Abstract
Background
Angiogenesis is one of the hallmarks of cancer. This complex mechanism of tumor progression provides tumors cells with essential nutrients. There have been a limited number of investigations of markers of angiogenesis in Glioblastomas (GBMs), and most previous studies have focused on VEGF-A. Recent evidence suggests that there is a complex lymphatic system in central nervous system (CNS), which suggests VEGF-C and VEGF–D as interesting biomarker candidates. This study was designed to evaluate the expressions of VEGF-A, −C, −D and their co-receptors, VEGFR-1, VEGFR-2, and VEGFR-3 by immunohistochemistry (IHC) using a series of GBMs. In addition, we evaluate any putative correlations between IHC expression levels of VEGF and clinical data of patients.
Methods
Tumor samples of 70 GBM patients (64 isocitrate dehydrogenase-1 wildtype (wtIDH-1) and 6 mutant (mutIDH-1)) were assessed by IHC using tissue microarray platforms for VEGF subunits and their co-receptors. The medical records were reviewed for clinical and therapeutic data.
Results
All VEGF subunits and receptors were highly expressed in GBMs: 57 out of 62 (91.9%), 53 out of 56 (94.6%) and 55 out of 63 cases (87.3%) showed VEGF-A, VEGF-C and -D imunoexpression, respectively. Interestingly, we had found both nuclear and cytoplasmic localization of VEGF-C staining in GBM tumor cells. The frequency of immunoexpression of VEGF receptors was the following: VEGFR-1, 65 out of 66 cases (98.5%); VEGFR-2, 63 out of 64 cases (98.4%); VEGFR-3, 49 out of 50 cases (90.0%). There were no significant differences in the patient overall survival (OS) related to the VEGF staining. A weak and monotonous correlation was observed between VEGF and its cognate receptors. The pattern of VEGF IHC was found to be similar when GBM mutIDH-1 subtypes were compared to wtIDH-1.
Conclusion
Both VEGF-C and –D, together with their receptors, were found to be overexpressed in the majority GBMs, and the IHC expression levels did not correlate with OS or IDH status. To understand the significance of the interactions and increased expression of VEGF-C, VEGF-D, VEGFR-2, and VEGFR-3 axis in GBM requires more extensive studies. Also, functional assays using a larger series of GBM is also necessary to better address the biological meaning of nuclear VEGF-C expression in tumor cells.
Similar content being viewed by others
Background
Glioblastoma (GBM) remains the most common and deadliest primary malignant brain tumor in adults (Ostrom et al. 2018). Histological characteristics as tumor cell anaplasia, microvascular proliferation and necrosis are hallmarks of GBM (Louis et al. 2016). The well-known pronounced vascular permeability and the high expression of vascular endothelial growth factor (VEGF) present in the abnormal vasculature, highlights the importance of angiogenesis in the tumor pathogenesis and therefore, constitute a rationale to target therapy (Lu-Emerson et al. 2015; Hundsberger et al. 2017).
Although great efforts have been implemented to develop new treatment strategies for GBM patients, the standard therapy is based on a multimodal approach comprising surgery, radiotherapy and chemotherapy, mostly centered on Temozolomide (TMZ) (Stupp et al. 2005). Due to its critical role in tumor biology, VEGF signaling pathways was proposed as an attractive target in cancer therapy since the 70’s (Folkman 1971). Thus far, and despite its established importance in GBM pathogenesis, only one anti-angiogenic therapy – the monoclonal anti-VEGF-A antibody, Bevacizumab is FDA approved despite its unreliable results (Friedman et al. 2009; Kreisl et al. 2009; Chinot et al. 2014; Diaz et al. 2017; Hundsberger et al. 2017; Wick et al. 2017). Indeed, as the complexity of angiogenesis has been unveiled and other VEGF family members were also implicated, alternative signaling pathways were considered as potential targets, fueling the development of new therapies, such as multitarget inhibitors – pan-vascular endothelial growth factor receptors (VEGFR) and tyrosine kinase inhibitors (TKIs). However, the benefits are unclear (Lu-Emerson et al. 2015).
The complexities and biological obstacles curb the use of angiogenesis as a potential therapeutic target in GBM patients. This might be partially explained by the intricate interaction of distinct signaling pathways and the higher number of involved molecules. Primarily, seven VEGF subunits are well distinguished, VEGF-A, −B, −C, −D, −E, −F and placental growth factor (PGF) – which can bind to their common receptors VEGFR-1, − 2, and − 3 and trigger an elaborate process of angiogenesis and lymphangiogenesis. Theses processes are invariably present in embryological, physiological and pathological conditions, such as tumorigenesis and tumor progression (Roy et al. 2006).
For instance, VEGF-A – the prototype member of the VEGF family – mediates VEGFR-1 and VEGFR-2 activation and predominantly regulates the process of angiogenesis in the central nervous system (CNS) (Carmeliet and Jain 2011). VEGF-C is a precursor protein that, in its cleaved form, has high affinity for both VEGFR-2 and VEGFR-3 that, when upregulated, may be associated with more unfavorable prognosis in GBM patients (Grau et al. 2007; Kessler et al. 2015; Zhao et al. 2016). Interestingly, VEGF-C, was initially described as primarily involved in the formation and maintenance of lymphatic vessels (Veikkola et al. 2001), which were classically thought to be absent from the CNS (Antila et al. 2017). However, increasing evidence indicates that VEGF-C is also expressed in the brain and may regulate several biological processes in physiological and pathological settings (Jenny et al. 2006; Grau et al. 2007; Shin et al. 2008; Zhao et al. 2016). From structural and functional perspective, VEGF-D is closely related to VEGF–C (Achen et al. 1998; Stacker and Achen 2018) and binds to the same receptors, i.e., VEGFR-2 and -3 (Roy et al. 2006) and thus, exerting an overlap role by inducing angiogenesis and lymphangiogenesis (Fagiani et al. 2016).
Emerging data has pointed out an increasing importance of VEGF-D, along with -C, as an alternative pro-angiogenic pathway in cancer (Grau et al. 2011; Yang et al. 2015; Stacker and Achen 2018), although their role in CNS tumors is barely understood (Debinski et al. 2001; Moffat et al. 2006; Grau et al. 2007; Ramani et al. 2012). Also, the precise implications of VEGF-B in cancer cells are not fully known (Roy et al. 2006; Lal et al. 2018) and VEGF-E and VEGF-F have not been reported in mammals (Roy et al. 2006). Additionally, only few studies have described the patterns of expression of angiogenic factors in human GBM, most of them just focusing on VEGF-A (Xu et al. 2013; Chen et al. 2015; Kessler et al. 2015; Baumgarten et al. 2016), VEGF-C and VEGF–D.
Therefore, this study aimed to evaluate the expression of VEGF-A, −C, −D and its receptors, VEGFR-1, − 2, and − 3 in a series of GBMs as well as whether any correlation exists between immunostaining and clinical features. Also, it was investigated the potential prognostic value of distinct VEGF family members.
Methods
We retrospectively review consecutive adult patients (> 18 years old) with newly diagnosed GBMs according to the current World Health Organization Classification of Tumors of Central Nervous System (WHO, 2016) (Louis et al. 2016). These patients underwent to neurosurgical procedures, aiming maximal safe resection whenever possible, between January 2003 and December 2011, from two Brazilian hospital databases: Hospital Israelita Albert Einstein (HIAE) and Hospital São Paulo - Universidade Federal de São Paulo (HSP-UNIFESP). All tumor specimens were overnight fixed in buffered formalin and subsequently embedded in paraffin blocks.
Tumor samples from 97 patients were available and carefully reviewed by a reference neuropathologist (LN). Samples with predominantly necrotic tissue or small sample sizes as those from patients with inconsistent clinical and/or therapeutic data were excluded. Thus, the study group comprised tumor tissue from 70 GBM samples (HSP-UNIFESP, n = 57; HIAE, n = 13).
Tissue microarray (TMA) construction
At least two different and of the most representatives areas of each tumor were selected for analysis. Cylindrical cores of 2-mm were removed and used in the construction of tissue microarray (TMA) blocks, as previously described (Saggioro et al. 2014). Eleven TMA blocks were constructed using a Beecher tissue array instrument™ (Beecher Instruments, Silver Spring, MD, USA) in accordance to the manufacturer’s instructions. For each immunostaining, 4 μm slices were cut from TMA blocks by using a Leica microtome and placed on glass charged slides. Slides were cut consecutively to minimize the influence of tissue heterogeneity when comparing the expression of the different VEGF family members within each patient tumor sample. As control, a normal prostate tissue prostate section was added in each TMA block.
Immunohistochemistry assays and evaluation
The immunohistochemical (IHC) procedures were performed on 4-μm-thick TMA sections and mounted on charged slides. Briefly, for immunostaining, the slides were deparaffinized, and rehydrated through a graded ethanol series. The antigen retrieval was done using Diva Decloaker solution (Biocare Med™, Concord, CA, USA), for 40 min at 98 °C in a steamer chamber. The slides were incubated with the pre-diluted antibody overnight at room temperature and subsequently washed with Tris-buffered saline with Tween 20, as shown in Table 1.
The immunohistochemistry test was chosen as the preferable evaluation method because of its practical approach, cost-effectiveness, widely availability, replicability, and has a proven usefulness in previous studies (Debinski et al. 2001; Jenny et al. 2006; Grau et al. 2007).
The IHC stains of VEGF and its cognate receptors were blindly evaluated at ×200 magnification using a DX-51 Olympus microscope, according to the following semiquantitative grading score: negative (0), absence or up to 10% IHC staining of the core area; score 1+, focal expression in > 10% to up to 25% of the core area; score 2+, intermediate expression in > 25% to up to 75% of the core; score 3+, diffuse expression in more than 75% of core area. At least a quarter of the core area had to be evaluable for scoring and faint immunostains were not considered. For each case (patient), the definitive IHC score was determined by the mean IHC stain obtained from the cores evaluated (up to 3 cores per case). The intensity of IHC stains was not considered. Finally, for each VEGF member the cellular location of IHC staining was noted, i.e., membrane, cytoplasmic and/or nuclei staining as well as the neoplastic and non-neoplastic endothelial cells.
The isocitrate dehydrogenase-1 (IDH-1) status was evaluated by IHC using the anti-IDH-1 mutant antibody for R132H (H09 clone, Dianova™, Hamburg, Germany) at 1:20, according to manufacturer’s recommendations.
Clinical profile and outcomes
The medical records of 70 patients were reviewed for the following data: gender, age at diagnosis, Karnofsky Performance Status (KPS), date of the first symptom, date of death or last evaluation, date and extent of resection and radiation therapy (RT) and chemotherapy regimens.
Neurosurgery was performed to attain the maximum safe and feasible resection according to the guidelines of both institutions, which have suitably equipped surgical centers. The extension of tumor resection was defined based on immediate (48 h) postoperative imaging (CT or MR), as radical resection (absence of residual contrast enhancement), partial resection or biopsy. Patients underwent 3D localized external beam RT delivered to the contrast-enhancing lesion shown at CT/T1-weightedimagesor T2/fluid attenuated inversion recovery sequence MR. The RT dose was prescribed according to the guidelines of the International Commission of Radiological Units fields, once daily at 2 Gy per fraction, 5 days a week, for a total of 60 Gy. The treatment protocols and personnel varied over time and between centers. Chemotherapy regimens also varied between centers. At HIAE, all patients were treated with concomitant and adjuvant Temozolomide according to the European Organization for Research and Treatment of Cancer–National Cancer Institute of Canada protocol (Stupp et al. 2005). At HSP-UNIFESP until 2008, patients received 200 mg/m2 carmustine (bis-cloroethylnitrosourea [BCNU]) at 6-week intervals starting 6 weeks after RT. Since 2009, TMZ has been available and patients could be treated with the EORTC-NCIC protocol. The patients who underwent chemotherapy treatment according to the EORTC-NCIC protocol were categorized as “RT concurrent with chemotherapy.” The patients who received BCNU were defined as having “RT and sequencing chemotherapy.” The patients who did not received RT and/or chemotherapy were defined as having “best supportive care”.
Statistical analyses
Data were described using absolute and relative frequencies for categorical data. Quantitative data were described using median and range, due to skewness. Overall survival (OS) was calculated from date of surgery until death or last follow-up and the cut-off date was November 30th 2018.
Survival curves were constructed according to the Kaplan-Meier method and comparison between groups by using log-rank test to explore relationships between well-recognized prognostic factors (age, KPS, extent of tumor resection, adjuvant treatments) and survival time in the univariate analysis. A conditional stepwise proportional hazard analysis (Cox-regression model) was used to identify independent predictors of survival. The level of significance was 0.05 (p < 0.05). [p ≤ 0.05].
For statistical analyses, only cases with consistent IHC expression (scores 2+ and 3+) were considered “positive or high expression”, i.e., cases with at least > 25% of IHC staining in the core area at definitive score. Cases showing < 25% IHC staining were considered as “negative or low expression”. Plausible associations among the IHC score of multiple VEGFs and their receptors were assessed using Spearman rank correlation coefficient.
The statistical analysis was performed using the statistical software R (R Core Team, 2017 added to the Car graphic package (Fox and Weisberg 2011) and survival (Therneau 2015).
Results
Clinicopathological features and patient outcomes
The median age of the patients at surgery was 59.5 years (range, 18–78 years), with 44 males (62.8%, male/female ratio of 1.7:1) (Table 2). The median follow-up time of all survivors was 8.21 months (range, 1.2–95.8 months) and the estimated OS was 7.25 months (range, 5.33–14.08 months).
VEGF-A, −C, −D and VEGFR-1, −2, −3 IHC expression and patient outcomes
The overwhelming majority of GBM cases showed consistent VEGF expression, regardless of its subunit or receptor, as shown in Table 3.
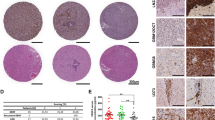
Even though several TMA cores were lost, of which the IHC score was not performed, mostly in regards to VEGFR-3 (28.6%) and VEGF-C (20.0%), the majority of GBM cases showed “high expression” for all VEGF subunits and their cognate receptors (> 25%, 2+ or 3+), as shown Table 3. Interestingly, VEGF-C showed conspicuous nuclear immunostaining (repeated three times) (Fig. 1). The remaining VEGF subunits and receptors showed cytoplasmic staining (data not shown).
Morphological and immunohistochemical features of VEGF-C and –D in GBM tissue microarray (TMA) sections. A) Cylindrical core of 2-mm from a representative GBM area (H&E, × 100). B) TMA design in a 4-um tissue section used for VEGF-C immunostaining (lower magnification). C to E) VEGF-C staining. Note the nuclear IHC expression of VEGF-C in tumor cells and in neoplastic endothelium of glomeruloid vessels (1C, arrows). F) VEGF-D expression in cytoplasm of GBM tumor cells and endothelial cells (× 400)
Overall, there were no significant differences in OS regardless the VEGF staining score on tumor cores (Table 4).
Additionally, other univariate analyses was performed using patients grouped according a binary IHC category: “no or low expression” (up to 1+) and “high expression” (2+ or 3+) for each VEGF subunit and its cognate receptors. The model was created to statiscally strengthen groups and overcome the hurdles as a result of a small sample size due to the missing TMA cores. However, no significant differences were found between OS and these IHC categories (Table 5).
Finally, regarding the IDH-1 mutation 6 out 70 patients harbor the canonical R132H mutation that is detected by IHC (8.6%). However, the IHC expression of VEGF subunits and cognate receptors did not differ from those with wtIDH-1, which constitutes the overwhelming majority of casuistry.
Evaluation of the relationship between distinct VEGF subunits and receptors
Table 6 shows the Spearman rank correlation coefficients among the IHC expression of VEGFs. The VEGF and its cognate receptors showed a weak and monotonous correlation pattern. A moderate correlation was only found for the VEGF-A and VEGFR-2 (Spearman = 0.478), as well as for VEGF-D and VEGFR-2 (Spearman = 0.456).
Discussion
In the current study, VEGF-C and -D along with its receptors VEGFR-2 and -3 were overexpressed in the majority of GBM tumor samples, ranging from 87.3% (VEGF-D) to 98.5% of the cases (VEGFR-1). However, there were no significant differences in the OS according to the VEGFs and their receptors and the immunostaining on tumor cells. This finding was expected since the VEGFs were overexpressed and our casuistry was overwhelmed by cases of GBMs with wtIDH-1 (only 6 out of 70 showed the IHD-1 R132H mutation).
Our results emphasized the hurdles for establishing angiogenic prognostic and predictive biomarkers in CNS tumors, which is in line of unclear benefits no matter the anti-angiogenic drug prescribed for unselected CNS tumor patient population (Winkler et al. 2018). Hopes persist to rest on the discovery of predictive biomarkers on tumor tissue, blood and/or radiographic parameters, which could merit the broad use of angiogenesis inhibitors, particularly for GBM patients (Lu-Emerson et al. 2015; Winkler et al. 2018). On the other hand, VEGF-C, −D, VEGFR-2 and -3 were not fashionable research targets due to their established role in primarily promoting the growth and remodeling of lymphatic vessels, which were considered absent in CNS, until recently. However, a considerable amount of evidence suggests the existence of a complex lymphatic system in CNS, mainly within the meninges, which drains cerebrospinal fluid into the deep cervical lymph nodes (Aspelund et al. 2014, 2015; Louveau et al. 2015; Weller et al. 2016; Antila et al. 2017). The development and modeling of that specific lymphatic system are closely related to the interactions between VEGF-C and VEGFR-3 (Antila et al. 2017). The emerging data brought upfront the exploration of VEGF-C and its receptors – VEGFR-2 and -3 – as its partially homologous VEGF, the VEGF-D (Achen et al. 1998; Grau et al. 2011; Michaelsen et al. 2018). To the best of our knowledge, this study represents the first to focus on the immunohistochemical expression of the expanded axis of proangiogenic factors simultaneously – VEGF-C, −D and VEGFR-2, − 3 – and that theoretically relate to lymphangiogenesis in GBM. However, a nuclear VEGF-C staining was found in GBM tumor cells, as was similarly previously described (Cai et al. 2012). Whether this nuclear VEGF-C staining means an abnormal cell reprograming or reflects the functional status of VEGF-C in the tumor cell is unknown. This finding deserves further investigations.
The accumulating data from experimental models and resected human tumor samples further described the expression of various VEGFs and their cognate receptors in glial tumors (Machein and Plate 2000; Huang et al. 2005). The first description of VEGF-D immunoreactivity in GBM was reported by Debinksi et al. (2001) who used GBM cell lines and ten tissue sections. They demonstrated that nearly all the GBM cells produce VEGF-D what may be partially implicated in oncogenic transformation and appeared to be an attractive target for novel treatment strategies. Following these results, Jenny et al. (2006) observed VEGF-C, −D and VEGFR-3 expression in normal brain tissue and in most brain tumors, as such glioblastomas and hemangioblastomas. The expression of VEGF-C and -D has been demonstrated to be diverse in GBM tissue samples, since the VEGF-C is found to be overexpressed compared to VEGF-D (Grau et al. 2011; Michaelsen et al. 2018).
Consistently, our results indicate that VEGF-C is proportionally overexpressed (94.6%), but is without any significant influence in patient prognosis. In contrast, low expressions of the VEGF-D (HR of 4.27 (CI 95%, 1.30 – 13.95) impaired the patient’ OS significantly (p = 0.016). Weickhardt et al. 2015 related that the anti-angiogenic therapy (i.e. Bevacizumab) might exhibited superior efficacy when VEGF-D is low expressed in other clinical scenario. On the other hand, despite its wide variability, previous reports suggested that VEGF-D, when upregulated, is associated with higher tumor grade of malignancy (Grau et al. 2011; Jiang et al. 2018). It is important to note that when the VEGF-D evaluations were grouped in a binary category (“absence/low expression” and “high expression”) no significant OS influence was found (HR of 1.22 (CI 95% 0.52–2.88), p = 0.64). We should emphasize that caution must be taken when interpreting this study’s VEGF-D findings, which might be partially explained by the restricted number of tumor samples with weak VEGF-D IHC expression (8.6%). In the same way, it was not found any significant differences on OS for the expression of others VEGF factors or cognate receptors. This finding might be partially explained by the relatively small number of patients in our series.
Substantial data support the correlation of the expressions of VEGF receptors, such as VEGFR-3 and the tumor grading (Grau et al. 2007, 2008). Baumgarten et al. (2016) have shown an overexpression of VEGFR-1, − 2 and, − 3 in GBM. Moreover, the expression of VEGFR-3 trends to turn positive as the pathologic grade of malignancy increases (Jiang et al. 2018). These previous findings were compatible with our results, as it was found overall higher immunoexpression of VEGFRs (VEGFR-1, 92.9%; − 2, 90%; − 3, 70%). However, in the present study it was found only a weak and monotonous correlation pattern between VEGF and its cognate receptors.
Overall, it is still unknown whether VEGFs and their receptors expression profiles are plausible biological markers to predict response and/or patient prognosis. Nevertheless, the are indications that VEGF-C, −D and VEGFR-2, − 3 in particular may have implications on alternative signaling pathway of primary and acquired resistance to anti-VEGF therapy (Moffat et al. 2006; Grau et al. 2011; Li et al. 2014; Michaelsen et al. 2018). Our results did not support this hypothesis since no recurrent or progressive GBM tumor samples were investigated. However, the overexpression of the entire axis of VEGF-C, −D, VEGFR-2 and -3 might be interpreted as an investigational target to clarify that theory, considering the recent data that suggests that there is a complex lymphatic system in the CNS (Aspelund et al. 2014, 2015; Louveau et al. 2015; Choy and Rahul Jandial 2016; Antila et al. 2017) and its development and modeling may be closely related to the interactions between VEGF-C and VEGFR-3 (Antila et al. 2017).
Conclusions
VEGF-C and -D and its receptors were overexpressed in the overwhelming majority GBMs but their expressions did not correlate with patient’s OS. The overexpression of the axis of VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 might be interpreted as a potential target for further studies, particularly the interactions between VEGF-C and VEGFR-3 as well the mild nuclear VEGF-C expression. Whether this nuclear staining means an abnormal cell reprograming or reflects the functional status of VEGF-C in the GBM cell is unknown. Additional studies with more extensive series of GBM are still necessary to better evaluate the roles of VEGF-C, VEGF- D and their cognate receptors, VEGFR-2 and VEGFR-3, in GBMs.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GBM:
-
Glioblastoma
- VEGF:
-
Vascular endothelial growth factor
- TMZ:
-
Temozolomide
- VEGFR:
-
Endothelial growth factor receptors
- TKIs:
-
Tyrosine kinase inhibitors
- PGF:
-
Placental growth factor
- CNS:
-
Central nervous system
- HIAE:
-
Hospital Israelita Albert Einstein
- HSP-UNIFESP:
-
Hospital São Paulo - Universidade Federal de São Paulo
- TMA:
-
Tissue microarray
- IHC:
-
Immunohistochemical
- KPS:
-
Karnofsky Performance Status
- CT:
-
Computer tomography
- MR:
-
Magnetic resonance imaging
- RT:
-
Radiotherapy
- BCNU:
-
Carmustine (bis-cloroethylnitrosourea)
- OS:
-
Overall survival
References
Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF et al (1998) Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A 95(2):548–553
Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T et al (2017) Development and plasticity of meningeal lymphatic vessels. J Exp Med 214(12):3645–3667
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M et al (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212(7):991–999
Aspelund A, Tammela T, Antila S, Nurmi H, Leppänen V, Zarkada G et al (2014) The Schlemm’s canal is a VEGF-C/VEGFR-3–responsive lymphatic-like vessel. J Clin Invest 124(9):3975–3986.
Baumgarten P, Blank A-E, Franz K, Hattingen E, Dunst M, Zeiner P et al (2016) Differential expression of vascular endothelial growth factor A, its receptors VEGFR-1, −2, and −3 and co-receptors neuropilin-1 and -2 does not predict bevacizumab response in human astrocytomas. Neuro-Oncology 18(2):173–183
Cai X, Ma S, Gu M, Zu C, Qu W, Zheng X (2012) Survivin regulates the expression of VEGF-C in lymphatic metastasis of breast cancer. Diagn Pathol 7(1):1–8
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307
Chen W, He D, Li Z, Zhang X, Pan D, Chen G (2015) Overexpression of vascular endothelial growth factor indicates poor outcomes of glioma: a systematic review and meta-analysis. Int J Clin Exp Med 8(6):8709–8719
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R et al (2014) Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722
Debinski W, Slagle-Webb B, Achen MG, Stacker SA, Tulchinsky E, Gillespie GY et al (2001) VEGF-D is an X-linked/AP-1 regulated putative onco-angiogen in human glioblastoma multiforme. Mol Med 7(9):598–608
Diaz RJ, Ali S, Qadir MG, De La Fuente MI, Ivan ME, Komotar RJ (2017) The role of bevacizumab in the treatment of glioblastoma. J Neuro-Oncol 133(3):455–467
Fagiani E, Lorentz P, Bill R, Pavotbawan K, Kopfstein L, Christofori G (2016) VEGF receptor-2-specific signaling mediated by VEGF-E induces hemangioma-like lesions in normal and in malignant tissue. Angiogenesis 19(3):339–358
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186
Fox J, Weisberg, S (2011) An R Companion to Applied Regression. (2 ed.) Thousand Oaks: Sage.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Grau S, Thorsteinsdottir J, von Baumgarten L, Winkler F, Tonn J-C, Schichor C (2011) Bevacizumab can induce reactivity to VEGF-C and -D in human brain and tumour derived endothelial cells. J Neuro-Oncol 104(1):103–112
Grau SJ, Trillsch F, Herms J, Thon N, Nelson PJ, Tonn J-C et al (2007) Expression of VEGFR3 in glioma endothelium correlates with tumor grade. J Neuro-Oncol 82(2):141–150
Grau SJ, Trillsch F, Von Lüttichau I, Nelson PJ, Herms J, Tonn JC et al (2008) Lymphatic phenotype of tumour vessels in malignant gliomas. Neuropathol Appl Neurobiol 34(6):675–679
Huang H, Held-feindt J, Buhl R, Mehdorn HM (2005) Expression of VEGF and its receptors in different brain tumors. System 27:371–377
Hundsberger T, Reardon DA, Wen PY (2017) Angiogenesis inhibitors in tackling recurrent glioblastoma. Expert Rev Anticancer Ther 17(6):507–515
Jenny B, Harrison JA, Baetens D, Tille J-C, Burkhardt K, Mottaz H et al (2006) Expression and localization of VEGF-C and VEGFR-3 in glioblastomas and haemangioblastomas. J Pathol 209(1):34–43
Jiang J, Wang S, Chen Y, Wang C, Qu C, Liu Y (2018) Immunohistochemical characterization of lymphangiogenesis-related biomarkers in primary and recurrent gliomas A STROBE compliant article. Medicine (United States) 97(39):e12458
Kessler T, Sahm F, Blaes J, Osswald M, Rübmann P, Milford D et al (2015) Glioma cell VEGFR-2 confers resistance to chemotherapeutic and antiangiogenic treatments in PTEN-deficient glioblastoma. Oncotarget 6(31):31050–31068
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Lal N, Puri K, Rodrigues B (2018) Vascular endothelial growth factor B and its signaling. Front Cardiovasc Med 5(April):39
Li D, Xie K, Ding G, Li J, Chen K, Li H et al (2014) Tumor resistance to anti-VEGF therapy through up-regulation of VEGF-C expression. Cancer Lett 346(1):45–52
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341
Lu-Emerson C, Duda DG, Emblem KE, Taylor JW, Gerstner ER, Loeffler JS et al (2015) Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol 33(10):1197–1213
Machein MR, Plate KH (2000) VEGF in brain tumors. J Neuro-Oncol 50(1–2):109–120
Michaelsen SR, Staberg M, Pedersen H, Jensen KE, Majewski W, Broholm H et al (2018) VEGF-C sustains VEGFR2 activation under bevacizumab therapy and promotes glioblastoma maintenance. Neuro-Oncology 20(11):1462–1474
Moffat BA, Chen M, Kariaapper MST, Hamstra DA, Hall DE, Stojanovska J et al (2006) Inhibition of vascular endothelial growth factor (VEGF)-a causes a paradoxical increase in tumor blood flow and up-regulation of VEGF-D. Clin Cancer Res 12(5):1525–1532
Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS (2018) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol 20(suppl_4):iv1–i86
Ramani P, Nash R, Radevsky L, Patel A, Luckett M, Rogers C (2012) VEGF-C, VEGF-D and VEGFR-3 expression in peripheral neuroblastic tumours. Histopathology 61(6):1006–1016
Roy H, Bhardwaj S, Ylä-Herttuala S (2006) Biology of vascular endothelial growth factors. FEBS Lett 580(12):2879–2887
Saggioro FP, Neder L, Stávale JN, Paixão-Becker ANP, Malheiros SMF, Soares FA et al (2014) Fas, FasL, and cleaved caspases 8 and 3 in glioblastomas: a tissue microarray-based study. Pathol Res Pract 210(5):267–273
Shin Y-J, Choi J-S, Lee J-Y, Choi J-Y, Cha J-H, Chun M-H et al (2008) Differential regulation of vascular endothelial growth factor-C and its receptor in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol 116:517–527
Stacker SA, Achen MG (2018) Emerging roles for VEGF-D in human disease. Biomolecules 8(1):1–17
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Therneau TM (2015) A package for survival analysis in S. version 2.38
Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV et al (2001) Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J 20(6):1223–1231
Weller M, Cloughesy T, Jr P, Stand WW, Choy C, Jandial R (2016) Science times: lymphatics in the brain?! Neurosurgery 78(2):N14
Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I et al (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963
Weickhardt AJ, Williams DS, Lee CK, Chionh F, Simes J, Murone C, et al. (2015) Vascular endothelial growth factor D expression is a potential biomarker of bevacizumab benefit in colorectal cancer. Br J Cancer 113(1):37–45.
Winkler F, Osswald M, Wick W (2018) Anti-angiogenics: their role in the treatment of glioblastoma. Oncol Res Treat 41(4):181–186
Xu Y, Zhong Z, Yuan J, Zhang Z, Wei Q, Song W et al (2013) Collaborative overexpression of matrix metalloproteinase-1 and vascular endothelial growth factor-C predicts adverse prognosis in patients with gliomas. Cancer Epidemiol 37(5):697–702
Yang Z, Wang Y-G, Su K (2015) VEGF-C and VEGF-D expression and its correlation with lymph node metastasis in esophageal squamous cell cancer tissue. Asian Pac J Cancer Prev 16(1):271–274
Zhao H, Hou C, Hou A, Zhu D (2016) Concurrent expression of VEGF-C and neuropilin-2 is correlated with poor prognosis in glioblastoma. Tohoku J Exp Med 238(2):85–91
Acknowledgements
Deise Lusca Chesca for the immunohistochemical reactions and TMA building blocks.
Elivane da Silva Victor and Gisele Cristine Eugenio for kindly perform statistical and analyses. Cristovao Luis Pitangueiras Mangueira for kindly allowing the necessary tests to be performed in the laboratory.
Funding
AmigoH (Amigos Einstein da Oncologia e Hematologia)
Author information
Authors and Affiliations
Contributions
LVML: acquisition of clinical data, interpretation of data, and drafted the manuscript. LN: perform pathological assays and interpretation, and drafted the manuscript; DCF: drafted the manuscript; LOK: drafted and manuscript revision; JNS: acquisition of pathological data and interpretation; SMFM: design of the study, acquisition of clinical data and interpretation, and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics review board of both institutions approved this study under the reference #33741714.4.0000.0071. This work does not represent a clinical trial and was therefore not registered as such.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Loureiro, L.V.M., Neder, L., Callegaro-Filho, D. et al. The immunohistochemical landscape of the VEGF family and its receptors in glioblastomas. Surg Exp Pathol 3, 9 (2020). https://doi.org/10.1186/s42047-020-00060-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-020-00060-5