Abstract
Background
Adenoid cystic carcinoma and primary squamous cell carcinoma are both rare breast neoplasms, which possess drastically different morphological and molecular features as well as distinguishing clinical behaviors and prognosis.
Case presentation
In this report, we described a rare case in which concurrent adenoid cystic carcinoma and keratinizing squamous cell carcinoma were diagnosed in contralateral breasts in an 85 year-old female patient. The patient had a history of adenoid cystic carcinoma diagnosed 11 years ago, which was treated by partial mastectomy followed by whole breast radiation. The recurrent carcinoma on the same side of the breast was small in size but appeared to involve an intraductal papilloma. Also, a newly occurred large cystic mass was identified on the contralateral breast, which histologically presented as a keratinizing squamous cell carcinoma with no glandular differentiation. No in situ or invasive carcinoma was identified in the overlying skin of the lesion, and no malignancy in a second site was found by PET-CT. Therefore, this lesion was mostly likely a primary squamous cell carcinoma of the breast.
Conclusion
The concurrence of two such rare neoplasms was likely an incidental finding or was therapy-related. However, more mechanistic studies are needed in order to understand whether predisposing genetic alterations exist in this rare case. Besides, cases of both breast adenoid cystic carcinoma and carcinoma with predominant squamous differentiation diagnosed in our institution were reviewed, which help to better characterize their clinicopathological features.
Similar content being viewed by others
Background
Adenoid cystic carcinoma (ACC) is a malignant neoplasm mostly diagnosed in salivary glands and is a rare finding in the breast. Histologically, ACC possesses a dual population of luminal epithelial and myoepithelial cells, and has different morphological variants including cribriform, tubular or solid patterns. Breast ACC is mostly triple-negative, but generally has an indolent clinical behavior and favorable prognosis. In contrast, squamous cell carcinoma (SCC) is an even rarer entity diagnosed in the breast, with a much worse prognosis. The main differential diagnosis for breast SCC are metaplastic carcinoma and metastatic SCC from a second site. Primary squamous cell carcinoma (PSCC) is a type of metaplastic breast carcinoma with predominant squamous differentiation. Molecularly, ACC is featured by MYB-NFIB translocation, while no specific genetic alteration has been described in SCC. Here, we reported a rare case in which ACC and PSCC were concurrently diagnosed in contralateral sides of the breast in an elderly female patient.
Case presentation
A 85-year-old female patient with no significant medical history was initially diagnosed with right breast ACC in 2009. The lesion was 5 cm in size and was negative for estrogen receptor (ER), progesterone receptor (PR), and HER2. She was treated by partial mastectomy and sentinel lymph node excision, and all three lymph nodes examined were negative for metastatic carcinoma. She was then treated by whole breast radiation to the right breast. The patient did not undergo recommended routine mammograms for several years and represented to the hospital with enlarging left breast mass 11 years after the initial diagnosis.
On physical examination, no mass was identified in the right breast on palpation. However, a large and firm mass could be palpated in the upper outer quadrant of the left breast. The mass appeared to move independently of the underlying chest wall and overlying skin. Diagnostic mammogram identified a subcentimeter circumscribed equal density mass in the central right breast (Fig. 1a). Besides, a big hyperdense partially circumscribed mass with associated scattered calcifications was identified in the upper outer quadrant of the left breast (Fig. 1b, c). The left breast skin showed abnormal thickening in the anterolateral aspect. Both masses were PET avid, and no hypermetabolic lesions at another site was detected by PET-CT. The right mass was then removed by partial mastectomy, and the left mass was treated by a total mastectomy followed by radiation.
Radiologic findings. a Mammography for right breast showing a circumscribed subcentimeter mass (arrowhead). Previous surgical site can also be identified (arrow). b Mammography for left breast showing a partly circumscribed mass with thickened overlying skin. No connection between the mass and skin was noted. c Computed tomography scan showing the lesions in both breasts
For the right partial mastectomy specimen, microscopic examinations showed a well-circumscribed papillary lesion with fibrovascular cores within the dilated duct, which were consistent with intraductal papillomas (Fig. 2a). Within the thickened fibrous septae of the breast tissue, clusters of basaloid-appearing small cells with cribriform structures and abundant intraluminal mucinous material were identified. Focal areas of sebaceous differentiation were also identified (Fig. 2b). Examination of the papillary lesions on higher magnification showed florid replacement of papilloma epithelium by the basaloid cells, with areas of luminal formation, mucinous material deposition, and focal sebaceous differentiation (Fig. 2c). At the periphery of the papillary lesion, an intact myoepithelial layer could be identified (Fig. 2d). Immunostains of the cell clusters in fibrous septae showed that the basaloid cells were mostly positive for p63 (Fig. 2e) and a small subset were positive for CD117 (Fig. 2f). The dual populations of epithelial and myoepithelial cells supported the diagnosis of ACC. Immunostains also highlighted the p63-positive myoepithelial cells (Fig. 2g) at the basal layer and the CD117-positive luminal epithelial cells (Fig. 2h) around luminal structures within the papillary lesion. SOX10 is a sensitive marker for both breast and salivary gland ACC (Yang et al. 2019). The SOX10 immunostain in this case showed positivity in both the glandular structures (Fig. 2i) as well as the papillary structures (Fig. 2j), which helped to confirm the diagnosis of ACC. Therefore, the right breast mass was diagnosed as ACC involving intraductal papilloma. The lesion was negative for ER, PR, and was not HER2-amplified. It was most likely a recurrence of the previous right breast ACC.
Microscopic and immunohistochemical examinations for the right breast lesion. a A large papillary lesion was present within a dilated duct. b Groups of cribriform basaloid cells were present within the thickened fibrous septae adjacent to the papillary lesion, which showed bland basaloid cells with prominent cribriform architecture, intraluminal mucin and foci of sebaceous differentiation. c High power examination of the papillary lesion revealed similar basaloid cells with area of sebaceous differentiation. d Periphery of the papillary lesion showed intact layer of myoepithelial cells. e Immunostain for p63 and (f) CD117 in glandular structures showed positivity for dual population of myoepithelial cells and luminal epithelial cells. g Immunostain for p63 (g) and (h) CD117 in the papillary lesion also highlighted dual population of myoepithelial cells and luminal epithelial cells. i Both the glandular structures and the (j) papillary lesion were also positive for SOX10 immunostain. (Hematoxylin-eosin, original magnification ×2 [a], × 20 [b], ×40 [c, d]; Immunohistochemistry, original magnification × 20 [e, f, g, h]. × 20 [i, j])
For the left mastectomy, gross examination identified a hyperemic area of the breast skin with a central ulceration (Fig. 3a). Sections of the specimen revealed an ill-defined firm, tan-white lesion with a 9.1 cm central cyst filled with red-brown fluid (Fig. 3b). The nipple was grossly unremarkable. Microscopically, the hyperemic skin revealed congested vessels in the superficial and deep dermis and an area of skin ulceration. However, there was no evidence of in situ or invasive carcinoma (Fig. 4a). Examination of the cystic lesion identified invasive SCC (Fig. 4b), which showed areas of prominent keratinization (Fig. 4c). No connection between SCC and the overlying epidermis was identified on multiple deeper level sections. The stromal component of SCC was composed of haphazardly arranged spindle cells with abundant infiltration of neutrophils, lymphocytes, and scattered eosinophils. Rare mitosis could be found within the stromal spindle cells (Fig. 4d). No glandular component was identified within the lesion. The nipple was also unremarkable by microscopic examination. Immunostains showed positivity for CK5/6 (Fig. 4e), p63 (Fig. 4f), and CAM5.2 (Fig. 4g) in the SCC, while the stromal spindle cell cells were negative for these markers. Both the epithelial and the stromal component were negative for SOX 10 (Fig. 4h). In addition, the lesion was negative for ER, PR, and was not HER2-amplified. Collectively, this lesion was diagnosed as a moderately differentiated keratinizing SCC.
Gross examinations for left breast lesion. a Gross image for the left breast. An area of skin disruption was present in a background of erythematous skin change. b Cut surface of the left breast lesion showing an irregular tan-white lesion with central cystic change. The lesion was not grossly connected with the overlying skin
Microscopic and immunohistochemical examinations for the left breast lesion. a Skin ulceration with congested blood vessels in dermis. Squamous epithelium adjacent to the ulceration appeared unremarkable. b Examination of the central cyst of the left breast lesion revealed cystic lining by squamous cell carcinoma. c Nests of squamous cell carcinoma with prominent keratinizing was noted diffusely in the lesion. d Stromal spindle cells were mixed with acute and chronic inflammatory cells. Very rare mitotic figure was noted (arrow). e Immuostains for CK5/6, f p63 and g CAM5.2 showed positivity in squamous cell carcinoma, but were are negative in the spindle cell component. h Both the squamous cell carcinoma and the spindle cell component were negative for SOX10 immunostain. (Hematoxylin-eosin, original magnification × 10 [a, b, c], 20x [d, h]; Immunohistochemistry, original magnification × 10 [e, f, g], × 20 [h])
Discussion and conclusions
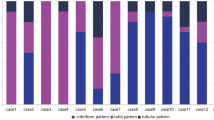
Both ACC and SCC are rare breast lesions. ACC accounts for less than 0.1% of breast cancer (Ghabach et al. 2010), and most cases are negative for hormonal receptors and are not HER2-amplified. The average size of reported ACCs is 1.6 cm (Treitl et al. 2018). The diagnosis of ACC is usually not challenging by identifying a dual population of luminal epithelial and myoepithelial cells. Mucinous and basement membrane materials are also usually present. Similar to their counterparts in salivary glands, different morphologic variants exist for breast ACC, including cribriform, tubular/trabecular, or solid. Perineural invasion is also a common finding. Genetically, ACC is characterized by MYB-NFIB fusion (Brill 2nd et al. 2011). However, a subset of ACC can progress to high-grade triple-negative breast cancer with the acquisition of additional genetic mutations, like TAG2, KDM6A, and CDK12 (Fusco et al. 2016). The solid variant of ACC is characterized by predominantly solid architecture composed of basaloid cells with moderate to marked nuclear atypia (Zhou et al. 2012). This subtype is more aggressive than conventional cribriform or tubular ACC, and demonstrates more frequent recurrence and lymph node metastasis (Foschini et al. 2016; Shin and Rosen 2002). Therefore, identification of the solid variant may provide prognostic implications for breast ACC patients. Despite of their triple-negative status, most cases of breast ACC have an indolent clinical course (Treitl et al. 2018; Bhutani et al. 2018; Romeira et al. 2016; Page 2005), with breast conserving surgery and postoperative radiotherapy being the most commonly used therapy (Soon et al. 2008; Sun et al. 2017).
We summarized 9 cases of breast ACC diagnosed in our institution from 2003 to 2015 (Table 1), and the result showed that most cases were treated by partial mastectomy followed by radiation. Chemotherapy was used for only 2 out of 9 cases. Tamoxifen was used for only 1 case, which had 10% positivity for ER (case No. 2). Besides this one, all the rest 8 cases were triple-negative carcinomas. Besides, all 9 cases did not develop lymph node metastasis. For follow-up, 8 out of 9 cases did not show recurrence. The only recurrent case (case No. 9) was the one described in this report. As noted, this recurrent case had the largest size (5 cm) when diagnosed initially, which indicated the possible correlation between tumor size and the recurrence risk.
In this case, the recurrent right breast ACC showed predominantly cribriform architecture with areas of sebaceous differentiation, which was a known associated phenomenon (Tavassoli and Norris 1986). Histologically, the neoplastic cells were relatively bland with no mitosis identified. Therefore, it was considered to be a low-grade lesion. However, the ACC was found to involve an intraductal papilloma, which was the main reason for it to become radiologically visible.
Breast PSCC is a type of metaplastic breast carcinoma with more than 90% of malignant cells being the squamous type (Hennessy et al. 2005). PSCC accounts for less than 0.1% of breast neoplasm (Gupta et al. 2006) and has a very aggressive clinical course. The median overall survival for these patients is about 40 months (Soliman 2019). Over 88% of breast PSCC cases are negative for hormonal receptors (Soliman 2019), and HER2 amplifications are rarely detected. About half of PSCC patients presented with complex cystic masses by imaging, with a mean size of 4.3 cm (Benoist et al. 2018). No specific genetic alteration has been described for SCC. The mean differential diagnosis for breast PSCC is metastatic SCC from breast skin or other sites. The etiology of PSCC is still not clear, though it may originate from squamous metaplasia of breast ducts. This theory was supported by the identification of SCC in situ in breast, in which no glandular differentiation was identified (Arafah et al. 2016).
In our case, although the left breast skin showed an area of ulceration, no in situ or invasive carcinoma was identified within the adjacent skin. The nipple was also unremarkable by gross and microscopic examination. The SCC had no direct connection with the overlying skin. Besides, we noticed prominent vascular congestion within the tumor and the overlying dermis. This finding was possibly due to the mass effect of the lesion which impeded the blood circulation in the left breast. The skin ulceration was most likely secondary to irritation by both the dermal vascular congestion and the underlying carcinoma. Meanwhile, a sub-areolar hemangioma was also found in the left breast, which was likely a nonspecific incidental finding. PET-CT also did not identify any malignancy in a second site. Collectively, this lesion was not considered to be a metastatic SCC from the skin or another site. Since the patient received radiotherapy for her right breast ACC 11 years ago, it was possible that the occurrence of her left breast lesion was correlated with her previous therapy.
Breast squamous cell carcinoma is a rare diagnosis in daily practice. Here, we summarized 11 cases of carcinoma that showed pure squamous differentiation on breast biopsies (Table 2), which were diagnosed in our institution from 2008 to 2020 (Table 2). It turned out that 6 out of 11 cases (54.5%) were eventually diagnosed as metaplastic carcinoma on excisions, and 3 of these 6 cases (50%) had ductal carcinoma in situ (DCIS) within the lesion. Besides, 4 out of 11 cases (36.4%) were eventually diagnosed as metastatic SCC to the breast, with a primary SCC being identified in either cervix or skin. Only 1 out of the 6 metaplastic cases (16.7%) was diagnosed as breast PSCC. Finally, 1 out 11 cases (9.1%) showed pure squamous differentiation but failed to identify a primary lesion outside of the breast site; however, the lesion was connected with the surface breast skin. Therefore, it was difficult to tell if the lesion was originated from the breast parenchyma or the breast skin. In terms of surface markers’ expression, 8 out the 11 cases (72.2%) were triple-negative carcinoma, and only 2 cases (18.2%) showed positivity for ER and/or PR. Overall, this cohort indicated that most breast cancer with pure squamous morphology on biopsies would turn out to be metaplastic carcinoma on follow up pathological examinations. The diagnosis for metastatic SCC was easier to be made if a primary site other than breast skin could be identified. When breast skin involvement was present, it was difficult to differentiate between breast PSCC and breast skin SCC with parenchymal involvement.
Due to the rarity of breast SCC, there is limited experience in terms of treatment. However, compared to invasive ductal carcinoma (IDC), radiotherapy and breast-conserving surgery are less commonly used for breast SCC. Chemotherapy is used more often for patients with low-risk SCC, but SCC is generally less responsive to chemotherapy compared to IDC. Hormonal therapy is also not applicable to most patients since breast SCC are usually triple-negative carcinomas. Overall, breast SCC is associated with poorer clinical outcomes (Zhu and Chen 2019).
In summary, we described a rare case of concurrent breast ACC and keratinizing PSCC in contralateral sides of breasts in an elderly female patient. The ACC was most likely recurrent, while the SCC could be an incidental finding or be secondary to the patient’s previous radiotherapy. Both lesions were negative for hormonal receptors and were not HER2-amplified. Further studies, for example next-generation sequencing of both lesions, may be helpful to rule out potential predisposing genetic alterations in this rare case.
Availability of data and materials
Not applicable.
Abbreviations
- ACC:
-
Adenoid cystic carcinoma
- SCC:
-
Squamous cell carcinoma
- PSCC:
-
Primary squamous cell carcinoma
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- DCIS:
-
Ductal carcinoma in situ
- IDC:
-
Invasive ductal carcinoma
- R:
-
Right
- L:
-
Left
- NA:
-
Not available
- TNBC:
-
Triple-negative breast cancer
- CA:
-
Carcinoma
- LN:
-
Lymph node
References
Arafah M, Ginter PS, Taylor DC, Hoda SA (2016) Squamous cell carcinoma in situ of the breast: report of a case. Breast J 22(5):573–577
Benoist P, Mureau A, Joueidi Y et al (2018) Management and prognosis of pure primary squamous cell carcinoma of the breast. J Gynecol Obstet Hum Reprod 47(7):275–280
Bhutani N, Kajal P, Singla S (2018) Adenoid cystic carcinoma of the breast: experience at a tertiary care centre of Northern India. Int J Surg Case Rep 51:204–209
Brill LB 2nd, Kanner WA, Fehr A et al (2011) Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol 24(9):1169–1176
Foschini MP, Rizzo A, De Leo A, Laurino L, Sironi M, Rucco V (2016) Solid variant of adenoid cystic carcinoma of the breast: a case series with proposal of a new grading system. Int J Surg Pathol 24(2):97–102
Fusco N, Geyer FC, De Filippo MR et al (2016) Genetic events in the progression of adenoid cystic carcinoma of the breast to high-grade triple-negative breast cancer. Mod Pathol 29(11):1292–1305
Ghabach B, Anderson WF, Curtis RE, Huycke MM, Lavigne JA, Dores GM (2010) Adenoid cystic carcinoma of the breast in the United States (1977 to 2006): a population-based cohort study. Breast Cancer Res 12(4):R54
Gupta C, Malani AK, Weigand RT, Rangineni G (2006) Pure primary squamous cell carcinoma of the breast: a rare presentation and clinicopathologic comparison with usual ductal carcinoma of the breast. Pathol Res Pract 202(6):465–469
Hennessy BT, Krishnamurthy S, Giordano S et al (2005) Squamous cell carcinoma of the breast. J Clin Oncol 23(31):7827–7835
Page DL (2005) Adenoid cystic carcinoma of breast, a special histopathologic type with excellent prognosis. Breast Cancer Res Treat 93(3):189–190
Romeira D, Cardoso D, Miranda H, Martins A (2016) Adenoid cystic carcinoma: triple negative breast cancer with good prognosis. BMJ Case Rep 2016:bcr2015213704
Shin SJ, Rosen PP (2002) Solid variant of mammary adenoid cystic carcinoma with basaloid features: a study of nine cases. Am J Surg Pathol 26(4):413–420
Soliman M (2019) Squamous cell carcinoma of the breast: a retrospective study. J Cancer Res Ther 15(5):1057–1061
Soon SR, Yong WS, Ho GH, Wong CY, Ho BC, Tan PH (2008) Adenoid cystic breast carcinoma: a salivary gland-type tumour with excellent prognosis and implications for management. Pathology. 40(4):413–415
Sun JY, Wu SG, Chen SY et al (2017) Adjuvant radiation therapy and survival for adenoid cystic carcinoma of the breast. Breast. 31:214–218
Tavassoli FA, Norris HJ (1986) Mammary adenoid cystic carcinoma with sebaceous differentiation. A morphologic study of the cell types. Arch Pathol Lab Med 110(11):1045–1053
Treitl D, Radkani P, Rizer M, El Hussein S, Paramo JC, Mesko TW (2018) Adenoid cystic carcinoma of the breast, 20 years of experience in a single center with review of literature. Breast Cancer 25(1):28–33
Yang C, Zhang L, Sanati S (2019) SOX10 is a sensitive marker for breast and salivary gland adenoid cystic carcinoma: immunohistochemical characterization of adenoid cystic carcinomas. Breast Cancer (Auckl) 13:1178223419842185
Zhou RJ, Hu CY, Yu L, Bi R, Yang WT (2012) Solid variant of mammary adenoid cystic carcinoma with basaloid features: a clinicopathologic and immunohistochemical study. Zhonghua Bing Li Xue Za Zhi 41(12):803–807
Zhu L, Chen K (2019) Clinicopathological features, treatment patterns, and prognosis of squamous cell carcinoma of the breast: an NCDB analysis. BMC Cancer 19(1):26
Acknowledgements
Authors sincerely acknowledge the technical help from our histology lab.
Funding
No funding is provided.
Author information
Authors and Affiliations
Contributions
Dr. AH designed the study and revised the manuscript. Dr. MY conducted data gathering and manuscript writing. Dr. HG and Dr. PB provided valuable guidance in study design and manuscript writing. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Obtained from institutional review board.
Consent for publication
Not applicable.
Competing interests
The authors declare that that have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, M., Bomeisl, P., Gilmore, H. et al. Concurrent breast adenoid cystic carcinoma and primary squamous cell carcinoma: report of a rare case with single institutional case reviews. Surg Exp Pathol 3, 20 (2020). https://doi.org/10.1186/s42047-020-00073-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-020-00073-0