Abstract
Predatory nematodes are important as they fed upon several prey nematode species. The diplogasterid predators are characterized by distinguished alterations in feeding apparatus. The diplogasterid predator, Fictor composticola, feeds on different categories of nematodes like microbivorous, mycophagous, and plant-parasitic nematodes. This study was undertaken under in-vitro conditions, in 1% water-agar plates, and in pots under screen house. In lab. study, 250, 500, 1000, 2000 freshly hatched juveniles of Meloidogyne incognita, and 2 females and 2 males of F. composticola were released in each plate. Observations on prey consumed were recorded after 24 and 48 hrs. of deliverance. Prey consumption of F. composticola boosted as the number of prey increased from 250 to 2000 per plate. At prey density of 500 per plate, maximum per cent consumption was observed and minimum at prey density 2000 per plate. Maximum predation found at a prey population density of 2000 individuals and minimum in 250 individuals per plate. This finding led to the conclusion of increased contacts between prey and predator at higher densities. In the pot experiment, plant height and shoot weight were reduced significantly over untreated control at 500 J2 and above. Root weight increased at 500 and 1000 levels, though it was similar to the control at 2000 level due to poor root development at this level. The number of galls and egg masses of M. incognita and final population of predator increased with increasing inoculum level of M. incognita.
Similar content being viewed by others
Background
Biocontrol of nematodes has been pondering as a promising management approach in recent years. This management option in an integrated nematode management system taken as an important component because it least disturbs the ecosystem and may fetch economic and effective control measures (Sikora 1992). Predaceous nematodes can turn out to be important among all biocontrol agents in nematode management, if they get equal opportunity and importance. The advocacy of predatory nematodes for biocontrol potential was known since Cobb (1917) who discovered their capacity in controlling plant-parasitic nematodes. Christei (1960) speculated on predaceous nematodes for their role in the management of plant-parasitic nematodes. Further studies on them were done by Cohn and Mordechai (1974) and Small (1979).
Predatory nematodes are also important because of their presence as plant, fungal, and bacterial feeders in the soil ecosystem process though they are present in a little amount of the available soil biomass. The four major taxonomic groups of predatory nematodes are Mononchida, Dorylaimida, Diplogasterida, and Aphelenchida. These groups have been dissimilar in their respective feeding apparatus, feeding mechanisms, and food preferences. The majority of studies on predaceous nematodes are based on agar plate experiments on their feeding mechanism and prey catching. To achieve success in biocontrol, prey (nematodes) should be susceptible to predation. Prey specificity in biocontrol is an important factor, and diplogasterids are known to be highly prey specific (Chitambar and Noffsinger 1989). Achieving successful biological control programs are based on prey range, knowledge of the predator-prey relationship, and proper utilization of different species of predators.
The diplogasterid predators are needed to explore by nematologists for their potential as biocontrol agents (Steel et al. 2010). These predators gained prominence after Yeates (1969) assessed the predatory abilities of Mononchus potohikus. Decomposing organic manures are home to a large number of diplogasterids. Prospects of using diplogasterid predatory nematodes seem promising because they are easily cultured in the in vitro conditions, bi-phasic feeding, high rate of predation, high fecundity, short life cycle, ability to perceive and respond to prey secretions (Bilgrami et al. 2005), thereby, act as promising biocontrol agents. Diplogasterid predators have been found effective for controlling root-knot nematode (Fauzia et al. 1998; Bajaj and Kanwar 2015).
Fictor composticola, a diplogasterid predator, is a voracious feeder and found mostly in compost or high organic matter. It predates upon different groups of nematodes (Bajaj and Kanwar 2015; Keshari 2016). It was found commonly in compost used for cultivating button mushroom (Agaricus bisporus (Lange) Singer) in Haryana and Bihar states of India, which has the potential to control the population of mushroom feeding nematodes in vitro (Kanwar et al. 2009). Keshari (2016) studied the predation efficiency of F. composticola with different prey density levels of mushroom nematodes viz. Aphelenchus avenae, Aphelenchoides swarupi, Ditylenchus myceliophagous, and Panagrolaimus sp. Nevertheless, the knowledge of predation efficiency of predacious nematodes in biocontrol study is still limited.
The aim of the present work was to evaluate the predation efficiency of F. composticola, using different levels of J2 of root-knot nematode, M. incognita as prey, under laboratory and greenhouse conditions.
Material and methods
An experiment was carried out in the laboratories and screen house of the Department of Nematology, Chaudhary Charan Singh Haryana Agricultural University (CCS HAU), Hisar, Haryana during 2018-19.
Nematode culture
Culture of M. incognita was maintained on brinjal plants (cv. BR 112) in pots. The roots were washed gently in water to remove the adhering soil. Nematode egg masses collected from roots were hatched by the modified Baermann-funnel technique to get juveniles. Freshly hatched juveniles were used for inoculation. The juveniles were counted under a stereo-zoom binocular microscope by dilution method, and the required number was inoculated.
The culture of F. composticola was maintained on 1 % water agar medium in Petri plates. M. incognita J2 were added in plates as prey from time to time. These predators in agar plates were incubated for multiplication in a BOD incubator at 25±2 °C, and this culture was used for experimentation.
Extraction and inoculation of nematodes
The predatory nematodes were placed in each agar plate from culture plates of F. composticola by picking needle. The inoculated agar plates were maintained at room temperature. For inoculation in pots, the predatory nematodes from culture plates were extracted in sterile water and inoculated. For extracting predatory nematodes from agar plates, they were filled with sterile water and kept for five minutes. After shaking well, the suspension of nematodes was collected in a beaker. This process should be repeated thrice. The predatory nematodes in the beaker were counted and used for the inoculation as per the requirement of the experiments. Freshly hatched M. incognita J2 were inoculated in pots around the roots by the pencil hole method.
In vitro study
Two males and 2 females of F. composticola were released in each plate, with different prey density levels i.e. 250, 500, 1000 or 2000 of M. incognita J2. After 24 and 48 hrs. of release, left over populations of prey were recorded under stereo-microscope. There were 4 replicates for each level. Nematode counting in agar plates was done by directly placing the plate under the microscope. For convenience in counting, Petri-plates were divided into small squares (about 6 -7 mm) by marking with a marker pen on the lower side of the bottom.
Pot study
This experiment was carried out under screen house conditions in pots containing sterilized soil. Seeds of cucumber (cv. CCH-1) were sown in 1 kg earthen pot, and after emergence, one healthy seedling was maintained per pot. Pots were inoculated by 4 inoculum levels (250, 500, 1000, or 2000 J2 per pot) of freshly hatched J2 of root-knot nematode and 400 F. composticola per pot, simultaneously. One un-inoculated control was kept for comparison. Observations were recorded on plant height, fresh shoot, and root weight, number of galls per plant, number of egg masses per plant, and final soil nematode population 45 days post inoculation. The treatments were replicated 4 times in a completely randomized design (CRD).
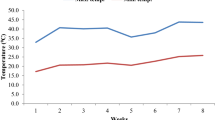
Fertilizers were applied as per recommendations of CCS HAU, Hisar (Anonymous 2018). Hand hoeing was done at weekly intervals for proper aeration. Yellow trap cards were installed to manage the whitefly population, and 3 sprays of Nimbicidin @ 0.2% were applied to protect the crop from insects. Temperature data during the experimental period were collected from the Department of Agricultural Meteorology, CCS HAU, Hisar.
Final nematode population estimation in soil
Soil at each pot was mixed properly, labelled, and brought to the laboratory. 200 cc soil was drawn from these samples, and extraction of nematodes was done by Cobb’s sieving and decanting method (Cobb 1918) followed by modified Bearmann’s funnel technique (Schindler 1961). Nematode population was counted at 40x magnifications, using a stereoscopic binocular microscope.
Statistical analysis
The data were analysed by Completely Randomised Design (CRD), using OPSTAT software available online at CCS HAU, Hisar University website (www.hau.ac.in). Treatment means were compared with the critical difference (CD) at 5 per cent level of significance. Nematode data were transformed using arcsine and square root transformation, where required.
Results and Discussion
In vitro study
After 24 hours, the maximum per cent consumption (64.5 %) was recorded at 500 density level and the minimum (29 %) was at 2000 J2 per plate. Considering the number of J2 of M. incognita consumed by F. composticola, the consumption was maximum at 2000 prey density level. The per cent consumption of prey at different prey density levels after 24 hrs. was non-significantly different from each other. After 48 hours of release, the per cent consumption was maximum (85%) at 500 prey density level and minimum (48.7%) at 2000 prey density level, but the consumption of prey was higher at this level. The per cent consumption of prey at 250 and 500 density levels were similar. M. incognita J2 consumed by F. composticola increased as the prey density increased from 250 to 2000 per plate. The per cent consumption of the prey was maximum (74.8 %) when the prey density was 500 and the minimum (38.9%) at prey density 2000 per plate (Table 1). Thus the result showed that increasing the prey density led to an increased number of prey consumed but not the per cent consumption. These observations are similar to the findings of Keshari (2016), indicating that an increasing population of prey increased predation efficiency of F. composticola, and the predation depended on the prey number. This increased predation may be attributed to increased chances of contact between predator and prey at higher densities. More prey were killed by F. composticola, when the prey population was increased from 100 to 1600 individuals. Maximum predation occurred in the population of 1600 prey individuals and the minimum was in 100 individuals per plate.
The data showed that irrespective of time, the per cent consumption (M. incognita as prey) differed at all 4 prey density levels, and it was the maximum (74.8) at 500 J2 per plate and the minimum (38.9) at 2000 J2 per plate. Considering the number of J2 of M. incognita consumed by F. composticola, it increased with increasing the prey density level. The predators, Enoploides longispiculosus and Adoncholaimus fuscus showed strong prey density-dependent predation rates (Moens et al. 2000). E. longispiculosus had a maximal predation rate on the 4 monhysterid prey nematodes at a prey density of 200 individuals per Petri dish or higher. The higher predation rate may be because at higher densities, there were more predator-prey encounters, resulting in reduced search duration, increased predation, and increased feeding duration. Chance encounters are opportunities over which predation rate depends and thus density-dependent (Yeates 1969; Osman 1988; Bilgrami 1993). Bilgrami et al. (2005) also reported that the predation of Laimydorus baldus and Discolaimus major was density-dependent.
In the present study, irrespective of prey density levels, the mean percentage of prey consumption of M. incognita were significantly higher at 48 hrs than at 24 hrs. Contrary to the present study, Bilgrami et al. (1984) did not observe any effect of prey density on its rate of predation, while working with Mononchus aquaticus, as they used lower prey densities i.e. 25, 50, 100 and 150 specimens of Chiloplacus symmetricus and Cephalobus sp.
Pot study
Cucumber plants inoculated with 250 J2 of root-knot nematode showed better plant height than other treatments. But, as the inoculum level of root-knot nematode increased, there was a decrease in plant height from 250 to 2000 J2/kg soil, although at 250, it was statistically at par with a non-inoculated check. A significant reduction in plant height was observed at the inoculum level of 500 J2 per kg soil and above. Minimum fresh shoot weight was observed in the inoculum level of 2000 J2 and maximum in non-inoculated check, which was observed statistically at par with 250 J2 but significantly different from other inoculum levels. There was a significant decrease in fresh shoot weight from 500 to 2000 J2/kg soil, as the inoculum level increased. The maximum and significantly highest fresh root weight was recorded at 1000 J2. Fresh root weight was at par in non-inoculated check, 250 and 2000 J2/kg soil (Table 2).
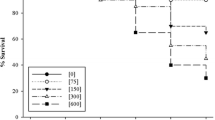
With an increase in the inoculum level from 250 to 2000 J2, there was an increase in the number of galls per plant. Minimum numbers of galls (12.50) were found at 250 J2 per plant. The maximum number of galls (87.25 per plant) were observed at 1000 J2 inoculum level, although it was similar to the galls at the 2000 level. The root galling at the 2000 level was more severe than at 1000 (Fig. 1). As the inoculum level increased from 250 to 2000 J2, the number of egg masses and the final nematode population also increased. The maximum and significantly highest final nematode population was observed at 2000 J2 (Table 3). There was a significant difference in the nematode population at 2000, 1000 and 500 J2 inoculum level. The final nematode population was at par at 250 and 500 levels. The minimum population of M. incognita was observed at 250 J2, which was similar to 500 level but less than at 1000 and 2000 levels. F. composticola might have lowered the root-knot nematode numbers. Fauzia et al. (1998) when studying the biocontrol potential of predatory nematode, Mononchoides longicaudatus on M. incognita on tomato plants, also found that the population of M. incognita was significantly decreased in all inoculum levels (100, 200 and 400 per pot) of predatory nematode after 45 days.
Partial control of M. incognita was achieved as the numbers of galls caused on tomato roots were reduced in the treatments, where the predator, Prionchulus sp., was added (Small 1979). Osman (1988), in a pot experiment, found that by adding predatory nematode, Diplogaster sp., the parasitic population of Tylenchulus semipenetrans or M. javanica in the roots of sour orange and tomato, respectively, were significantly reduced. Diplogasterid predator, Koerneria sudhausi cultured on bacteria, resulted in a reduced tomato root galling index when the predator was introduced into the M. javanica-infested soil in pots (Bar-Eyal et al. 2008).
The population of predator recovered was a maximum at 2000 density level and minimum at 250 level. The population of the predator increased with an increase in prey density level (Fig. 2). The final population of the predator was small; the reason for this may be high temperature (27.8 to 42.6 0C) during the experimental period, which might have affected its multiplication adversely. Another reason for the low population could be the unavailability of prey after the penetration of root-knot larvae, endo-parasitic nematode, in the roots.
Conclusion
In the present study, F. composticola proved to be a very efficient predator under lab conditions, where the prey are easily, abundant and available to the predator, while under natural conditions, several environmental factors, as the temperature is a crucial factor that affects the predation efficiency. Further studies at different temperatures/ crop seasons are needed to exploit the predatory potential of F. composticola for the management of root-knot nematode.
Availability of data and materials
All data generated and/or analyzed during the present study are available in the manuscript, and the corresponding author has no objection to the availability of data and materials.
Abbreviations
- F. composticola :
-
Fictor composticola
- M. javanica :
-
Meloidogyne javanica
- M. incognita :
-
Meloidogyne incognita
- E. longispiculosus :
-
Enoploides longispiculosus
- BOD:
-
Biological Oxygen Demand
References
Anonymous (2018) Package of practices for horticultural crops in Haryana. Directorate of Extension Education, CCS HAU, Hisar.
Bajaj HK, Kanwar RS (2015) Biology and predatory attributes of a diplogasterid nematode, Fictor composticola Khan et al., 2008. Helminthologia 52:50–57
Bar-Eyal M, Sharon E, Spiegel Y, Oka Y (2008) Laboratory studies on the biocontrol potential of the predatory nematode, Koerneria sudhausi (Nematoda : Diplogasteridae). Nematology 10:633–637
Bilgrami AL (1993) Analysis of relationships between predation by Aporcelaimellus nivalis and different trophic categories. Nematologica 39:356–365
Bilgrami AL, Ahmad I, Jairajpuri MS (1984) Observations on the predatory behaviour of Mononchus aquaticus. Nematol Mediterr 12:41–45
Bilgrami AL, Gaugler R, Brey C (2005) Prey preference and feeding behaviour of the diplogasterid predator Mononchoides gaugleri (Nematoda: Diplogasterida). Nematology 7:333–342
Chitambar JJ, Noffsinger EM (1989) Predacious behaviour and life history of Odontopharynx longicaudata (Diplogasterida). J Nematol 21:284–291
Christei JR (1960) Biological control-predaceous nematodes. In: Sasser JM, Jenkins WR (eds) Nematology: Fundamentals and recent advances with emphasis on plant parasitic and soil forms. University of North Carolina Press, Chapel Hill, pp 466–468
Cobb NA (1917) The mononchs (Mononchus Bastian 1865): a genus of free-living predatory nematodes. Soil sci 3:431–486
Cobb NA (1918) Estimating the nema population of the soil. Agricultural Technology Circular-1, Bureau of plant industry, U.S.D.A California, USA, pp 48.
Cohn P, Mordechai E (1974) Experiments in suppressing citrus populations by use of marigold and a predacious nematode. Nematol Mediterr 2:43–53
Fauzia M, Jairajpuri MS, Khan Z (1998) Biocontrol potential of Mononchoides longicaudatus on Meloidogyne incognita on tomato plants. Int J Nematol 8:89–91
Kanwar RS, Bajaj HK, Nandal SN (2009) Potential of a predatory nematode, Fictor composticola as biocontrol agent of nematode pest of mushroom. In: Abstract of the National Symposium on Nematode Problems of India, Y.S. Parmar University of Horticulture and Forestry, Solan. 8-9 July 2009.
Keshari N (2016) Predatory behaviour of Fictor composticola Khan et al. and its potential for the management of nematode pests of button mushroom. Dissertation, CCS Hayana Agricultural University, Hisar, India.
Moens T, Herma P, Verbeeck L, Steyaert M, Vincx M (2000) Predation rates and prey selectivity in two predaceous estuarine nematode species. Marine Ecology-Progress series 205:185–193
Osman GY (1988) Studies on the potential use of the predator, Diplogaster sp. (Nematoda: Diplogasteridae) on certain root parasitic nematodes. Anz Schädlingsk Pflanz Umweltschutz 61:70–73
Schindler AF (1961) A simple substitute for Baermann funnel. Plant Disease Reporter 45:747–748
Sikora RA (1992) Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Ann Rev Phytopathol 30:245–270
Small RW (1979) The effect of predatory nematodes on population of plant parasitic nematodes in pots. Nematologica 25:94–103
Steel H, de la Pena E, Fonderie P, Willekens K, Borgonie G, Bert W (2010) Nematode succession during composting and the potential of the nematode community as an indicator of the composting maturity. Pedobiologia 53:181–190
Yeates GW (1969) Predation by Mononchoides potohikus (Nematoda: Diplogasteridae) in laboratory culture. Nematologica 15:1–9 https://doi.org/10.1163/187529269X00029
Acknowledgement
Temperature data for the experimental period provided by the Department of Agricultural Meteorology, CCS HAU, Hisar, India, is gratefully acknowledged.
Funding
This study is a part of a PhD Thesis of the senior author. No funding was obtained from any source.
Author information
Authors and Affiliations
Contributions
Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted on predatory and plant-parasitic nematode species that are abundant in the environment and does not require ethical approval.
Consent for publication
The authors agree to publish this paper. The data has not been published completely or in part in any other journal.
Competing interests
Harjot Singh Sidhu declares that he has no conflict of interest. Rambir Singh Kanwar declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sidhu, H.S., Kanwar, R.S. Effect of prey density of meloidogyne incognita on the predation efficiency of the predator, fictor composticola (nematoda: diplogasteridae). Egypt J Biol Pest Control 30, 72 (2020). https://doi.org/10.1186/s41938-020-00274-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00274-5