Abstract
Background
Xylanase enzyme plays an important role in nature as being a part of protecting the environment from pollution. It has also various industrial applications.
Main body of abstract
Marine fungal isolate was recovered from red sea water at Sharm El-Sheikh province, Egypt, and tested for xylanase activity, using different agricultural wastes as a substrate. It was found that rice straw was the best substrate for xylanase production (0.37 U/ml). Thus, it was subjected for identification by 18S rDNA gene. The phylogenetic analysis results indicated that this fungal isolate belonging to Aspergillus species with a similarity of 99% and named as A. oryzae SS_RS-SH (MN894021). The regular two-level factorial design was used to optimize the important medium components, which significantly affected the xylanase production. The model in equation suggested optimal conditions of 2% of rice straw, 8 g/l of yeast extract, 4 g/l of (NH4)2SO4, 2 g/l K2HPO4, and 2.5 g/l MgSO4.7H2O for a maximum xylanase yield. The antifungal activity of crude xylanase on mycelial growth of some pathogenic fungi isolated from different hosts was investigated. The results showed that xylanase T1 had a potent antifungal activity than control. Greenhouse experiments indicated that all treatments with xylanase at different concentrations significantly decreased infection occurrence of beans, which have been effectively infected with root rot pathogens, compared to unprocessed control treatments.
Short conclusion
Xylanase yield increased 2.43-folds than initial screening. The xylanase had a potential antifungal activity both in vitro and under greenhouse conditions. The outcome of this study ensured that this fungal strain could be used as biological control for plant disease.
Similar content being viewed by others
Background
Xylanase (E.C.2.8.1.8) is defined as a set of hemicellulose, which is necessary for the hydrolysis of 1, 4-xylans present in lignocellulostic materials (Jae et al. 2009). It plays an important role in nature as being a part of protecting the environment from pollution because of its alternative way to chemical hydrolysis (El Shamy et al. 2016). Industrial applications of xylanase depended upon its ability to hydrolyze xylan, which is the abundant natural polysaccharide (Polizeli et al. 2005). Xylanase from different microorganisms such as fungi, bacteria, and few yeast strains have gained interest owing to their prospective uses in numerous industrialized manners such as hydrolyses production, nutritive enhancement of lignocellulosic feedstuff, wines and liquids clearing up, and bio-bleaching of craft soft tissue in paper manufacturing, food additives, poultry, improving the handling of dough used for extraction of coffee, plant oils and extraction of starch (Shabeena et al. 2017 and Nitin et al. 2017).
The greatest shared industrialized xylanase producing microorganisms are the species of Trichoderma and Aspergillus along with the bacterial strains as Bacillus species (Sapag et al. 2002). Fungi have been broadly used to deliver hydrolytic enzymes for industrialized applications, comprising xylanases, whose levels in fungi are commonly a lot higher than in yeast and microscopic microorganisms (Atalla et al. 2020).
Some of the most commonly agro-residues used as a cheap and renewable carbon source for xylanase production and for developing biotechnological processes of industrial interest; as wheat bran (El Shamy et al. 2016), wheat husk (Kumar et al. 2018), different and numerous of agricultural wastes, and different vegetable leaf industries and groundnut shell (Rosmine et al. 2017; Sindhu et al. 2017). A recent study also showed that wastewater from the pulp industry was reused as medium for xylanase production (de Queiroz-Fernandes et al. 2017).
Plackett-Burman design (PBD) is a powerful statistical technique to screen “n” variables in just “n + 1” in a shake flask experiment, which is used to reduce the total number of experiments, as it commonly used to optimize fermentation processes. This technique cannot determine the interaction effect, but it is very useful for the first step of an optimization procedure. As well as, it evaluates the essential of each factor in moderately few experiments (Bharti 2016; Plackett and Burman 1946).
The aim of this work was xylanase production from different agriculture wastes by genetically identified marine fungal Aspergillus oryzae strain. Then, the significant nutritional elements of Plackett-Burman design and assessment of their optimum concentrations in the cultivation medium were selected for competent production. Finally, its application as a biological control for faba bean root diseases.
Materials and methods
Microorganism
Marine fungal strain, Aspergillus oryzae MN894021, was isolated from the red sea water at Sharm El-Sheikh province, Egypt, and identified by 18S rRNA gene. The fungal culture was maintained on a medium containing glucose 1.0 g/l, peptone 0.5 g/l, yeast extract 0.1 g/l, agar 15 g/l, 800 ml sea water, and 200 ml distilled water (Jenkins et al. 1998), incubated at 30 °C for 7 days and then kept at 4 °C for storage.
Molecular identification of fungal isolate
DNA isolation, PCR amplification, and sequencing
DNA extraction was done, using the protocol of Gene Jet genomic DNA purification Kit (Thermo# K0791), following the manufacture of the kit. The PCR amplification of 18S rDNA region was carried out, following the manufacture of Maxima Hot Start PCR Master Mix (Mix (Thermo) #K0221). The 18srDNA was amplified by polymerase chain reaction (PCR), using primers designed to amplify 1500 bp fragment of the 18SrDNA region. The ITS1–5.8S–ITS2 genomic region was amplified from genomic DNA, using the forward primer ITS1 (5-TCCGTAGGTGAACCTGCGG-3) and the reverse primer ITS4 (5-TCCTCCGCTTATTGATATGC-3) (Hamed et al. 2015; White et al. 1990).
The PCR reaction was performed with 2 μl of 10 X Taq PCR buffer, 1.6 μl from 2.5 mM dNTP mixture, 1 μl of both forward and reverse primers (10 pmol/μl), 2 μl template genomic DNA (20 ng/μl), 0.2 μl from KOMA-Taq. (2.5 U/μl), and distilled water (HPLC grade) up to 20 μl. The reaction mixture was as follows: initial denaturation at 95 °C for 1 min, 30 cycles dent. 95 °C for 30 s, annealing 55 °C for 2 min, extension 68 °C for 1.5 min, final extension 68 °C for 10 min for 1 cycle. After completion, the PCR products were electrophoresed on 1% agarose gels, containing ethidium bromide (10 mg ml), to ensure that a fragment of the correct size had been amplified.
The amplification products were purified by Montage PCR clean up kit (Millipore). The purified PCR products were sequenced, using the 2 primers that were used before in the PCR reaction. Sequencing was performed by big Dye Terminator Cycle Sequencing kit V.3.1 (Applied Biosystems, USA). PCR products were resolved on an Applied Biosystems model 3730XL automated DNA sequencing system (Applied Biosystems, USA) at the Macrogen, INC, Seoul Korea.
Phylogenetic analysis and tree construction
Phylogenetic data were obtained by aligning the nucleotides of different 18S rRNA retrieved from BLAST algorithm (www.ncbi.nlm.nih.gov/BLAST), using the CLUSTAL W program version 1.8 with standard parameters. Phylogenetic and molecular evolutionary analyses were conducted, using Mega 6 program (Tamura et al. 2013). All analyses were performed on a bootstrapped data set containing 100 replicates (generated by the program).
Pathogenic fungi
The tested soil-borne pathogenic fungi were Fusarium (F) solani, F. chlamydosporum, F. incranatum, Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolina, Sclerotinia sclerotiorum, and Botrytis cinerea. These fungi were isolated from various hosts, showing root rot and or damping-off disease symptoms. All isolates were identified according to Booth (1985); Barnett and Hunter (1986), and Simmons (2007).
Substrates
Different agricultural wastes (potato peel, orange peel, corn cobs, rice straw, soybean, sawdust, and wheat bran) were used as substrates for xylanase production under greenhouse conditions. All wastes were washed, dried at 70 °C in an oven, and powdered, using a blender before use. One substrate further was selected to give the maximum xylanase production (El Shamy et al. 2016).
Production medium
According to Cunha et al. (2018), the medium used for xylanase production was composed of (%): 0.4 peptone, 0.4 yeast extract, 0.2 KH2PO4, 0.8 (NH4)2SO4, 0.25 MgSO4.7H2O, 2% substrate at pH 7.0. A 50 ml of fermentation medium into a 250-ml Erlenmeyer flasks was inoculated by two disks in diameter from the fungal strain and incubated on rotary shaker at 150 rpm, on 30 °C for 7 days.
Xylanase assay
Determination of enzyme activity was carried out according to the method of Monreal and Reese (1969): One milliliter of 1% birch wood xylan (Sigma Aldrich, St. Louis, USA) in acetate buffer (pH 4.6) in test tubes was added to 1 ml of the culture filtrate and mixed by shaking. The mixture was incubated in a water bath at 50 °C for 30 min, then cooled and centrifuged before assaying. The amount of reducing sugars was determined with 1 ml of 3, 5-dinitrosalicylic acid (DNS). One unit of enzyme activity was taken from the catalyst one micromole of substrate in 1 min under specific conditions.
Experiment design and statistical analysis
The regular two-level factorial design was used to optimize the important medium components, which converting affected on xylanase production by A. oryzae MN894021. Five indented variables (different substrate concentrations, yeast extract, (NH4)2SO4, K2HPO4, MgSO4.7H2O) were investigated, using regular two-level factorial design at two levels, low level (−1) and high level (+1) (Table 1).
Factorial experiment design is based on the first-order model equation: Y = β∘ + ∑βixi, where: Y is the response (enzyme activity), β∘ is the model coefficient, βi is the linear coefficient, and xi is the level of the independent variable.
In the present study, 5 factors were screened in 32 experimental designs. All experiments were carried out in duplicate and the averages of xylanase activity were taken as response (Table 2). An analysis of variance (ANOVA) for the obtained results was used. For designing the experiments, analysis of variance and the Design-Expert 12 software from StatEase, Inc. were applied.
Evaluation of the efficiency of crude xylanase grown on rice straw against different fungal isolates by agar well diffusion method
Crude xylanase from A. oryzae MN894021 was screened for antifungal activity, using a sterile cork borer of size 6.0 mm in diameter according to Bobbarala et al. (2009). Potato dextrose agar (PDA) (Sigma Aldrich, St. Louis, USA) plates were inoculated by old cultures grown for 72 h (PDA) of the selected pathogenic fungal strains. An aliquot (0.02 ml) of inocula was introduced to molten PDA and poured into Petri dish by pour plate technique. A distance 500 μl of filtrate of crude xylanase solution was homogenized, filled in deep blocks, and incubated at 25 °C for 48-72 h. The antifungal activity was evaluated by measuring inhibition of mycelial growth (in millimeter) and the experiment was carried out in triplicates. The toxicity of the extracts to fungi growth, in terms of percentage inhibition of mycelial growth was calculated, using the formula: % inhibition = dc − dt/dc × 100, where dc = average increase in mycelial growth in control, dt = average increase in mycelial growth in treatment (Singh and Tripathi 1999).
Green house experiment
Source of faba bean seeds
Faba bean (Vicia fabae L.) cultivar Giza 40 used in this study was obtained from Legume Crop Research Department, Field Crop Research Institute, Agriculture Research Centre, Ministry of Agriculture, Egypt. Three fungal pathogens, F. solani, M. phaseolina, and R. solani showed high percentages of infection and evaluated the antifungal activity of crude xylanase on different concentrations of rice straw waste under greenhouse conditions on faba bean were evaluated. Sandy clay soil was transferred in pots. Inoculum from each of the above cultures was colonized separately and was infected at the rate of 3 g/100 g soil. The disinfected bean seeds were coated with crude xylanase at the rate of 5 ml/kg seeds. Seed dressing was carried out by applying the xylanase to the gum-moistened seeds in polyethylene bags and shaked well to ensure even distribution of the added materials. The treated seeds were then left on a plastic tray to air dried. Three replicates were used per treatment. Pathogen free-seeds were surface sterilized and planted (5 seeds/pot) in both inoculated and non-inoculated soils. All pots were maintained in greenhouse conditions. Fifteen days after sowing, the disease ratios were determined by recording the number of non-emerged seeds (pre-emergence damping-off), while post-emergence root rot was recorded from (30 to 45 days after sowing). The equation described by Khalifa (1987) was followed:
Statistical analysis
Tukey test for multiple comparisons among means was utilized (Neler et al. 1985).
Results and discussion
Molecular identification of the isolate
DNA isolation and PCR amplification
The DNA content of SS_RS-SH strain was endangered to PCR via common primers to magnify the ITS1 and ITS4 sections among the minor and major genomic rDNA, comprising the 18S rDNA. The present primers improved a DNA piece of about 579 bp. The present outcome was in contract with Rasul et al. (2007) who originated that these primers were exact for fungus and an augmented DNA piece of about 560 bp expending some fungus. Aspergillus oryzae SS_RS-SH nucleotide sequence (520 bp) was blasted by the presented GenBank data via NCBI-BLAST search (www.ncbi.nlm.nih.gov/BLAST) to associate the SS_RS-SH isolate with individuals of Aspergillus sp. strains. The consequences presented the extraordinary sequence correspondence species (99%) with A. oryzae.
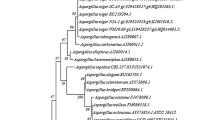
Phylogenetic analysis and GC%
The phylogenetic tree (Fig. 1) disclosed that SS_RS-SH isolate was the greatest nearly correlated to A. oryzae. Thus, it was suggested a name, A. oryzae SS_RS-SH. The G + C% was unique of numerous universal structures utilized to identify the fungi genomes. The GC content of the SS_RS-SH isolate was 58 mol% achieved from the phylogenetic analysis. This outcome was in agreement with those by Nakase and Komagata (1971) who revealed that the G + C content of fungus varied from 31.5 to 63%, depending on each class, genus, and species.
GenBank ID
The nucleotide sequences of 18S rRNA gene of A. oryzae SS_RS-SH were dumped in the Gen Bank under accession number: MN894021.
Production xylanase using different agricultural wastes
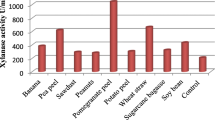
Different agricultural wastes (potato peel, orange peel, corn cobs, rice straw, soybean, saw dust, and wheat bran) were used as substrates for xylanase production from A. oryzae MN894021. The results in Fig. 2 showed that a maximum xylanase activity was at 0.37 U/ml, using rice straw waste as substrate, followed by soybean and corn cobs produced (0.32, 0.29 U/ml), respectively, while saw dust, orange peel, and wheat bran produced (0.25, 0.46 and 0.17 U/ml), respectively and exhibited low xylanase production. Comparatively less xylanase production observed with potato peel waste produced 0.18 U/ml. These results proved that rice straw was the best substrate for xylanase production, using A. oryzae MN894021. de O Souza et al. (1999) proposed that xylanase production by few fungal strains utilizing agricultural wastes relied upon the substrate structures, selection of fermentation style, and conditions just as downstream handling of the delivered enzymes. These results were greater than that obtained by Soroor et al. (2013) who reported that total xylanase activity of 0.005 and 0.002 U/ml in a basal medium complemented with rice straw as C-source. As well as, obtained results were coincided with Anthony et al. (2003) who found that A. fumigatus formed extraordinary levels of xylanase from rice straw.
Optimization of medium components for xylanase production
The statistical design was used to improve medium constituents that improving xylanase production from A. oryzae MN894021, using the rice straw as substrate. The two-level factorial design optimized significant variables along with their interactions on xylanase production. Several studies reported that statistical methods have been applied for optimization of microbial enzyme production and the enzyme yield was increased (Vishwanatha et al. 2010).
In the present study, a two-level factorial design was successfully applied to test the relative importance of medium components on xylanase production. The two-level factorial design with both the actual and predicted responses for the 32 experimental runs was presented in Table 2. The data indicated that there was a wide variation from 0.35 to 0.90 U/ml of xylanase enzyme in the 32 runs, which reflected the caused variations due to the presence of different factors affecting the activity at low and high importance of levels of medium optimization to achieve higher productivity. The results showed that the maximal xylanase yield of 0.90 U/ml was realized in run 14 achieved under optimal experimental conditions with 2.5% rice straw and 9.0 g/l yeast extract, 6.0 g/l (NH4)2SO4, 1.5 g/l K2HPO4, and 2.0 g/l MgSO4.7H2O, while the lowest yield was found in run 31 (0.35 U/ml). Obtained results were greater than those of Park et al. (2002) who denoted that the enhanced xylanase activity of 0.5 U/ml, using rice straw by A. niger culture, was achieved by fractional factorial design of optimization medium conditions and process parameters. As well, the present results were the most close to those obtained by Salihu et al. (2015) who reported that the maximum xylanase activity varied from 1.00 to 1.5 U/ml, using soybean hulls by A. fumigatus NITDGPKA3 and A. niger, respectively, using statistical methods.
The relationship between the medium components and the response obtained from the 32 experiments was predicted by the first-order model equation which was used to explain the xylanase production,
where Y predicted response and A, B, C, D, and E are the coded values of substrate concentration, yeast extract, (NH4)2SO4, KH2PO4, and MgSO4.7H2O, respectively.
Analysis of the data from the factorial experiments involved a first-order (main effects) model. The main effects of the examined factors on the xylanase activity were calculated and presented in the Pareto graph (Fig. 3). The Pareto graph was an important tool for analyzing all the parameters, then to focus on the most significant factors. On the analysis of the regression coefficients of the 5 variables, values for (NH4)2SO4, yeast extract, and substrate concentration were significant showing a positive effect on xylanase activity, while KH2PO4 and MgSO4.7H2O were contributed negatively. The analysis of variance (ANOVA) of the main effects of factors showed that the model F value of 24.62 implying the model was significant. There was only a 0.01% chance that an F value this large could occur due to noise, probability values < 0.05, (p < 0.05) indicated that model terms were significant, in these cases A, B, C, CD, ABD, BCD, ABCDE, whereas p values > 0.1 (p > 0.1) indicated that model terms were not significant as shown in Table 3.
The p value was the probability that the magnitude of a contrast coefficient was due to random process variability and served as a tool for checking the significance of each of the coefficients, when the concentration effect of the tested variable was positive, the influence of the variable upon xylanase enzyme production was greater at a high concentration. The fit of the model can be checked by the “determination coefficient” R2, R2 = 0.9676. Normally, a regression model having an R2 value higher than 0.90 was considered to have a very high correlation (Haaland 1989). The value of the “Predicted R2” was found to be 0.8309 which has a reasonable agreement with the R2 of 0.9676 and adjusted R2 of 0.9283. This revealed that there was good agreement and a high correlation between the experimental and the theoretical values predicted by the model, and the regression model provided an excellent explanation of the relationship between the independent variables (factors) and the response (xylanase production) and almost all the variation could be accounted for by the model equation. The equation in terms of coded factors can be used to make predictions about the response for given levels of each factor. By default, the coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients.
Figure 4 shows that the actual response values agreed well with the predicted response ones. This model can be used to predict the xylanase production within the limits of the experimental factors. The interaction effects of variables on xylanase were studied by plotting a three-dimensional (3D) surface plot against any two independent variables. The 3D response surface plots described by the regression model were drawn to illustrate the effects of the independent variables and the interactive effects of each independent variable on the variable responses. The interaction among the independent variables such as the substrate concentration, and yeast extract; substrate concentration and MgSO4.7H2O; substrate concentration and K2HPO4; (NH4)2SO4 and K2HPO4; (NH4)2SO4 and MgSO4.7H2O; K2HPO4 and MgSO4.7H2O showed a significant effect on xylanase activity by A. oryzae (Fig. 5a-f). The effect of concentration of substrate and one of the other variables was shown in Fig. 5a-c. Results indicated that the xylanase activity significantly increased with increasing substrate concentration, yeast extract, MgSO4.7H2O, and K2HPO4 and produced (0.90 U/ml). The same results in Fig. 5d-f showed that maximum xylanase activities were at 4 g/l (NH4)2SO4, 2 g/l K2HPO4, and 2.5 g/l MgSO4.7H2O. Obtained results were in agreement with Kanagasabai and Thangavelu (2013) who reported that the ammonium salts ((NH4)2SO4) improved the growth level as well as enhanced the protein appearance by mediating ammonium conforming enzymes; thereafter, xylanase activity was amended.
3D surface plot showing the interaction between significant factors affecting xylanase production. a Substrate concentration and yeast extract. b Substrate concentration and MgSO4. 7H2O. c Substrate concentration and KH2PO4. d (NH4)2SO4 and KH2PO4. e (NH4)2SO4 and MgSO4. 7H2O. f KH2PO4 and MgSO4. 7H2O
The obtained results indicated that the high yeast extract level had a high contribution to the xylanase yield. These results were in accordance to Cui and Zhao (2012) who denoted that increasing the yeast extract concentration in the medium enhanced xylanase activity by A. awamori NRRL 3112 due to it contained a lot of vitamins, minerals, and amino acids, which are usually used as growth stimulants or growth factors for microbes and may play an important role for the enzyme metabolism resulting in an increase in xylanase activity (Vimalashanmugam and Viruthagiri 2013).
The present results indicated the importance of the substrate concentration for xylanase production. The xylanase activity produced by Penicillium sp. WX-Z1 increased with gradual increase of wheat bran concentration as a substrate (Cui and Zhao 2012). Obtained results showed the xylanase activity was enhanced at a higher level of MgSO4. These results were in agreement with Cui and Zhao (2012) who indicated that the xylanase production could achieve a higher activity when the concentration of MgSO4 was at a higher level between −0.3 and 0.1 (coded value). This enhancement referred to Mg2+ has a positive effect on the stabilization of the ribosome and cellular membranes which relatively enhanced the activity of xylanase. The same phenomenon was observed by Vimalashanmugam and Viruthagiri (2013).
In vitro antifungal activity of crude xylanase
Effect of crude xylanase produced from A. oryzae MN894021, which was grown on rice straw on mycelial growth of some pathogenic fungi isolated from different hosts. Results showed that xylanase T1 showed higher antifungal activity than control and different treatments (Table 4). The highest inhibition with T1 against F. incranatum, B. cinrea, S. rolfsii, and R.solani were (100.0, 66.6, 66.6, 55.5%), respectively. Also, a moderate effect on F. solani, F. chlamydosporum, and M. phaseolina (50.0, 50.0, and 33.3%), respectively, and the weakest effect on S. sclerotiorum (10.0%). In case of T2, T3 and T4 had significant effects on all pathogenic fungi than the control. As also shown in Table 4, the antifungal activity of xylanase extract against some phyto-pathogenic fungi decreased the linear growth of A. alternata, F. oxysporum, Phoma destructive, R. solani, and Sclerotium rolfsii with different degrees of activity against the tested fungi.
Greenhouse experiments
The data in Table 5 showed that all treatments with xylanase at different concentrations could highly significantly reduce disease incidence of beans, which have been artificially infected with root rot pathogens, than the untreated control treatment. Seed coating with xylanase (T1) gave a significant protection to emerged bean seeds against invasion of pathogenic fungi at the pre-emergence stage which recorded (71.5, 100.0, and 90.0% protection) on F. solani, M. phaseolina, and R. solani, respectively. Seed coating recorded more than 70.0% protection than the untreated control. At the post-emergence stage, data also showed that treatment with xylanase (T1 and T2) could reduce the percentages of root-rot incidence, which was higher than the untreated control (treatment with pathogens alone). Treatment with xylanase (T1) caused a reduction in the percentage of root-rot incidence recorded as 75.0, 100.0, and 90.9% in soils infected with F. solani, M. phaseolina, and R. solani, respectively.
Similar results were obtained by Das et al. (2013) who isolated the highest xylanase production by A. carneus and the highest cellulase production by A. fumigatus and were successfully used in the biodegradation of rice straw compost, which was rich in nitrogen, potassium and silicon. It enhanced plant growth, development, and disease suppression in chili cultivation (Dukare et al. 2011). Obtained results were in agreement to Kausar et al. (2013) who indicated that the rice straw compost was used for chili farming under glasshouse conditions. Chili seeds cv. Kulai were planted in Sclerotium rolfsii tested soil where microbial straw manure expanded seed germination, seedling foundation, plant development, and smothered improvement of foot rot disease which contrasted with utilizing commercial fertilizer and fungicide, Benomyl.
Conclusions
A marine fungal was recovered from the red sea water in Egypt, identified as A. oryzae MN894021, produced about 0.37 U/ml xylanase, using rice straw waste. Statistical optimization using two-level factorial design for xylanase production by A. oryzae using rice straw as raw material showed an improvement of the production on the medium constitutes. Applied treatment of crude xylanase is applicable, safe, and cost-effective method as an antifungal compound. It could have promise success as an alternative to conventional chemical fungicides for the management of plant diseases.
Abbreviations
- PDA:
-
Potato dextrose agar
- PCR :
-
Polymerase chain reaction
- Rpm:
-
Round per minute
- bp:
-
Base pair
- GC:
-
Guanine and cytosine
- Pp:
-
Pomegranate peel
References
Anthony T, Raj KC, Rajendran A, Gunasekaran P (2003) High molecular weight cellulase-free xylanase from alkali-tolerant Aspergillus fumigatus AR1. Enzyme Microbial Technol 32(6):647–654
Atalla MMS, EL Gamal GN, Awad MH (2020) Chitinase of marine Penicillium chrysogenum MH745129: isolation, identification, production and characterization as controller for citrus fruits postharvest pathogens. J J Biol Sci (JJBS) 13(1):19–28
Barnett HI, Hunter BB (1986) Illustrated general of imperfect fungi, 4th edn. Macmillan Publishing, New York
Bharti A (2016) Screening of important factors for xylanase and cellulase production from the fungus C. cinerea RM-1 NFCCI-3086 through Plackett-Burman experimental design. Bioresorce 11(4):8269–8276
Bobbarala V, Katikala PK, Naidu KC, Penumaj S (2009) Antifungal activity of selected plant extracts against phytopathogenic fungi Aspergillus niger f2723. Indian J Sci Technol 2:87–90
Booth C (1985) The genus Fusarium 2nd ed. Kew, surrey: Commonwealth Mycological Institute
Cui F, Zhao L (2012) Optimization of xylanase production from Penicillium sp. WXZ1 by a two-step statistical strategy: Plackett-Burman and box-behnken experimental design. Int J Mol Sci 13:10630–10646
Cunha L, Martarello R, de Souza PM, de Freitas MM, Barros KVG, Ferreira Filho EX, Homem-de-Mello M, Magalhães PO (2018) Optimization of xylanase production from Aspergillus foetidus in soybean residue. Enzyme Res 2018:1–7
Das A, Paul T, Halder SK, Jana A, Maity C, Das Mohapatra PK, Pati BR, Mondal KC (2013) Production of cellulolytic enzymes by Aspergillus fumigatus ABK9 in wheat bran-rice straw mixed substrate and use of cocktail enzymes for deinking of waste office paper pulp. Biores Technol J 128:290–296
de O Souza MC, Roberto IC, Milagres AMF (1999) Solid-state fermentation for xylanase production by Thermoascus aurantiacus using response surface methodology. Appl Microbiol Biotechnol 52:768–772
de Queiroz-Fernandes GM, Martins BL, Rustiguel CB (2017) Reuse of wastewater from pulp industry for the optimization of fungal xylanase production. Acta Sci Biol Sci 39:21–26
Dukare AS, Prasanna R, Dubey SC, Nain L, Chaudhary V, Singh R, Saxena AK (2011) Evaluating novel microbe amended composts as biocontrol agents in tomato. Crop Prot 30:436–442
El Shamy AR, El Gamal GN, Atalla MMS (2016) Effect of different agricultural wastes on xylanase production by Saccharomyces cerevisiae and its application on citrus fruit. J Pure App Microb 10:897–904
Haaland PD (1989) Experimental design in biotechnology. Marcel Dekker, New York
Hamed ER, Awad HM, Ghazi EA, El-Gamal NG, Shehata HS (2015) Trichoderma asperellum isolated from salinity soil using rice straw waste as biocontrol agent for cowpea plant pathogens. J Appl Pharm Sci 5:091–098
Jae WL, Jun YP, Mi K (2009) In G purification and characterization of a thermostable xylanase from the brown rot fungus Laetiporus sulphureus. J Biosci Bioeng 107:33–37
Jenkins R, Bebbington P, Burgha ST, Farell M (1998) British psychiatric morbidity survey. British J Psychiatric 173:4–7
Kanagasabai V, Thangavelu V (2013) Medium optimization for solid state fermentative production of xylanase by Aspergillus terreus using central composite. Innov Roman Food Biotechnol 13:18–29
Kausar H, Ismail MR, Saud HM, Othman R, Habib SH (2013) Use of lignocellulolytic microbial consortium and pH amendment on composting efficacy of rice straw. Compost Sci Util 21:121–133
Khalifa EZ (1987) Further studies on some soil borne fungi affecting soybean and their control. Ph.D. Thesis, Fac. of Agric. Menouif. Univ, Egypt, p 148
Kumar BA, Amit K, Alok K, Dharm D (2018) Wheat bran fermentation for the production of cellulase and xylanase by Aspergillus niger NFCCI 4113. Res J Biotechnol 13:5
Monreal J, Reese ET (1969) The chitinase of Serratia marcescens. Can J Microbiol 15:689–696
Nakase T, Komagata K (1971) DNA base composition of some species of yeasts and yeast-like fungi. J Gen Appl Microbiol 17:363–369
Neler J, Wassermann W, Kutner MH (1985) Applied linear statistical models. In: Richard D (ed) Regression Analysis of Variance and Experimental Design, 2nd edn. Irwin Inc, Homewood Illinois, pp 117–155
Nitin KS, Vivek KT, Santosh KM (2017) The production of xylanase enzyme (E.C. Number=3.2.1.8) using solid substrate fermentation. Biotechnol: An Ind J:134–145
Park YS, Kang SW, Lee JS, Hong SI, Kim SW (2002) Xylanase production in solid state fermentation by Aspergillus niger mutant using statistical experimental designs. Appl Microbiol Biotechnol 58:761–766
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 37:305–325
Polizeli ML, Rizzatti CS, Monti R, Terenzi HF, Jorge J, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol:67–577
Rasul MG, Hiramatsu M, Okubo H (2007) Genetic relatedness (diversity) and cultivar identification by randomly amplified polymorphic DNA (RAPD) markers in teasle gourd (Momordica dioica Roxb.). Sci Hortic 111:271–279
Rosmine E, Sainjan NC, Silvester R et al (2017) Statistical optimization of xylanase production by estuarine Streptomyces sp. and its application in clarification of fruit juice. J Genet Eng Biotechnol 15:393–401
Salihu A, Shuaibu M, Bala OA (2015) A statistical design approach for xylanase production by Aspergillus niger using soybean hulls: optimization and determining the synergistic effects of medium components on the enzyme production. Jordan J Biol Sci 8:319–323
Sapag A, Wouters J, Lambert C, de Ioannes P, Eyzaguirre J, Depiereux E (2002) The endoxylanases from family 11: computer analysis of protein sequences reveals important structural and phylogenetic relationships. J Biotechnol 95:109–131
Shabeena KS, Ravi M, Jayaraj YM (2017) Microbial production of xylanase using regional agro wastes. Int J Pharm Bio Sci 8(3):796–804.
Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW (2007) A common neural substrate for perceiving and knowing about color. Neuropsychologia 45:2802–2810
Sindhu R, Binod P, Mathew AK, Abraham A, Gnansounou E, Ummalyma SB, Thomas L, Pandey A (2017) Development of a novel ultrasound assisted alkali pretreatment strategy for the production of bioethanol and xylanases from chili post-harvest residue. Bioresour Technol 242:146–151
Singh J, Tripathi NN (1999) Inhibition of storage fungi of black gram (Vigna mungo L.) by some essential oils. Flavour Frag J 14:1–4
Soroor MAM, Ghazy AEH, Mahdy ESMS, El-badry MO, Shousha WG, ELKhonezy MI (2013) Purification and characterization of cellulase-poor xylanases from Trichoderma reesei F418 grown on rice straw by solid-state fermentation. J Appl Sci Res 9:1702–1713
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Vimalashanmugam K, Viruthagiri T (2013) Optimization 0f mineral nutrient supplements for production of xylanase by Aspergillus niger under solid-state fermentation (SSF) using central composite design. Int J Pharm Chem Biol Sci 3:615–626
Vishwanatha KS, Rao AGA, Singh SA (2010) Acid protease production by solid-state fermentation using Aspergillus oryzae MTCC 5341: optimization of process parameters. J Ind Microbiol Biotechnol 37(2):129–138
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Acknowledgements
The authors acknowledge National Research Centre especially Chemistry of Natural and Microbial Products Department, Pharmaceutical and Drug Industries Research Division for their support and assurance.
Availability of supporting data
All information generated or analyzed during this work are incorporated in this manuscript.
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
SA selected the marine fungi, optimization of xylanase, enzyme assay, writing fabricated the XYZ sample for the experiment, NA selected the microorganism, enzymes assay, optimization conditions, enzyme immobilization, fabricated the XYZ sample for the experiments, AE participated in the enzyme immobilization, enzyme assay, writing immobilization part, NG applied the antagonistic effects, green house experiment, HM participated in the strain identification, writing identification part, formatting, and revising the manuscript. All authors have approved the final article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atalla, S.M.M., Ahmed, N.E., Awad, H.M. et al. Statistical optimization of xylanase production, using different agricultural wastes by Aspergillus oryzae MN894021, as a biological control of faba bean root diseases. Egypt J Biol Pest Control 30, 125 (2020). https://doi.org/10.1186/s41938-020-00323-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00323-z