Abstract
Background
Programmed cell death ligand 1 (PD-L1) is a predictive biomarker of the response of immunotherapy in some types of cancer. In the last years it was described its expression in breast cancer, namely in triple-negative breast cancer (TNBC) cases. In order to better understand the prognostic value of PD-L1 in breast cancer, this study analysed its expression in a series of primary tumours and respective nodal metastases.
Methods
PD-L1 expression was evaluated by immunohistochemistry in a series of 52 breast cancer cases and paired lymph node metastasis and correlate with the clinicopathological features of the primary tumour. The TNBC cases were re-classified using morphological and immunohistochemistry surrogate markers and the expression of PD-L1 was correlate with the different subtypes.
Results
It was observed that the majority of the cases with PDL-1 positive in the nodal metastasis did not express PD-L1 in the primary tumour (90.0%, 10 out of 11 cases). In addition, from the cases with a negative PD-L1 expression in the primary tumour, 23.8% expressed PD-L1 in the metastasis (10 out of 42 cases).No relationship was found between the PD-L1 expression in nodal metastasis and the clinicopathological features of the primary tumour. Finally, basal-like immunosuppressed (BLIS) TN tumours seem to be less prone to express PD-L1.
Conclusion
Although without statistical significance, there is a gain in terms of the PD-L1 expression in the nodal metastasis when compared to the primary tumour. This may have therapeutic implications on immunotherapy that blocks the PD-1/PD-L1 pathway.
Similar content being viewed by others
Background
Breast cancer is the most common tumour among women all over the world, representing, according to International Agency for Research on Cancer (IARC) data, the second leading cause of death in developing countries (Globocan 2012).
Although the risk factors for breast cancer are well known, in most cases it is not possible to understand the disease aetiology, as it is considered heterogeneous and with different phenotypes (Dong et al. 2002). Furthermore, its development and progression seems to be dependent on the epigenetic alterations and its tumour microenvironment (Martins and Schmitt 2018; Polónia et al. 2017).
Breast carcinomas have been classified in four subtypes: Luminal A, Luminal B, HER 2 (with overexpression of the Human Epidermal growth factor Receptor type 2 – HER 2) and triple-negative (TN) (Matos et al. 2005). The TN subtype has the most aggressive clinical characteristics and there is no standard treatment for these patients (Burstein et al. 2015; Matos et al. 2005; Nanda and Saha 2016). This subtype can be categorized according to its molecular characteristics, namely luminal androgen receptor (LAR), mesenchymal (MES), basal-like immunosuppressed (BLIS) or basal-like immune-activated (BLIA) (Burstein et al. 2015). It has been demonstrated that the TN subytpe most frequently shows intra-tumour lymphocytes and also expresses the programmed cell death ligand 1 (PD-L1) (Székely et al. 2017; Dill et al. 2017; Li et al. 2018; Nanda and Saha 2016; Polónia et al. 2017).
The PD-L1 is a transmembrane protein whose expression can be found at the surface of a variety of cells, including the tumour cells (Botti et al. 2017; Iwai et al. 2017). The PD-1/PD-L1 signalling pathway has been described as an escaping mechanism for the tumour cells out of the immune system (Botti et al. 2017; Polónia et al. 2017). The detection of PD-L1 can be used as a biomarker helping in predicting the response to the targeted immunotherapy against the PD-L1 and its receptor. This has been confirmed for certain types of tumour such as melanoma, non-small-cell lung carcinoma, and renal cell carcinoma (Javed et al. 2017; Kim et al. 2017; Liu et al. 2017; Topalian et al. 2016; Wang et al. 2017).
The PD-L1 expression in breast cancer has been associated with clinicopathological features of worse prognosis. Studies suggest the PD-L1 expression is associated with tumours with lymph nodes metastasis, Oestrogen Receptor (RE) negative, high histological grades (G3) and TN subtype (Li et al. 2018; Polónia et al. 2017; Zhang et al. 2017). However, the results reported by the literature regarding the PD-L1 expression in the breast cancer are still controversial due to the use of different antibody clones, different scores, evaluation in tumour cells and lymphocytes, as well as the molecular heterogeneity (Dill et al. 2017; Polónia et al. 2017; Yu et al. 2017).
Nevertheless, the PD-1/PD-L1 immune checkpoint blockade therapy with pembrolizumab and atezolizumab in the TN breast cancer has been recently used in phase I clinical trials demonstrating promising, durable responses, with minimal and tolerable side effects (Hartkopf et al. 2016; Polónia et al. 2017).
In order to better characterise the value of the PD-L1 expression in the breast cancer, the aim of our study was to determine the PD-L1 expression by immunohistochemistry in primary breast cancer tumours and their respective nodal metastasis, and investigate the association between PD-L1 expression and the clinical features of the cases.
Methods
Tumour samples
This retrospective study comes after a previous study that evaluated PD-L1 expression in a series of 466 cases of primary breast cancer diagnosed between 1978 and 1992 (Polónia et al. 2017). From this series were selected 52 cases where tissue from lymph node metastasis where available for evaluation of PD-L1 expression. Additionally, from the original 466 cases, 65 cases of TNBC cases were re-classified and studied for the expression of PD-L1. The subtyping of these cases were done using morphological and immunohistochemistry (IHC) criteria. To be consider as TN the cases were negative for estrogen receptor (ER), progesterone receptor (PR) and HER2. Cases with apocrine morphology and androgen receptor (AR) positive were consider “luminal AR” (n = 2); cases with spindle cell morphology and with low expression of claudins were consider “mesenchymal” (n = 1); cases of TN invasive carcinoma non-specific type lymphocyte-rich in the stroma and positive for CK5 and/or EGFR and/or P-cadherin were considered “basal-like” immune-activated (n = 33) and cases of TN invasive carcinoma non-specific type, without lymphocytes in the stroma and positive for CK5 and/or EGFR and/or P-cadherin were considered “basal-like” immunosuppressed (n = 21).
Table 1 shows the features captured for each case.
PD-L1 immunohistochemistry
IHC staining for PD-L1 was performed in 3 μm sections from paraffin blocks. The primary antibody was an anti-human PD-L1 rabbit monoclonal (clone SP142, dilution: ready to use; Ventana, Tucson, AZ). The assay was carried out on an automated immunostaining system, the Ventana BenchMark Ultra, using the OptiView DAB IHC Detection kit according to manufacturer’s instructions.
Positive (human tonsil) and negative staining controls (omission of the primary antibody) were performed in parallel with the paraffin sections.
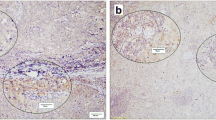
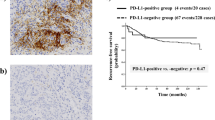
PD-L1 expression was independently assessed by two pathologists, without prior knowledge of the clinical information. Positivity for PDL-1 marker was defined according to the following criteria: membranous and cytoplasmic staining ≥1% in tumour cells, as previously described by our group (Polónia et al. 2017) (Fig. 1).
The analysis of the PLD-1 expression was performed in tumour cells only; immune cell staining was not considered.
Statistical analysis
The data statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software version 21.0 for Windows. The associations between variables were analysed using the Mann-Whitney’s U test, Fisher’s test and Pearson’s Chi-square test. The value of p < 0.05 was considered to indicate statistical significance.
Results
Relationship between the PD-L1 expression in the metastasis in relation to clinicopathological features of the primary tumour (Table 2)
It was observed that for the cases with positive PD-L1 expression, the average age is 61 years (± 11.36). Additionally, in terms of the tumour size and the histological grade, the positive PD-L1 expression was more frequent for the size around 2-5 cm and for the higher histological grades (G3). When analysed in relation with the other molecular biomarkers, no association was observed between the PD-L1 expression and that of the ER, PR, HER2 or the marker of proliferating cells (Ki 67). Additionally, no association was observed between the PD-L1 expression and breast cancer molecular subtype.
Relationship between the PD-L1 expression in the primary cancer and the nodal metastasis (Table 3)
It was observed that the majority of the cases with positive PD-L1 in the nodal metastasis did not express PD-L1 in the primary tumour (90.0%, 10 out of 11 cases). In addition, from the cases with a negative PD-L1 expression in the primary tumour, 23.8% expressed PD-L1 in the lymph node metastasis (10 out of 42 cases). From 52 cases, 7 cases were excluded due to the low amount of tissue from the primary tumour in the TMA and one additional case was excluded because the lymph node metastasis it was a micrometastasis.
There is a gain of around 23% in terms of PD-L1 expression in the metastasis when compared to that in the primary tumour. However, there is no statistically significant relationship supporting this interdependence.
Relationship between the PD-L1 expression in the TN primary tumour cases and their molecular subtype (Table 4)
The subtypes BLIS and BLIA were the most frequently observed with a relative frequency of 25.08 and 57.89%. From 65 classified and analysed cases, 9 were excluded because was not possible to assess the PD-L1 expression in the primary tumour and 1 additional case due to the inability to classify its molecular subtype. It was observed that the majority of the cases with positive PD-L1 expression in the TN primary tumour belongs to the molecular subtype TN BLIA (91.67%, 11 out of 12 cases) (Table 4).
Discussion
The gain observed in terms of PD-L1 expression in the metastasis when compared to that in the primary tumour may translate into new ways of treating metastatic tumours through targeted immunotherapy against the PD-L1 and its receptor as reported in recent studies using the PD-L1 inhibitor pembrolizumab as therapy (Yu et al. 2017). Curiously, despite previous studies evaluating PD-L1 in distant metastasis not showing difference of expression between primary tumour and respective metastasis (Dill et al. 2017), a recent study from Li et al. (Li et al. 2018) demonstrated a stronger and more frequent PD-L1 expression in lymph node metastases than in paired primary breast tumours. These results are in line with ours, suggesting that primary breast tumours can be not adequate surrogates for determining PD-L1 expression.
The discordance between the expression of PD-L1 in the primary tumour and metastases can be due several factors. First, it is very well know that the expression of PD-L1 in different tumours is dynamic and can be change during the tumour progression (Li et al. 2018; Wang et al. 2017). PD-L1 expression is upregulated in tumour cells by inflammatory cytokines and particularly interferons (IFNs) produced by infiltrating immune cells (Dong et al. 2002). In addition, it was demonstrated that basal-like breast cancer cells have the capacity to evade the immune response via upregulation of PD-1 ligands adapted to IFN-c, which is secreted by T helper lymphocytes (Karasar and Esendagli 2014). Therefore, the T cells in the lymph nodes may drive PD-L1 expression to induce adaptive immune resistance for the tumour metastatic cells (Li et al. 2018). The high molecular heterogeneity of breast cancer, through clonal selection can also promote discordance of PD-L1 expression in primary and metastatic cells. Additionally, the use of different types of specimens (i.e. TMAs in the primary tumour and full sections in lymph node metastasis) may explain differences in the observed PD-L1 expressions in the present study. There are studies in other types of cancer, some showing concordance of PD-L1 expression between primary and metastasis as for example in lung cancer (Kim et al. 2017) but others showing gain of expression in metastasis as for example in colon cancer (Wang et al. 2017).
Another find of our study is that the expression of PD-L1 is more frequent in the TNBC subtype BLIA. These findings are in concordance with our previous study (Polónia et al. 2017) and the study of Li et al. (Li et al. 2018) that showed association between PD-L1 expression and presence of tumour-infiltrating lymphocytes, one of the main characteristics of BLIA cases. In the molecular classification the BLIA subgroup are the tumours displaying upregulation of genes controlling B cell, T cell and natural killer functions (Burstein et al. 2015). From morphological point of view, these tumours are rich in stromal tumour-infiltrating lymphocytes (TILs). Stromal TILs include several types of T and B cell lymphocytes that can be present in different proportions (Martins and Schmitt 2018; Polónia et al. 2017). Cytotoxic (CD8+) T cells are increased in high grade breast cancer cases and cases with high proliferative index and it is associated with better clinical outcome (Polónia et al. 2017; Seo et al. 2013). CD4+/FOXP3+ T cells are associated with worse prognosis (Merlo et al. 2009; Polónia et al. 2017). Furthermore B cells are also associated with high histological grade and basal-like phenotype, as well as with better prognosis (Mahmoud et al. 2012; Polónia et al. 2017). This indicates that humoral and cell-mediated immune response acts in convergence to achieve a best antitumour response. Concerning PD-L1 expression, in addition to tumour cells, it has been reported that it can be also present in stromal lymphocytes, specifficaly in CD4+/FOXP3- T cells (Ghebeh et al. 2006; Polónia et al. 2017). However PD-L1 expression is not associated with T cell exhausted markers, which means that PD-L1 is only partially inhibiting T cells (Polónia et al. 2017; Sabatier et al. 2015). Nevertheless, this does not contradict the idea of blocking PD-L1 in order to reactive partially inhibited T cells and further increase the antitumour immune response. Following our findings, maybe the determination of PD-L1 in the lymph node metastasis can be more accurate to assess the response of immunotherapy.
Conclusions
This study analysed the PD-L1 expression in a series of breast cancer cases and subsequent lymph node metastasis, which is a heterogeneous disease with different prognosis. According to the obtained results, it was possible to conclude that:
-
We observed that lymph node metastases have more frequent PD-L1 expression than in primary tumours, suggesting that primary tumours cannot be adequate surrogates for determining PD-L1 expression in breast cancer; further studies using a large cohort of patients are needed to confirm these results that can lead new therapeutic implications on immunotherapy that blocks the PD-1/PD-L1;
-
. The PD-L1 expression in lymph node metastasis does not seem to be related to the clinicopathological features of the primary tumour;
-
Although without statistical significance, in the TN primary tumours, the molecular subtype “BLIA” seems to more frequently express PD-L1.
Abbreviations
- BLIA:
-
Basal-like immune-activated
- BLIS:
-
Basal-like immunosuppressed
- IARC:
-
Agency for Research on Cancer
- IHC:
-
Immunohistochemistry
- LAR:
-
Luminal androgen receptor
- MES:
-
Mesenchymal
- PD-L1:
-
Programmed cell death-ligand 1
- PR:
-
Progesterone receptor expression
- RE:
-
Oestrogen Receptor
- TMAs:
-
Tissue microarray
- TN:
-
Triple negative
References
Botti G, Collina F, Scognamiglio G, Rao F, Peluso V, De Cecio R et al (2017) Programmed death ligand 1 (PD-L1) tumor expression is associated with a better prognosis and diabetic disease in triple negative breast cancer patients. Int J Mol Sci 18:459. https://doi.org/10.3390/ijms18020459.
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA et al (2015) Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 21:1688–1698.
Dill EA, Gru AA, Atkins KA, Friedman LA, Moore ME, Bullock TN et al (2017) PD-L1 expression and intratumoral heterogeneity across breast cancer subtypes and stages: an assessment of 245 primary and 40 metastatic tumors. Am J Surg Pathol 41:334–342.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F et al (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800.
Ghebeh H, Mohammed S, Al-Omair A et al (2006) The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 8:190–198.
Globocan. Breast Cancer. Estimated Incidence, Mortality and Prevalence Worldwide in 2012. 2012 Available from: http://globocan.iarc.fr/old/FactSheets/cancers/breast-new.asp. Accessed 12 June 2018.
Hartkopf AD, Taran FA, Wallwiener M, Walter CB, Kramer B, Grischke EM et al (2016) PD-1 and PD-L1 immune checkpoint blockade to treat breast cancer. Breast Care (Basel) 11:385–390.
Iwai Y, Hamanishi J, Chamoto K, Honjo T (2017) Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci: 24(1)24–26. https://doi.org/10.1186/s12929-017-0329-9.
Javed A, Arguello D, Johnston C, Gatalica Z, Terai M et al (2017) PD-L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Immunotherapy 9:1323–1330.
Karasar P, Esendagli G (2014) T helper responses are maintained by basal-like breast cancer cells and confer to immune modulation via upregulation of PD-1 ligands. Breast Cancer Res Treat 145:605–614.
Kim S, Koh J, Kwon D, Keam B, Go H et al (2017) Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Eur J Cancer 75:141–149.
Li M, Li A, Zhou S, Xu Y, Xiao Y et al (2018) Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer 18:4. https://doi.org/10.1186/s12885-017-3916-y.
Liu D, Wang S, Bindeman W (2017) Clinical applications of PD-L1 bioassays for cancer immunotherapy. J Hematol Oncol: 10–11. https://doi.org/10.1186/s13045-017-0479-y
Mahmoud SM, Lee AH, Paish EC et al (2012) The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat 132:545–553.
Martins D, Schmitt F (2018) Microenvironment in breast tumorigenesis: friend or foe? Histol Histopathol:18021. https://doi.org/10.14670/HH-18-021..
Matos I, Dufloth R, Alvarenga M et al (2005) p63, cytokeratin 5, and P-cadherin: three molecular markers to distinguish basal phenotype in breast carcinomas. Virchows Arch 447:688–694.
Merlo A, Casalini P, Carcangiu ML et al (2009) FOXP3 expression and overall survival in breast cancer. J Clin Oncol 27:1746–1752.
Nanda R, Saha P (2016) Immune checkpoint inhibition for triple-negative breast cancer. Oncol Hematol Rev (US) 12. https://doi.org/10.17925/OHR.2016.12.01.31
Polónia A, Pinto R, Cameselle -Teijeiro J, Schmitt F, Paredes J (2017) Prognostic value of stromal tumor infiltrating lymphocytes and programmed cell death-ligand 1 expression in breast cancer. J Clin Pathol 70:860–867.
Sabatier R, Finetti P, Mamessier E et al (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6:5449–5464.
Seo AN, Lee HJ, Kim EJ et al (2013) Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 109:2705–2713.
Székely B, Silber ALL, Pusztai L (2017) New therapeutic strategies for triple -negative breast cancer. J Oncol 32:130–137.
Topalian SL, Taube JM, Anders RA, Pardoll DM (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275–287.
Wang HB, Yao H, Li CS, Liang LX, Zhang Y et al (2017) Rise of PD-L1 expression during metastasis of colorectal cancer: implications for immunotherapy. J Dig Dis 18:574–581.
Yu LY, Tang J, Zhang CM, Zeng WJ, Yan H, Li MP et al (2017) New immunotherapy strategies in breast cancer. Int J Environ Res Public Health 14(1), 68. https://doi.org/10.3390/ijerph14010068.
Zhang M, Sun H, Zhao S, Wang Y, Pug H, Wang Y et al (2017) Expression of PD-L1 and prognosis in breast cancer: a metaanalysis. Oncotarget 8:31347–31354.
Acknowledgements
The authors would like to acknowledge Dina Leitão from the Faculty of Medicine, University of Porto, for her contribution to the immunohistochemistry procedures required for this study and Dr. Jorge Cameselle-Teijeiro from Vigo for provide the cases for the study. FS is partially supported by the project NORTE-01-0145-FEDER-000003, supported by Norte Portugal Regional Programme (NORTE 2020), under the PORTUGAL 2020 Partnership.
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
AMA participated in the design of the study, observed microscopic expression, participated read and performed the statistical analysis. JP participated in the design of the study, participated in its design and coordination. FS conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with national regulative law for the handling of biological specimens from tumour banks, the samples being exclusively available for the research purposes in retrospective studies as well as under the international Helsinki declaration.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Alves, A.M., Paredes, J. & Schmitt, F. Expression of PD-L1 in primary breast carcinoma and lymph node metastases. Surg Exp Pathol 2, 7 (2019). https://doi.org/10.1186/s42047-019-0033-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-019-0033-z