Abstract
Background
Chronic Chagas cardiomyopathy (CCC) is characterized by the presence of a multifocal inflammatory response and myocardial damage, leading to fibrosis, arrhythmias and ventricular dysfunction. The expression of syndecan-4, a transmembrane proteoglycan, was previously found to be increased in the hearts of mice chronically infected with Trypanosoma cruzi. The possible involvement of syndecan-4 in the disease pathogenesis, however, remains unknown. Here we evaluated the pattern of expression of syndecan-4 in the heart tissue of T. cruzi infected mice and subjects with Chagas cardiomyopathy, correlating with the degree of inflammation and fibrosis.
Methods
The expression of syndecan-4 was evaluated by immunofluorescence and RT-qPCR in the hearts of C57Bl/6 mice at different time points after infection with the Colombian strain of T. cruzi. Immunostainings for syndecan-4 were performed in heart samples obtained from CCC patients and other etiologies of heart failure. The number of infiltrating inflammatory cells and area of fibrosis were also evaluated and quantified.
Results
In the experimental model, the number of infiltrating inflammatory cells and fibrosis area in the hearts progressively increased after the acute phase of infection, while syndecan-4 expression remained elevated in similar levels in both the acute and chronic phases. Confocal microscopy analysis demonstrated the localization of syndecan-4 expression in blood vessels, co-localized with α-SMA, a marker for vascular smooth muscle cells (VSMCs). Confocal microscopy analysis of human hearts samples showed a similar pattern of syndecan-4 expression in blood vessels. No correlation between syndecan-4 expression and inflammation or fibrosis was found in the hearts from subjects with CCC. We also compared the expression of syndecan-4 evaluated in subjects with CCC, idiopathic dilated cardiomyopathy and ischemic cardiomyopathy. No differences in the number of syndecan-4 positive vessels/mm2 were found comparing the three groups (P = 0.466), whereas CCC patients presented a higher number of infiltrating inflammatory cells, compared to the other etiologies of heart failure. Additionally, no correlation between syndecan-4 and fibrosis or numbers of inflammatory cells was found.
Conclusions
Syndecan-4 is expressed in the heart during the acute and chronic phases of Chagas disease, in association with VSMCs, independently of the degree of myocardial fibrosis or the number of infiltrating inflammatory cells.
Similar content being viewed by others
Background
Chagas disease (CD), caused by the protozoan parasite Trypanosoma cruzi, represents the third largest tropical disease burden, after malaria and schistosomiasis (Organization WH 2010). Despite the significant reduction in the number of infected people that has occurred worldwide, CD still represents a major public health problem in endemic countries, mainly Latin America, and is becoming an emerging problem in non-endemic countries, such as USA, Spain, Japan, and Australia, due to population migration (Bern 2015; Schmunis 2007). Chronic Chagas cardiomyopathy (CCC) is the most severe form of clinical presentation in CD and may occur up to 20 years after infection, in approximately 20–30% of infected subjects (Rassi and Rassi 2007; WHO 2015). The hallmark of CCC is the presence of a multifocal inflammatory reaction, which leads to myocardial fibrosis, often followed by ventricular dysfunction and arrhythmias (Marin-Neto et al. 2007; Rochitte et al. 2005; Mello et al. 2012). In this stage of the disease, the conventional therapy for CD has not shown significant benefits (Morillo et al. 2015), and there is no effective treatment other than orthotopic heart transplantation.
Although the pathogenesis of CD has not been fully understood, it is well known that the persistent cardiac damage that occurs during the chronic phase is, at least partially, a result of immune responses directed to the parasites, as well as to autoreactive cells, which recognize heart antigens (Soares et al. 2001; Bonney and Engman 2015). Previous studies indicate a role for Th1 lymphocytes, with a high production of IFN-γ, which has been associated with progression to severe forms of the disease in subjects with CCC (Soares et al. 2001; Gomes et al. 2003). IFN-γ and TNF-α are overexpressed in the hearts of mice chronically infected with T. cruzi (Soares et al. 2010) and can activate macrophages, an important cell population in inflammatory sites.
Our group demonstrated, through DNA microarray analysis, that several genes related to inflammation and fibrosis are upregulated in the hearts of mice with CCC (Soares et al. 2011). Among those genes, we described the upregulation of syndecan-4 gene transcription in the heart. Syndecan-4 expression was modulated after treatment with bone marrow mononuclear cells or granulocyte-colony stimulating factor (G-CSF), two protocols that significantly reduced cardiac inflammation and fibrosis in the mouse model of T. cruzi infection (Soares et al. 2011; Vasconcelos et al. 2013), thus suggesting a role of this protein in the pathogenesis of Chagas disease.
Syndecan-4 is a transmembrane protein capable of carrying heparan sulfate and chondroitin sulfate chains, and it is expressed by different cell types, including endothelial cells, smooth muscle cells, and cardiac myocytes, playing a role in processes such as fibroblast growth factor signaling, regulation of cell migration and control of cell adhesion (Tkachenko et al. 2005; Nikkari et al. 1994; Li et al. 1997). In clinical settings, syndecan-4 concentration has been shown to be increased in subjects with heart failure, inversely correlated with left ventricular ejection fraction (Ma et al. 2013), and also after acute myocardial infarction (Kojima et al. 2001). Takahashi and colleagues demonstrated that syndecan-4 might be useful as a possible biomarker to predict cardiovascular events, such as death and hospitalization caused by worsening of heart failure (Takahashi et al. 2011).
In the present study, we aimed to evaluate the pattern of expression of syndecan-4 in heart tissue of mice and subjects with Chagas disease (CD), correlating this expression with inflammation and myocardial fibrosis.
Methods
Animal procedures
Six-to-eight weeks old female C57BL/6 mice were used for T. cruzi infection and as normal controls. All animals were raised and maintained at the animal facility of the Center for Biotechnology and Cell Therapy, Hospital São Rafael, in rooms with controlled temperature (22 ± 2 °C) and humidity (55 ± 10%), and continuous air flow. Animals were housed in a 12 h light/12 h dark cycle (6 am - 6 pm) and provided with rodent diet and water ad libitum. Animals were handled according to the NIH guidelines for animal experimentation. All procedures described had prior approval from the local institutional animal ethics committee at Hospital São Rafael (01/13).
Trypanosoma cruzi infection
Trypomastigotes of the myotropic Colombian T. cruzi strain were obtained from culture supernatants of infected LLC-MK2 cells, as previously described (Federici et al. 1964). Infection of C57BL/6 mice was performed by intraperitoneal injection of 1000 T. cruzi trypomastigotes in saline, and was confirmed through evaluation of parasitemia at different time points after infection.
Real time reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was isolated from heart samples with TRIzol reagent (Invitrogen, Molecular Probes, Oregon, USA) and concentration was determined by photometric measurement. High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used to synthesize cDNA of 1 μg RNA following manufacturer’s recommendations. RT-qPCR assay was performed to detect the expression levels of SDC4. The RT-qPCR amplification mixtures contained template cDNA, Taqman Master Mix and probe (all from Applied Biosystems). The samples were normalized with HPRT1 (endogenous control). All reactions were run in triplicate on an ABI7500 Sequence Detection System (Applied Biosystems) under standard thermal cycling conditions. The threshold cycle (2-∆∆ct) method of comparative PCR was used to analyze the results.
Human samples
The study complied with the Declaration of Helsinki, and was approved by the Ethics Committee of the São Rafael Hospital under the number 51025115.3.0000.0048. Sixty samples were obtained at Messejana Hospital in Fortaleza, Ceará, a specialized medical center for orthotopic heart transplantation in Brazil. Samples consisted of fragments of explanted hearts from 15 subjects with CD, confirmed by serological assay, 21 subjects with ICM, and 24 subjects with IdDCM. Heart samples from left ventricle and septum were included in paraffin, stained with H&E and Sirius Red, and used for immunostaining for detection of syndecan-4, as described below.
Morphometry
Heart sections were analyzed by light microscopy after paraffin embedding, followed by standard hematoxylin and eosin (H&E) staining. Inflammatory cells infiltrating heart tissue were counted using a digital morphometric evaluation system. Images were digitized using the slide scanner Scan Scope (Leica). Morphometric analyses were performed using the software Image Pro Plus v.7.0 (Media Cybernetics). The inflammatory cells were counted in 10 fields (400× magnification) per heart. The percentage of fibrosis was determined using Sirius red-stained heart sections and the Image Pro Plus v.7.0. Two blinded investigators performed the analyses.
Immunofluorescence analysis
Histological sections of paraffin embedded tissues were dewaxed and submitted to a heat-induced antigen retrieval step by incubation in citrate buffer (pH = 6.0). Then, sections were incubated overnight at 4 °C with the following primary antibodies: goat anti-syndecan-4, diluted 1:400 (Santa Cruz Biotechnology), mouse anti-α-smooth muscle actin (1:200, Dako) and rabbit anti-von Willebrand factor (1:100, Diagnostic Biosystem, Pleasanton, CA). Next, the sections were incubated for 1 h with secondary antibodies anti-goat IgG Alexa fluor 488-conjugated, anti-mouse IgG Alexa Fluor 568-conjugated (1:600, Molecular Probes) or anti-rabbit IgG Alexa Fluor 568-conjugated (1:1000, Molecular Probes). Nuclei were counterstained with DAPI (Vector Labs). The presence of fluorescent cells was determined by observation on a FluoView 1000 confocal microscope (Olympus) and A1 confocal microscope (Nikon). Quantifications were performed in 10 random fields captured under 400× magnification, using the Image Pro Plus v.7.0 software (Media Cybernetics).
Statistical analysis
Categorical data were presented as percentages. Continuous variables were tested for normal distribution using Shapiro-Wilk test and were expressed as mean ± SEM or median (interquartile interval). Comparisons of continuous variables among groups were performed with analysis of variance (ANOVA) test, followed by Bonferroni post hoc test for multiple-comparison test, or Kruskal-Wallis, depending on normality assessed by Shapiro-Wilk test. Correlation between continuous variables was evaluated by Pearson or Spearman coefficients, depending on normality. Cases with missing data were not included in the analysis. Analyses were performed using SPSS version 20.0 (IBM) or Prism 6.0 (GraphPad Software), and p < 0.05 was considered statistically significant.
Results
Analysis of inflammation, fibrosis and syndecan-4 expression in the hearts of mice during the course of infection with T. cruzi
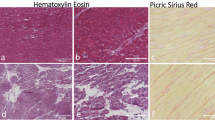
Groups of mice infected with T. cruzi were euthanized at different time points after infection for histological and immunofluorescence analyses. H&E-stained heart sections obtained from mice during the acute phase (30 days) of infection showed the presence of intense and diffuse inflammatory infiltrates mainly composed by mononuclear cells. Parasite nests and perivascular inflammation were also frequently present (Fig. 1a and Additional file 1: Figure S1A). A marked decrease on inflammation was seen 90 days after infection, followed by a slight increase of a multifocal inflammatory infiltrate, and areas of myocytolysis thereafter (Fig. 1a and c). The presence of arterioles and veins with thickened wall due to VSMC proliferation was frequently seen during the chronic phase (Additional file 1: Figure S1B). The presence of diffuse interstitial fibrosis was observed in Sirius red-stained sections, and morphometric analysis showed a progressive increase in cardiac fibrosis with time of infection (Fig. 1b and d).
Histopathological analysis of mouse hearts at different time points after infection. a, Heart sections stained with H&E, showing the presence of inflammatory infiltrate at 30, 90 and 180 days after infection with T. cruzi. b, Heart sections stained with Sirius red, showing areas of fibrosis at 30, 90 and 180 days after infection with T. cruzi. Arrows indicate the presence of parasite nests in the acute phase (30 days of infection). c and d, Quantification of inflammation and fibrosis by morphometric analysis in the hearts of infected mice. Data represent the mean ± SEM of 5–6 mice/group. * P < 0.05; ** P < 0.01; *** P < 0.001. Calibration bars = 50 μM
Sections of mouse hearts collected at different time points after infection and of uninfected controls were stained with anti-syndecan-4 antibodies. Syndecan-4 staining was seen in cardiomyocytes and blood vessels, both in mice euthanized during the acute and chronic phases of infection (Fig. 2a-d). A statistically significant increase in syndecan-4+ blood vessels was seen at the three time points evaluated, when compared to uninfected controls (Fig. 2e and 2f).
Expression of syndecan-4 in the heart is increased after T. cruzi infection. Heart sections of uninfected (a) and T. cruzi-infected mice, 30 (b), 90 (c) and 180 (d) days after infection were stained with anti-syndecan-4 antibody (green) and DAPI (blue) for nuclei, and observed by confocal microscopy. e, Quantification of syndecan-4+ blood vessels in heart sections. Data represent the mean ± SEM of 5–6 mice/group. f, Evaluation of cardiac Sdc4 gene expression by qRT-PCR. ** P < 0.01; *** P < 0.001 compared to other groups
To better characterize the expression of syndecan-4, we performed confocal microscopy analysis in heart sections co-stained for α-smooth muscle actin (α-SMA) and von Willebrand factor (vWF). Co-localization between vWF, a marker for endothelial cells, and syndecan-4 was observed (Fig. 3a and b). However, co-localization of syndecan-4 and α-SMA was much more intense and frequently found, indicating that vascular smooth muscle cells (VSMCs) are the main cell population expressing syndecan-4 in this model (Fig. 3c and d). We also performed double staining for the pan-leukocyte marker CD45 and syndecan-4, but did not observe co-staining (Fig. 3e and f).
Expression of syndecan-4 in endothelial cells, vascular smooth muscle cells. and leukocytes. Heart sections of mice 180 days after infection were stained with syndecan-4 and α-smooth muscle actin - α-SMA (a and b), von Willebrand factor - vWF (c and d) or CD45 (e and f). Sections were co-stained with DAPI and observed by confocal microscopy
Inflammation, fibrosis and syndecan-4 expression in human heart samples
Heart sections were prepared from explanted human hearts at end-stage CCC and stained with H&E for histological analysis. We observed the presence of foci of inflammatory infiltrates composed mainly of mononuclear cells near areas of myocytolysis (Fig. 4a, c and e). Alterations in the microvasculature, including perivascular inflammation, fibrosis, proliferation of microvessels and thickening of vessel walls, leading to occlusion, were also observed (Additional file 1: Figure S1C-E). Additionally, extensive areas of interstitial fibrotic scar were found in Sirius red-stained sections (Fig. 4b, d and f).
Presence of inflammation and fibrosis in explanted hearts from subjects with CCC. Heart sections of explants from three subjects with CCC were prepared. a, c and e, Staining with H&E, showing multifocal inflammatory infiltrate composed by mononuclear cells. b, d and f, Sirius red staining, showing extensive areas of interstitial fibrosis
The expression of syndecan-4 in human heart samples was evaluated by analysis using confocal microscopy. Syndecan-4 staining was also found in myocytes and mainly in blood vessels, co-localizing with αSMA staining, indicating expression on VSMCs (Fig. 5a and b). Co-localization between syndecan-4 and vWF was found in lower extent (Fig. 5c and d). Analysis of heart samples with CCC was performed to evaluate expression of syndecan-4 expression, inflammation and fibrosis (Table 1). Syndecan-4 expression varied and did not correlate with the degree of inflammation or fibrosis.
Expression of syndecan-4 in heart sections of subjects with CCC. Sections of explanted human hearts from subjects with CCC were stained with anti-syndecan-4 antibody and α-smooth muscle actin - α-SMA (a and b) or von Willebrand factor - vWF (c and d). Sections were co-stained with DAPI and observed by confocal microscopy
Comparison of syndecan-4 expression, inflammation and fibrosis among subjects with end-stage CCC, ICM and idDCM
Intense inflammation and fibrosis are hallmarks of CCC. To investigate if syndecan-4 expression was associated with features of CCC, we compared the expression of syndecan-4 in human heart samples. Sections from explanted hearts were obtained from 60 individuals with end-stage cardiomyopathy. The median number of inflammatory cells/mm2 was 31.0 cells/mm2 (IQI: 16.8–109.8). There was a statistically significant difference in this variable when we compared the three groups of heart samples. The median numbers of inflammatory cells/ mm2 were 127.8 cells/mm2 (IQI: 31.0–260.3), 20.1 cells/mm2 (IQI: 12.4–41.7) and 24.4 cells/mm2 (IQI: 19.4–64.3) in subjects with CD, ICM and IdDCM, respectively (P = 0.035; Fig. 6a).
Comparison of inflammation, fibrosis and syndecan-4 expression in the hearts of subjects with end-stage cardiomyopathy. Heart samples of subjects with Chagas disease (n = 7), ischemic cardiomyopathy (n = 5) and idiopathic dilated cardiomyopathy (n = 8). a, Number of inflammatory cells/mm2 (Kruskal-Wallis one-way analysis of variance, P = 0.035). b, Percentage of myocardial fibrosis (ANOVA, P = 0.610). c, Number of syndecan-4+ blood vessels/mm2 (Kruskal-Wallis one-way analysis of variance, P = 0.466)
The mean percentage of myocardial fibrosis was 17.4 ± 8.2%, with no statistically significant difference across the groups of heart samples. The mean percentage of myocardial fibrosis was 19.1 ± 7.7% in subjects with CD; 16.4 ± 8.6% in subjects with ICM; and 17.1 ± 8.4% in subjects with IdDCM (P = 0.610; Fig. 6b).
Regarding the expression of syndecan-4 assessment, only 20 samples were successfully stained for the immunostaining analysis (7 from subjects with Chagas disease, 5 from subjects with ischemic cardiomyopathy, and 8 from subjects with idiopathic cardiomyopathy). The overall median syndecan-4 positive vessels/mm2 was 0.54 vessels/mm2 (IQI: 0.27–1.45). No differences were detected comparing the three groups of heart samples. In subjects with CD, the median syndecan-4 positive vessels/mm2 was 0.76 vessels/mm2 (IQI: 0.52–1.66); while in subjects with ICM it was 0.46 vessels/mm2 (IQI: 0.30–1.17); and in subjects with IdDCM it was 0.43 vessels/mm2 (IQI: 0.13–1.70) (P = 0.466; Fig. 6c).
No correlation was found between the number of syndecan-4+ vessels/mm2 and either the degree of myocardial fibrosis (r = 0.261, P = 0.265; Fig. 7a) or the number of inflammatory cells/mm2 (r = 0.098, P = 0.680; Fig. 7b). When we analyzed the results per group of heart samples, we did not find any statistically significant correlation either.
Analysis of correlation between syndecan-4 expression, inflammation and fibrosis in explanted hearts from subjects with end-stage cardiomyopathy. a, Correlation between syndecan-4+ blood vessels/mm2 and number of inflammatory cells (n = 20, Spearman’s correlation). b, Correlation between syndecan-4+ blood vessels/mm2 and percentage of myocardial fibrosis (n = 20, Spearman’s correlation)
In CD heart samples (n = 7), we found no significant correlation between the number of syndecan-4 positive vessels/mm2 and either the degree of myocardial fibrosis (r = 0.219, P = 0.637) or the number of inflammatory cells/mm2 (r = 0.314, P = 0.494). The results of these correlations were, respectively, r = 0.835 (P = 0.079) and r = 0.276 (P = 0.653) for heart samples of subjects with ICM (n = 5), and r = 0.303 (P = 0.466) and r = 0.217 (P = 0.606) for heart samples of subjects with IdDCM (n = 8).
Discussion
Heart failure is the final common pathway of a variety of primary cardiovascular diseases regardless of the nature of the cardiomyopathy. Understanding the clinical and pathological differences among its main etiologies is crucial for achieving breakthroughs in the treatment of such a life-threatening disorder. Finding new biomarkers is of special interest, as they may also serve as molecular targets for the development of therapeutic strategies. The current study provides a characterization of syndecan-4 expression in the heart tissue during acute and chronic T. cruzi infection in mice. In addition to the analysis in the mouse model, it shows, for the first time, the expression of syndecan-4 in human tissue samples of subjects who underwent orthotopic heart transplantation due to chronic Chagas disease, ischemic cardiomyopathy, and idiopathic cardiomyopathy.
Syndecan-4 is expressed in a variety of cell types, including cardiomyocytes, endothelial cells, cardiac fibroblasts and smooth muscle cells (Li et al. 1997; Samarel 2013; Julien et al. 2007; Xie et al. 2012). In our study, we found that, although present in cardiomyocytes and endothelial cells, syndecan-4 expression was highly increased in VSMCs upon T. cruzi infection, both in mouse and human hearts. Previous studies have shown that syndecan-4 expression and shedding is increased by mechanical stress in VSMCs (Li and Chaikof 2002). Syndecan-4 co-localizes with integrin heterodimers in focal adhesion complexes, which are affected by physical forces, thus causing the regulating the expression of syndecan-4 (Li and Chaikof 2002). Therefore, it is possible that the increased expression of syndecan-4 is induced by mechanical stress in the vessel walls. The role of syndecan-4 as a mechanosensor has already been demonstrated (Bellin et al. 2009). The implications of this finding to the disease pathogenesis are currently unknown and speculative, and should be further explored in future studies using adequate tools to block or enhance cardiac syndecan-4 expression.
Syndecan-4 has a variety of roles, including cell-cell adhesion, cell-ECM adhesion, binding to growth factors, thus affecting the recruitment of inflammatory cells, as well as cardiac fibroblast activation and fibrosis deposition (Samarel 2013; Li and Chaikof 2002; Gopal et al. 2017; Okina et al. 2012; Strand et al. 2013). The extracellular domain of syndecan-4 has heparan sulfate chains, which bind to several molecules that modulate processes of tissue injury and repair, including cell migration and proliferation, cell-substrate interactions, extracellular matrix remodeling, binding of cytokines and growth factors (Li et al. 2002; Maillard et al. 2014). Recently, Xi and co-workers (2016) have shown that syndecan-4 influences the migration of endothelial progenitor cells. As shown in our study and in previous reports in the literature, CCC is associated with microvasculature alterations, including the proliferation of microvessels (Prado et al. 2011). Thus, it is possible that elevated expression of syndecan-4 participates in the promotion of microvessels formation found in Chagas heart disease.
Although syndecan-4 expression has been linked to inflammatory responses, we did not find a correlation between the degree of inflammation and syndecan-4 expression. Our results showing a higher inflammation in the hearts of subjects with CCC are in accordance with data in the literature regarding the presence of a well-established inflammatory reaction in chronic Chagas disease (Marin-Neto et al. 2007). Interestingly, a significantly higher number of inflammatory cells was found in the hearts of subjects with ICM and IdDCM, in the presence of similar degrees of cardiac fibrosis.
Myocardial fibrosis has been described as prominent not only for CD, but also for ICM and IdCM (Ottaviani et al. 2015; de Leeuw et al. 2001). Explanted hearts were previously examined by de Leeuw and colleagues to determine whether specific histopathologic features were present in the myocardium of patients with end-stage idiopathic dilated cardiomyopathy. Their group revealed marked fibrosis in the hearts of 21 of 37 patients with idiopathic cardiomyopathy and in 26 of 35 patients with heart diseases of known causes (de Leeuw et al. 2001). In 2015, Ottaviani and colleagues compared the gross and histopathological parameters in hearts explanted-native or previously transplanted-from patients with end-stage heart failure with the clinical hemodynamics parameters at the time of orthotopic heart transplantation. The authors showed that both ischemic and non-ischemic cardiomyopathy patients of end-stage heart failure requiring heart transplantation presented high grades of fibrosis, hypertrophy and myocytolysis (Ottaviani et al. 2015). Our study reinforces this finding, since we did not find significant differences in fibrosis area when hearts from the three etiological groups were evaluated.
Additionally, we did not find differences in the degree of myocardial fibrosis or in the density of syndecan-4 positive vessels among the three groups of heart samples. Likewise, we showed no significant correlation between the number of syndecan-4 density and the number of inflammatory cells or the percentage of myocardial fibrosis. These data are complementary and in accordance with our recently published study, which showed lack of association between serum syndecan-4 and myocardial fibrosis measured by magnetic resonance imaging in subjects with CCC (Larocca et al. 2017).
Regarding the human material, the present study was limited by the reduced sample size for the immunostaining analysis, as a result of a surprisingly high number of samples that were not successfully stained, probably due to non-standardized tissue processing or tissue overfixation. Moreover, the inclusion of samples obtained from subjects with advanced heart failure may decrease the ability to detect differences in syndecan-4 expression among different etiologies that could possibly be present in earlier stages of the diseases.
Conclusion
In conclusion, we demonstrated the expression of syndecan-4 on VSMCs in both mice and human hearts in Chagas cardiomyopathy, without any evidence of correlation between either the percentage of myocardial fibrosis or the number of inflammatory cells and the number of syndecan-4 positive blood vessels. Further work is needed to clarify the role of syndecan-4 in the pathogenesis of CCC and its usefulness as a disease serological biomarker.
Abbreviations
- CCC:
-
Chronic Chagas cardiomyopathy
- CD:
-
Chagas disease
- CNPq:
-
National Council for Research
- FAPESB:
-
Research Foundation of Bahia State
- G-CSF:
-
Granulocyte-colony stimulating factor
- H&E:
-
Hematoxylin and eosin
- ICM:
-
Ischemic cardiomyopathy
- idDCM:
-
Idiopathic dilated cardiomyopathy
- NIH:
-
National Institutes of Health
- RANTES:
-
Regulated on activation, normal T cell expressed and secreted
- RT-qPCR:
-
Real time reverse transcription polymerase chain reaction
- VEGF:
-
Vascular endothelial growth factor
- vWF:
-
Von Willebrand factor
- α-SMA:
-
α-smooth muscle actin
References
Bellin RM, Kubicek JD, Frigault MJ, Kamien AJ, Steward RL, Barnes HM et al (2009) Defining the role of syndecan-4 in mechanotransduction using surface-modification approaches. Proc Natl Acad Sci U S A 106(52):22102–22107
Bern C (2015) Chagas’ disease. N Engl J Med 373(19):1882
Bonney KM, Engman DM (2015) Autoimmune pathogenesis of Chagas heart disease: looking back, looking ahead. Am J Pathol 185(6):1537–1547
de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM (2001) Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int 14(5):299–306
Federici EE, Abelmann WH, Neva FA (1964) Chronic and progressive myocarditis and myositis in C3H mice infected with Trypanosoma cruzi. Am J Trop Med Hyg 13:272–280
Gomes JA, Bahia-Oliveira LM, Rocha MO, Martins-Filho OA, Gazzinelli G, Correa-Oliveira R (2003) Evidence that development of severe cardiomyopathy in human Chagas’ disease is due to a Th1-specific immune response. Infect Immun 71(3):1185–1193
Gopal S, Multhaupt HA, Pocock R, Couchman JR (2017) Cell-extracellular matrix and cell-cell adhesion are linked by syndecan-4. Matrix Biol 60(61):57–69
Julien MA, Wang P, Haller CA, Wen J, Chaikof EL (2007) Mechanical strain regulates syndecan-4 expression and shedding in smooth muscle cells through differential activation of MAP kinase signaling pathways. Am J Physiol Cell Physiol 292(1):C517–C525
Kojima T, Takagi A, Maeda M, Segawa T, Shimizu A, Yamamoto K et al (2001) Plasma levels of syndecan-4 (ryudocan) are elevated in patients with acute myocardial infarction. Thromb Haemost 85(5):793–799
Larocca TF, Macêdo CT, Noya-Rabelo M, Lemos Correia LC, Moreira MI, Caldas AC et al (2017) Lack of association between serum syndecan-4, myocardial fibrosis and ventricular dysfunction in subjects with chronic Chagas disease. PLoS One 12(12):e0189408
Li J, Brown LF, Laham RJ, Volk R, Simons M (1997) Macrophage-dependent regulation of syndecan gene expression. Circ Res 81(5):785–796
Li J, Partovian C, Hampton TG, Metais C, Tkachenko E, Sellke FW et al (2002) Modulation of microvascular signaling by heparan sulfate matrix: studies in syndecan-4 transgenic mice. Microvasc Res 64(1):38–46
Li L, Chaikof EL (2002) Mechanical stress regulates syndecan-4 expression and redistribution in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 22(1):61–68
Ma X, Ouyang P, Zhang Z, Lai W, Xu D (2013) Changes and clinical significance of serum level of syndecan-4 protein in patients with chronic congestive heart failure. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 29(8):866–869
Maillard L, Saito N, Hlawaty H, Friand V, Suffee N, Chmilewsky F et al (2014) RANTES/CCL5 mediated-biological effects depend on the syndecan-4/PKCα signaling pathway. Biol Open 3(10):995–1004
Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV (2007) Pathogenesis of chronic Chagas heart disease. Circulation 115(9):1109–1123
Mello RP, Szarf G, Schvartzman PR, Nakano EM, Espinosa MM, Szejnfeld D et al (2012) Delayed enhancement cardiac magnetic resonance imaging can identify the risk for ventricular tachycardia in chronic Chagas’ heart disease. Arq Bras Cardiol 98(5):421–430
Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Rosas F et al (2015) Randomized trial of Benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med 373(14):1295–1306
Nikkari ST, Järveläinen HT, Wight TN, Ferguson M, Clowes AW (1994) Smooth muscle cell expression of extracellular matrix genes after arterial injury. Am J Pathol 144(6):1348–1356
Okina E, Grossi A, Gopal S, Multhaupt HA, Couchman JR (2012) Alpha-actinin interactions with syndecan-4 are integral to fibroblast-matrix adhesion and regulate cytoskeletal architecture. Int J Biochem Cell Biol 44(12):2161–2174
Organization WH (2010) Chagas disease: control and elimination. World Health Organization. World Health Assembly report, Geneva, pp 1–4
Ottaviani G, Radovancevic R, Kar B, Gregoric I, Buja LM (2015) Pathological assessment of end-stage heart failure in explanted hearts in correlation with hemodynamics in patients undergoing orthotopic heart transplantation. Cardiovasc Pathol 24(5):283–289
Prado CM, Jelicks LA, Weiss LM, Factor SM, Tanowitz HB, Rossi MA (2011) The vasculature in chagas disease. Adv Parasitol 76:83–99
Rassi A, Rassi SG (2007) Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation 115(9):1101–1108
Rochitte CE, Oliveira PF, Andrade JM, Ianni BM, Parga JR, Avila LF et al (2005) Myocardial delayed enhancement by magnetic resonance imaging in patients with Chagas’ disease: a marker of disease severity. J Am Coll Cardiol 46(8):1553–1558
Samarel AM (2013) Syndecan-4: a component of the mechanosensory apparatus of cardiac fibroblasts. J Mol Cell Cardiol 56:19–21
Schmunis GA (2007) Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz 102(Suppl 1):75–85
Soares MB, de Lima RS, Rocha LL, Vasconcelos JF, Rogatto SR, dos Santos RR et al (2010) Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J Infect Dis 202(3):416–426
Soares MB, Lima RS, Souza BS, Vasconcelos JF, Rocha LL, Dos Santos RR et al (2011) Reversion of gene expression alterations in hearts of mice with chronic chagasic cardiomyopathy after transplantation of bone marrow cells. Cell Cycle 10(9):1448–1455
Soares MB, Pontes-De-Carvalho L, Ribeiro-Dos-Santos R (2001) The pathogenesis of Chagas’ disease: when autoimmune and parasite-specific immune responses meet. An Acad Bras Cienc 73(4):547–559
Strand ME, Herum KM, Rana ZA, Skrbic B, Askevold ET, Dahl CP et al (2013) Innate immune signaling induces expression and shedding of the heparan sulfate proteoglycan syndecan-4 in cardiac fibroblasts and myocytes, affecting inflammation in the pressure-overloaded heart. FEBS J 280(10):2228–2247
Takahashi R, Negishi K, Watanabe A, Arai M, Naganuma F, Ohyama Y et al (2011) Serum syndecan-4 is a novel biomarker for patients with chronic heart failure. J Cardiol 57(3):325–332
Tkachenko E, Rhodes JM, Simons M (2005) Syndecans: new kids on the signaling block. Circ Res 96(5):488–500
Vasconcelos JF, Souza BS, Lins TF, Garcia LM, Kaneto CM, Sampaio GP et al (2013) Administration of granulocyte colony-stimulating factor induces immunomodulation, recruitment of T regulatory cells, reduction of myocarditis and decrease of parasite load in a mouse model of chronic Chagas disease cardiomyopathy. FASEB J 27(12):4691–4702
WHO (2015) Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec:33–44. https://www.ncbi.nlm.nih.gov/pubmed/?term=25671846
Xie J, Wang J, Li R, Dai Q, Yong Y, Zong B et al (2012) Syndecan-4 over-expression preserves cardiac function in a rat model of myocardial infarction. J Mol Cell Cardiol 53(2):250–258
Funding
This study was supported by funds from National Council for Research (CNPq), Research Foundation of Bahia State (FAPESB), and Funding Authority for Studies and Projects (FINEP).
Availability of data and materials
Please contact author for data requests.
Author information
Authors and Affiliations
Contributions
TFL – Conceptualization, design of the study, methodology, data analysis, writing original draft. BSFS – Conceptualization, methodology, data analysis, writing – review and editing. CTM – Design of the study, methodology, data analysis. CMA – Methodology, data analysis. JFV – Methodology, data analysis. DNS - Methodology, data analysis. DCNP – Methodology, data analysis. FT – Methodology and resources. JDSL – Methodology and resources. WLC – Methodology, data analysis. RRS – Conceptualization, resources, funding acquisition. MBPS – Conceptualization, design of the study, methodology, data analysis, writing – review and editing, final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All of the experimental work in mice received prior approval by local Animal Experimentation Ethics Committee of Hospital São Rafael, with the reference number 01/13. The Ethics Committee of Hospital São Rafael, with the reference number 51025115.3.0000.0048, approved the use of human heart samples in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Vascular alterations in hearts during Chagas disease. Heart sections of mice 30 days (A) or 8 months (B) after T. cruzi infection, stained with H&E. Arrow indicates the presence of a parasite nest. C-F, Sections of explanted human hearts from end-stage Chagas disease heart failure subjects, stained with H&E (C-E) and Sirius red (F). Presence of perivascular inflammation (C), proliferation of microvessels (D), thickening of arteriole walls and perivascular fibrosis (E and F). Calibration bars = 50 μM. (TIFF 3081 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Larocca, T.F., Souza, B., Macêdo, C.T. et al. Assessment of syndecan-4 expression in the hearts of Trypanosoma cruzi-infected mice and human subjects with chronic Chagas disease cardiomyopathy. Surg Exp Pathol 1, 5 (2018). https://doi.org/10.1186/s42047-018-0012-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-018-0012-9