin which inhibits phospholipase A2 which decreases the prostaglandin synthesis (Yao et al., 1999).
The results of the current study showed that addition of both dexmedetomidine and dexamethasone to the hyperbaric bupivacaine in spinal anesthesia for CS decreased the incidence and the intensity of shivering as 5 patients only in dexmedetomidine group complained of grade 1 and grade 2 shivering out of 30 patients, while 7 patients only out of 30 in dexamethasone group complained of shivering grades 1, 2, and 3 (Fig. 1).
It seems that the antishivering effects of dexmedetomidine are mediated by binding to α2 receptors in the brain and the spinal cord which reduce central thermosensitivity via attenuating the conductance of neurons (Bajwa et al., 2012).
It was reported that there is a lower incidence of shivering (2 in 31) following SA by heavy bupivacaine 0.5% plus 5 μg intrathecal dexmedetomidine for lower abdominal surgeries compared with (12 of 31) in the control group (Abdelhamid & El-Lakany, 2013).
Regarding the intrathecal dexmedetomidine and its effect on shivering, information about that is limited; in a randomized controlled trial done, they investigated the efficacy of dexmedetomidine when added to local anesthetics intrathecally in the prevention of shivering in 80 patient who underwent transurethral resection of prostate with spinal anesthesia. Eighty patients were divided into 2 groups; one received 12.5 mg hyperbaric bupivacaine plus 0.5 ml isotonic saline; the other group received 12.5 mg hyperbaric bupivacaine plus 10 μg dexmedetomidine in 0.5 ml isotonic saline. The incidence of shivering was significantly lower (15%) in the dexmedetomidine group than the saline group (57%). They concluded that adding 10 μg dexmedetomidine to hyperbaric bupivacaine in the transurethral resection of the prostate procedure could reduce the incidence of shivering; this result agreed with the current study in spite of using higher dose of dexmedetomidine 10 μg (Moawad & Elawdy, 2015).
Another study compared the effects of 5 μg intrathecal dexmedetomidine with 100 μg morphine as supplements to 10 mg bupivacaine in 120 parturients undergoing elective CS under SA. Only 7.7% of patients in the dexmedetomidine group had shivering, while 30% and 35.9% of patients in the morphine and the bupivacaine groups experienced shivering, respectively. Also this result is in agreement with our current study (Qi et al., 2016b).
Also, the study compared intrathecal morphine and intrathecal dexmedetomidine in patient undergoing gynecological surgeries under SA, and has shown that dexmedetomidine may be safely used as an intrathecal supplement in cesarean delivery. Respiratory depression is a potential side effect of dexmedetomidine. But this side effect is just reported to occur by using high doses of dexmedetomidine. Intrathecal optimal dose 2.5 or 5 μg dexmedetomidine does not cause respiratory depression, and this dose agrees well with the dose used in our current study (Kurhekar et al., 2016).
Another study which used the same dose as our study, 5 μg intrathecal dexmedetomidine, as an adjuvant to hyperbaric bupivacaine in spinal anesthesia for patients undergoing CS concluded that the drug is safe and effective in decreasing the incidence and intensity of shivering using the dose of 5 μg, but 2.5 μg intrathecally did not alleviate shivering (He et al., 2017).
The same results agreed with the study which also used 5 μg intrathecal dexmedetomidine in spinal anesthesia in CS and concluded that it significantly reduced the incidence and intensity of shivering without major side effects (Nasseri et al., 2017).
Meta-analysis and trial sequential analysis done to investigate the effect of intrathecal dexmedetomidine on prevention of shivering in CS after spinal anesthesia showed that intrathecal dexmedetomidine reduced the risk of shivering in patients undergoing CS under spinal anesthesia and did not increase the incidence of side effects as PONV, hypotension, or bradycardia. However the trial sequential analysis indicated that further trials are still needed to confirm the abovementioned findings (Miao et al., 2018).
Another study compared intrathecal dexmedetomidine and intrathecal magnesium sulfate for the prevention of post spinal anesthesia shivering in uroscopic surgeries, in which 5 μg of dexmedetomidine was used as in our current study, and concluded that both drugs were effective in reducing the incidence of post spinal shivering; 5 patients (14.3%) in dexmedetomidine group, 8 patients (22.8%) in magnesium sulfate group, and 21 patients (60%) in control group developed shivering, and the use of MgSO4 was recommended as it is more physiologically, more readily available in most operating theaters and much less expensive than dexmedetomidne (Omar et al., 2019).
The results of our current study showed that dexamethasone 8 mg used intrathecally combined with hyperbaric bupivacaine in cesarean sections was effective and safe in decreasing the incidence and intensity of post-spinal shivering.
These results agree with study which investigated intrathecal dexamethasone versus meperidine for prevention of shivering during transurethral prostatectomy and concluded that intrathecal dexamethasone effectively prevented post-spinal shivering with similar effects as intrathecal meperidine and with less side effects (Moeen & Moeen, 2017).
As regards sedation score in the current study, there was high significance between both groups. As all the patient in dexmedetomidine group were sedated with different grades, 15 patients were mildly sedated (grade 1), 13 patients (grade 2), and only 2 patients were deeply sedated (grade 3) compared to only 6 patients in dexamethasone group who were mildly sedated (grade 1).
These results agreed with the two studies in which all the patients of dexmedetomidine group were sedated with different grades of sedation (Fig. 2) (Nasseri et al., 2017; Omar et al., 2019).
As regards side effects, most of the patients in both groups complaining of hypotension (blood pressure < 30% of the pre induction value) especially at the first 15 min after spinal anesthesia were treated with incremental doses of 5 mg IV ephedrine sulfate with no statistical difference between both groups. The same for bradycardia, three cases in group A and two cases in group B were treated with atropine sulfate 0.01–0.02 mg/kg with no statistical difference. Only two cases complaining of nausea and vomiting in group A and three cases in group B were treated with ondansetron 4 mg IV with no statistical difference, and these results for both groups agreed with the results of the studies (Nasseri et al., 2017; Moeen & Moeen, 2017; Miao et al., 2018).
Conclusion
This comparative study between dexmedetomidine and dexamethasone as an intrathecal adjuvant for prevention of perioperative shivering in cesarean sections showed that both drugs can be added safely to bupivacaine, and both dexmedetomidine and dexamethasone decreased the incidence and the intensity of shivering.
Dexamethasone was found to significantly prolong the duration of sensory block and delay opioid requirements in postoperative management, while dexmedetomidine is more effective in sedating the patients during the intra- and postoperative period.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CS:
-
Cesarean section
- SpO2 :
-
Oxygen plethysmography
- ECG:
-
Electrocardiogram
- PACU:
-
Post-anesthesia care unit
- SA:
-
Spinal anesthesia
- ASA-PS:
-
American Society of Anesthesiologists—physical status
- SPSS:
-
Statistical Package for Social Sciences
- NIBP:
-
Non-invasive blood pressure
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- MBP:
-
Mean blood pressure
- HR:
-
Heart rate
- NMDA:
-
N-methyl D-aspartate
References
Abdelhamid SA, El-Lakany MH (2013) Intrathecal dexmedetomidine: useful or not? J Anesth Clin Res 4:9
Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S (2012) Reduction in the incidence of shivering with perioperative dexmedetomidine: a randomized prospective study. J Anaesthesiol Clin Pharmacol 28:86–91
Bani-Hashem N, Hassan-Nasab B, Pour EA, Maleh PA, Nabavi A, Jabbari A (2011) Addition of intrathecal dexamethasone to bupivacaine for spinal anesthesia in orthopedic surgery. Saudi J Anaesth 5(4):382–386
Haque MM, Aleem MA, Haque FH, Siddique AB, Afrose R (2018) Efficacy of 0.5% hyperbaric bupivacaine with dexamethasone versus 0.5% hyperbaric bupivacaine alone in spinal anaesthesia for patient undergoing lower abdominal urological and lower limb orthopedic surgeries. Mymensingh Med J 27(2):375–381
He L, Xu JM, Liu SM, Chen ZJ, Li X, Zhu R (2017) Intrathecal dexmedetomidine alleviates shivering during cesarean delivery under spinal anesthesia. Biol Pharm Bull 40:169–173
Kurhekar P, Kumar SM, Sampath D (2016) Comparative evaluation of intrathecal morphine and intrathecal dexmedetomidine in patients undergoing gynaecological surgeries under spinal anaesthesia: a prospective randomised double blind study. Indian J Anaesth 60(6):382–387
Miao S, Shi M, Zou L, Wang G (2018) Effect of intrathecal dexmedetomidine on preventing shivering in cesarean section after spinal anesthesia: a meta-analysis and trial sequential analysis. Drug Des Devel Ther 12:3775–3783
Moawad HE, Elawdy M (2015) Efficacy of intrathecal dexmedetomidine in prevention of shivering in patients undergoing transurethral prostatectomy: a randomized controlled trial. Egypt J Anaesth 31:181–187
Moeen SM, Moeen AM (2017 Aug) Intrathecal dexamethasone vs. meperidine for prevention of shivering during transurethral prostatectomy: a randomized controlled trial. Acta Anaesthesiol Scand 61(7):749–757. https://doi.org/10.1111/aas.12920
Nasseri K, Ghadami N, Nouri B (2017) Effect of intrathecal dexmedetomidines on shivering after spinal anesthesia for cesarean sections: a double blind randomized clinical trial. Drug Des Devel Ther 11:1107–1113
Naziri F, Rabiee SM, Banihashem N, Hosseinjanzadeh K, Shirkhani Z, Solimanian SS (2010) Comparative study of intrathecal dexamethasone with epinephrine as adjuvants to lidocaine in cesarean section
Omar H, Aboella WA, Hassan MM et al (2019) Comparative study between intrathecal dexmedetomidine and intrathecal magnesium sulfate for the prevention of post-spinal anaesthesia shivering in uroscopic surgery; (RCT). BMC Anesthesiol 19(1):190
Qi X, Chen D, Li G et al (2016b) Comparison of intrathecal dexmedetomidine with morphine as adjuvants in cesarean sections. Biol Pharm Bull 39:1455–1460
Qi X, Meyer N, Huang X et al (2016a) Intrathecal dexmedetomidine as adjuvant to ropivacaine in hysteroscopic surgery; a prospective, randomized control study. Int J Clin Pharmacol Ther 54(3):185–192
Rai A, Bhutia MP (2017) Dexmedetomidine as an additive to spinal anaesthesia in orthopaedic patients undergoing lower limb surgeries: a randomized clinical trial comparing two different doses of dexmedetomidine. J Clin Diagn Res 11(4):UC09–UC12
Solphour A, Jafari A, Hashemi M et al (2016) A comparison of prophylactic use of meperidine, meperidine plus dexamethasone, and ketamine plus midazolam for preventing of shivering during spinal anesthesia: a randomized double blind placebo controlled study. J Clin Anesth 34:128–135
Varshney RK, Garg M, Kapoor K, Jheetay GS (2019) The role of ramosetron in the prevention of post-spinal shivering in obstetric patients. A prospective randomized double blind study. Rom J Anaesth Intensive Care 26(1):37–43
Wahdan AS, El-Sakka AI, Gaafar HMI (2017) The effect of addition of dexamethasone to levobupivacaine in parturients receiving combined spinal-epidural for analgesia for vaginal delivery. Indian J Anaesth. 61(7):556–561
Yanshuai M, Shuang Q (2017) Effects of dexmedetomidine in reducing post-cesarean adverse reactions. Exp Ther Med 14:2036–2039
Yao XL, Cowan MJ, Glawin MT, Lawrence MM, Angus CW, Shelhamer JH (1999) Dexamethasone alters arachidonate release from human epithelial cells by induction of P11 protein synthesis and inhibition of phospholipase a 2 activity. J Biol Chem 274:17202–17208
Zhang J, Zhang X, Wang H, Zhou H, Tian T, Wu A (2017) Dexmedetomidine as a neuraxial adjuvant for prevention of perioperative shivering: meta-analysis of randomized controlled trials. PLoS One 12(8):e0183154
Acknowledgements
We have no affiliations with or involvement in any organization or entity that we have any financial interests
Funding
Self-funding
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. Dr. MI: Literature search; Writing the article; Provision of materials, patients, and resources. Prof. Dr. OS: Conception and design; Critical revision of the article; Final approval of the article. Dr. AA: Statistical expertise; Critical revision of the article; Final approval of the article. Dr. OM: Literature search; Writing the article; Provision of materials, patients, and resources; Data collection. Dr. MA: Literature search; Provision of materials, patients, and resources; Analysis and interpretation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval of research ethical committee of Faculty of Medicine, Ain-Shams University was obtained (code number: FMASU M D 46/2018) and written informed consent from all participants was obtained.
Consent for publication
Written informed consent was obtained from all subjects or their legal surrogate.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ismaiel, M.A.M.A.N., El Safty, O.M.T., El-Agamy, A.E.S. et al. A comparative study between dexmedetomidine and dexamethasone as an intrathecal adjuvant for prevention of perioperative shivering in cesarean section. Ain-Shams J Anesthesiol 12, 53 (2020). https://doi.org/10.1186/s42077-020-00102-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-020-00102-w