Abstract
Background
Diadegma insulare (Cresson) is the most efficient parasitoid of Plutella xylostella (L.) in cruciferous crops. Considering its effectiveness, the present study was performed to investigate the impacts of cauliflower and cabbage cultivars under 3 different temperature regimes on the parasitism efficiency and offspring sex ratio of D. insulare.
Main body
This study revealed that D. insulare remained active at all the 3 temperatures (i.e., 19, 23, and 27 °C) and on all selected cruciferous cultivars. However, 23 °C (along with 65±5% RH and 16L: 8D hours) was the most suitable temperature, whereas relatively preferred host plant cultivars were the “White marble” (cauliflower) and “Asha” (cabbage) at which comparatively higher parasitism rates (79.39 and 73.31%, respectively) were recorded. Moreover, non-significant differences were observed in the offspring sex ratios of the parasitoid at different temperature regimes; however, minute differences were found among studied cruciferous cultivars.
Conclusion
Overall, “White marble” (cauliflower) and “Asha” (cabbage) were recommended to be used as potential host plant cultivars for mass rearing of D. insulare.
Similar content being viewed by others
Background
Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) is the most devastating pest of cruciferous crops all over the globe (Cobblah et al. 2012). P. xylostella has the potential to damage all plant parts above the soil causing crop yield losses up to 90% round the year (Oke 2008). Ecofriendly management practices can be performed against the insect pest by utilizing two key biotic factors, i.e., host plants and natural enemies (Kfir 1997).
Host plants act as the primary interface between insect pests and their parasitoids; moreover, they also interact and affect the parasitoid’s efficiency (Howe and Schaller 2008). Besides direct impacts of host plant characteristics on the parasitoids, plants may interact indirectly through multitrophic associations (Stiling and Moon 2005). Host plants play a significant role during the bio-ecological processes related to the parasitoids such as parasitism, survival, and growth rates (Sarfraz et al. 2008). According to Bjorkman (2000), leaf quality of plants affects biological traits and host plant selection of parasitoids. It has also been reported that parasitoid complex of P. xylostella has different parasitism efficiencies in different cruciferous crops (Liu et al. 2000).
Diadegma insulare (Cresson) (Hymenoptera: Ichneumonidae) is one of the most efficient biocontrol agents of P. xylostella (Dosdall et al. 2011). It is a tiny ichneumon, koinobiont, host-specific wasp and larval endoparasitoid which may cause up to 80% parasitism in the field populations of P. xylostella (Braun et al. 2004). The D. insulare is considered to be the most beneficial parasitoid for the integrated management programs of P. xylostella because of its synchronization with host life cycle and comparatively outstanding efficiency (Xu et al. 2001). Considering the potential of this parasitoid, enhancement of its populations is desired for sustainable management of its host insect pest. Despite the fact that D. insulare has a key role in the management of field populations of P. xylostella, research studies regarding its parasitism efficiency, sex ratio, and multitrophic associations are not common.
This study aimed to evaluate the impact of different temperature regimes and host plant cruciferous cultivars on the parasitism efficiency and sex ratio of D. insulare and to enhance the mass rearing procedure of this important parasitoid.
Materials and methods
Experimental studies were initiated by the development of 3 types of cultures, i.e., plant culture of cauliflower and cabbage cultivars, P. xylostella culture, and D. insulare culture. Insect rearing jars made up of transparent plastic sheets and muslin cloth were used. Different temperature regimes were maintained according to the need of experimental studies, and to acclimatize with the abiotic and biotic conditions, insects’ cultures (P. xylostella and D. insulare) were reared for 3 generations under similar conditions before performing a certain experimental study.
Plant culture of cauliflower and cabbage cultivars
Five cultivars, each of cauliflower (i.e., Smilla, White marble, Cashmere, White magic, and TSX-C22) and cabbage (i.e., Quisor, Stonehead, Baseball, Asha, and Delight ball), were selected and grown from seeds into seedling trays in glasshouse. Peltracom® growing media were utilized to grow plants. After 5 weeks, plants were transplanted into plastic pots having 7.5cm diameter until hardening stage. At the age of 45–50 days, plants were utilized for P. xylostella and D. insulare rearing and further experimental studies.
Plutella xylostella culture
Larvae and pupae of P. xylostella were collected from cauliflower/cabbage fields at Rawalpindi (Punjab, Pakistan) sites. Collected larvae were brought into insect growth chamber (25 °C, 65±5% RH, and 16L: 8D hours) and released on fresh and untreated potted plants of cauliflower/cabbage and placed in rearing jars, whereas collected pupae were placed singly in glass vials, provided with honey solution (20%) soaked cotton plugs. Plants were replaced (where needed) until the pupation of feeding larvae, and then, developed pupae were shifted to glass vials for adult emergence. To obtain the moth’s progeny of homogenous age and acclimatize with the host plant cultivars as well as abiotic controlled conditions, separate plant cultivars were placed in separate rearing jars and mated P. xylostella were released for 6 h to oviposit. Emerged larvae were fed on relevant plant cultivars, and developed pupae were collected in marked glass vials singly. Emerging moths were used for further rearing and research studies.
Diadegma insulare culture
Parasitized larvae and pupae of P. xylostella were collected from cauliflower/cabbage crops at Rawalpindi (Punjab, Pakistan) sites. Collected larvae were brought into insect growth chamber (25 °C, 65±5% RH, and 16L: 8D hours) and released on fresh and untreated potted plants of cauliflower/cabbage and placed in rearing jars whereas collected pupae were placed singly in glass vials. From this field collection, emerged adults of D. insulare were identified and released into rearing jars for mating, provided with honey solution (20%) soaked cotton swabs in petri plates. To obtain the parasitoid’s progeny of homogenous age and acclimatize with the host plant cultivars as well as abiotic controlled conditions, nine potted plants from each cultivar were placed in separate rearing jars along with ten third instar larvae of P. xylostella on each plant. After that, 10 pairs of mated D. insulare were released for parasitization in each rearing jar for 24 h. Plants were replaced (where needed) until the pupation of all the larvae, and then, developed parasitized pupae were harvested and kept in marked glass vials for adult emergence. Emerging parasitoids were used for further rearing and research studies.
Parasitism and sex ratio of D. insulare
Twenty potted plants of each cultivar of cauliflower and cabbage were used singly in separate rearing jars, and each plant was infested with 10 newly molted 3rd instar larvae of P. xylostella. One pair of 1-day-old mated D. insulare was released for 24 h on 10 plants of each cultivar whereas the remaining 10 plants received herbivores only and served as control (without parasitoids). Insects were observed daily, and the number of surviving larvae was recorded until pupation. Afterwards, pupae were harvested and kept in the labeled glass vials provided with honey solution (20%) soaked cotton plugs for emerging adults and placed under required controlled conditions. After adults’ emergence, parasitism (%) was recorded and corrected by using control mortality. Females and males of parasitoid were identified on the basis of ovipositor and counted for sex ratio determination. Similar experimental studies were performed under 3 different temperature regimes (i.e., 19, 23, and 27 °C) along with 65±5% RH and photoperiod of 16L: 8D hours in SANYO incubator, MIR-254 (Netherlands).
Biochemical analysis
Plant leaves from each cultivar were collected, washed with distilled water, and oven dried at 65 °C for 48 h. After the achievement of constant weight, samples were ground and subjected to different tissue nutrient analyses, following recommended procedures. Kjeldahl’s (1883) method was followed for the determination of total nitrogen (N) contents, whereas potassium (K) contents were assessed by flame photometer (Knudsen et al. 1982) and phosphorus (P) contents were analyzed by spectrophotometer (Anderson and Ingram 1993).
Statistical analysis
Parasitism (%) was determined using the following equation:
whereas corrected mortalities (MC) were obtained using the Schneider-Orelli (1947) equation:
Sex ratio was determined using the following equation:
Completely randomized design was followed in factorial manner for experimental layout and analysis of variance for parasitism and sex ratio in offspring of the parasitoid. All the data were subjected to arcsine transformation prior to ANOVA. Means of significant treatments were compared using Tukey’s HSD test. Analysis of the collected data was computed using Statistix 8.1 software (McGraw-Hill 2008).
Results and discussion
Parasitism efficiency and host plant preference by D. insulare
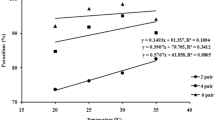
Parasitism efficiency of D. insulare was observed to differ significantly on cruciferous cultivars under different temperature regimes (Fig. 1). It was observed that under 19 °C (F = 8.21, DF = 90, P < 0.05), relatively higher parasitism rate (43.06%) was recorded on “White marble,” whereas it was lower (20.06%) on “Smilla.” Under 23 °C (F = 12.9, DF = 90, P < 0.05), the highest parasitism (79.39%) was observed on “White marble” which was found non-significantly different to “Asha” (73.31%); however, parasitism was minimum (50.0%) on “Delight ball.” Under 27 °C (F = 7.24, DF = 90, P < 0.05), relatively higher parasitism (61.49%) was observed on “White marble,” while it was lowest (37.46%) on “Delight ball.” Considering the parasitism efficiency of D. insulare in relation to host plant cultivars, “White marble” and “Asha” were found as the relatively preferred cultivars, while “Smilla” and “Delight ball” were the comparatively less preferred cultivars. It was evaluated that among the 3 temperature regimes, the best performance of D. insulare was explored at 23 °C; however, 19 °C was the least preferred.
Phytochemical analysis of all the studied cruciferous cultivars was performed. According to the obtained results, higher nitrogen contents were found in the cultivars “White marble” and “Asha” (viz. 0.340 and 0.326%, respectively), while lower nitrogen contents were found in “Smilla” and “Delight ball” (viz. 0.213 and 0.175%, respectively). Similarly, higher phosphorus contents were documented in cultivars “White marble” and “Asha” (viz. 0.046 and 0.044%, respectively), whereas lower phosphorus contents were found in “Smilla” and “Delight ball” (viz. 0.023 and 0.024%, respectively). In contrast to these, higher potassium contents were observed in cultivars “Smilla” and “Delight ball” (viz. 0.294, 0.271%, respectively), while lower potassium contents were found in cultivars “White marble” and “Asha” (viz. 0.169 and 0.164%, respectively).
Obtained results demonstrated that variation in temperature regimes along with different cruciferous cultivars influenced the parasitism efficiency of D. insulare. It has also been reported in the literature that both host plants (Vinson and Iwantsch 1980) and the temperature regimes (Golizadeh et al. 2014) are the responsible factors to affect the parasitoid’s performance. During the present study, “White marble” and “Asha” cultivars showed higher nitrogen and phosphorus contents than the rest. It has been well documented that nitrogen and phosphorus are responsible for vigorous growth and development in insect pests (Lu et al. 2007). This higher level resulted in greater biological potential in host insect (i.e., P. xylostella), which in turn result in better parasitism rate. Present findings are comparable to the findings of Sarfraz et al. (2009) who have reported similar results regarding efficient parasitism of D. insulare. Potassium is inversely proportional to the growth potential of insect pests according to the previous studies conducted by Amtmann et al. (2008). So, lower potassium contents in cultivars have contributed to higher parasitism. These findings are also in accord with the previous studies of Shah (2017) who reported higher resistance in insect pests, and alternatively, this resistance results in lower parasitism.
The findings concerning the temperature regimes revealed that parasitism (%) on cruciferous cultivars was observed to be fluctuating and it was found minimum under 19 °C and maximum under 23 °C, followed by 27 °C. Regarding temperature, similar trends in the fluctuation of parasitism by Oomyzus sokolowskii on P. xylostella were observed by Wang et al. (1999) and it was recorded that parasitism remained increasing from 20 to 25 °C, while it decreased afterwards. Efficacy of O. sokolowskii in the field populations of China was observed by Liu et al. (1997), and when it was correlated with temperature, relevant findings were documented.
Sex ratio in parasitoid’s progeny
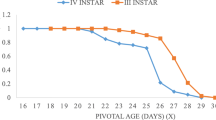
During this study, sex ratio in the progeny of D. insulare was non-significantly different under different temperature regimes; however, minute variations were reported among the studied cruciferous cultivars (Fig. 2). These findings are comparable to the findings of Pandey and Tripathi (2008) who reported a little variation in most of the desired qualities like sex ratio of C. chlorideae in the range of 17–27 °C. Similarly, obtained results showed minor changes in the sex ratio in the studied cultivars which all belong to cruciferous crops. So, it was well documented that parasitoids do not show significant change in sex ratio within the similar plant family as reported by Idris and Grafius (1993). It happens usually in the case of parasitoids which are adaptive to wide range of temperatures like D. insulare. According to Harcourt (1960), it is the general trend in the life cycle of parasitoids that their male emergence proportion remains higher than females and similar findings were observed during the present study in most of the cases. The present study reported that even varying levels of nitrogen, phosphorus, and potassium in cabbage and cauliflower cultivars did not affect the sex ratio in the progeny of parasitoid. It can be concluded that in case of D. insulare, sex ratio in the progeny may be a natural phenomenon which does not alter by the cultivars within the same family.
Conclusions
D. insulare was an active biocontrol agent of P. xylostella in almost all utilized cruciferous cultivars and at all the 3 temperature regimes. The “White marble” (cauliflower) and “Asha” (cabbage) were the relatively preferred host plant cultivars for D. insulare, which may be utilized to manage P. xylostella.
Availability of data and materials
The data used and analyzed during this project are available from the corresponding author on reasonable request.
Abbreviations
- RH:
-
Relative humidity
- P Di :
-
Number of D. insulare pupae
- L t :
-
Number of introduced P. xylostella larvae
- M C :
-
Corrected mortality
- L:D:
-
Light to dark ratio
References
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133(4):682–691
Anderson JM, Ingram JS (1993) Tropical soil biology and fertility. A Handbook of Methods. CAB International, Wallingford, United Kingdom, pp 1–321
Bjorkman C (2000) Interactive effects of host resistance and drought stress on the performance of gall making aphid living on Norway spruce. Oecologia 123:223–231
Braun L, Olfert O, Soroka J, Mason P, Dosdall LM (2004). Diamondback moth biocontrol activities in Canada. In: Kirk AA, Bordat D (eds) Improving biocontrol of Plutella xylostella. Proc Int Symp p 144-146.
Cobblah MA, Afreh-Nuamah K, Wilson D, Osae MY (2012) Parasitism of Plutella xylostella (L.) (Lepidoptera: Plutellidae) populations on cabbage Brassica oleracea var. capitata (L.) by Cotesia plutellae (Kurdjumov) (Hymenoptera: Braconidae) in Ghana. W Afr J Appl Ecol 20(1):37–45
Dosdall LM, Soroka JJ, Olfert O (2011) The diamondback moth in canola and mustard: current pest status and future prospects. Prairie Soils Crops J 4:66–76
Golizadeh A, Kamali K, Fathipour Y, Abbasipour H (2014) Life table and temperature-dependent development of Diadegma anurum (Hymenoptera: Ichneumonidae) on its host Plutella xylostella (Lepidoptera: Plutellidae). Environ Entomol 37(1):38–44
Harcourt DG (1960) Biology of the diamondback moth, Plutella maculipennis (Curt) (Lepidoptera: Plutellidae), in eastern Ontario. III Natural enemies. Can Entomol Res 29:419–428
Howe GA, Schaller A (2008) Direct defenses in plants and their induction by wounding and insect herbivores. In: Schaller A (ed) Induced plant resistance to herbivory. Springer, Berlin, pp 7–29
Idris AB, Grafius E (1993) Pesticides affect immature stages of Diadegma insulare (Hymenoptera: Ichneumonidae) and its host, the diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 86(4):1203–1212
Kfir R (1997) Parasitoids of Plutella xylostella (Lep.: Plutellidae) in South Africa: an annotated list. Entomophaga 42:517–523
Kjeldahl J (1883) A new method for the determination of nitrogen in organic matter. Z Anal Chem 22:366–382
Knudsen D, Peterson GA, Hatt PF (1982) Lithium, sodium, and potassium. Part 2. In: A. L. Page et al. (2nd eds). Methods of soil analysis. American Society of Agronomy, Madison 9:225–246
Liu S-S, Wang X-G, Guo S-J, He J-H, Song H-M (1997) A survey of insect parasitoids of Plutella xylostella and the seasonal abundance of the major parasitoids in Hangzhou, China. In: The management of Diamondback moth and other crucifer pests, Proceedings of the 3rd International Workshop. Mardi, Malaysia, pp 61–66
Liu S, Wang X, Guo S, He J, Shi Z (2000) Seasonal abundance of the parasitoid complex associated with the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) in Hangzhou, China. Bull Entomol Res 90:221–231
Lu ZX, Yu XP, Heong KL, Cui HU (2007) Effect of nitrogen fertilizer on herbivores and its stimulation to major insect pests in rice. Rice Sci 14(1):56–66
McGraw-Hill C (2008) Statistix 8.1 (Analytical Software, Tallahassee, Florida). Maurice/Thomas text.
Oke OA (2008) Evaluation of the effectiveness of three insecticides to control Diamondback moth (Plutella xylostella) in cabbage (Brassica oleracea var. capitata L). Eur J Sci Res 22(3):391–395
Pandey AK, Tripathi CPM (2008) Effect of temperature on the development, fecundity, progeny sex ratio and life-table of Campoletis chlorideae, an endolarval parasitoid of the pod borer, Helicoverpa armigera. BioControl 53(3):461
Sarfraz M, Dosdall LM, Keddie BA (2008) Host plant genotype of the herbivore Plutella xylostella (Lepidoptera: Plutellidae) affects the performance of its parasitoid Diadegma insulare (Hymenoptera: Ichneumonidae). Biol Control 44:42–51
Sarfraz M, Dosdall LM, Keddie BA (2009) Host plant nutritional quality affects the performance of the parasitoid Diadegma insulare. Biol Control 51(1):34–41
Schneider-Orelli O (1947) Entomologisches Praktikum. Sauerlander HR Aarau Switzerland.
Shah TH (2017) Plant nutrients and insects development. Internat J Entomol Res 2:54–57
Stiling P, Moon DC (2005) Quality or quantity: the direct and indirect effects of host plants on herbivores and their natural enemies. Oecologia 142:413–420
Vinson SB, Iwantsch GF (1980) Host suitability for insect parasitoids. Annu Rev Entomol 25:397–441
Wang XG, Liu SS, Guo SJ, Lin WC (1999) Effects of host stages and temperature on population parameters of Oomyzus sokolowskii, a larval-pupal parasitoid of Plutella xylostella. Biocontrol 44:391–402
Xu J, Shelton AM, Cheng X (2001) Comparison of Diadegma insulare (Hymenoptera: Ichneumonidae) and Microplitis plutellae (Hymenoptera: Braconidae) as biocontrol agents of Plutella xylostella (Lepidoptera: Plutellidae): field parasitism, insecticide susceptibility, and host searching. J Econ Entomol 94:14–20
Acknowledgements
Authors are very much thankful to Shehnaz Parveen Qureshi, Shagufta Parveen Qureshi, Dr. Altaf Ahmad, Wali Muhammad, Salman Ghuffar, Abdul Mannan Hamzah, Muhamad Shehzad, Muhammad Saeed, Muhammad Nadir Naqqash, and Muhammad Amad Hussain for their continuous support during the whole experimental studies.
Funding
Experimental studies were funded by the Higher Education Commission, Islamabad (Pakistan).
Author information
Authors and Affiliations
Contributions
MSQ and AM planned and designed the research experiments. MSQ performed the experiments and wrote the research article. MN and KNS performed the data interpretation and statistical analysis. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qureshi, M.S., Mohsin, A.u., Naeem, M. et al. Parasitism and sex ratio of Diadegma insulare (Cresson) (Hymenoptera: Ichneumonidae) against Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) on cruciferous cultivars under different temperatures. Egypt J Biol Pest Control 30, 122 (2020). https://doi.org/10.1186/s41938-020-00324-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00324-y