Abstract
Background
The size-specific dose estimate (SSDE) is a dose-related metrics that incorporates patient size into its calculation. It is usually derived from the volume computed tomography dose index (CTDIvol) by applying a conversion factor determined from manually measured anteroposterior and lateral skin-to-skin patient diameters at the midslice level on computed tomography (CT) localiser images, an awkward, time-consuming, and not highly reproducible technique. The objective of this study was to evaluate the potential for the use of body mass index (BMI) as a size-related metrics alternative to the midslice effective diameter (DE) to obtain a size-specific dose (SSDE) in abdominal CT.
Methods
In this retrospective study of patients who underwent abdominal CT for the investigation of inflammatory bowel disease, the DE was measured on the midslice level on CT-localiser images of each patient. This was correlated with patient BMI and the linear regression equation relating the quantities was calculated. The ratio between the internal and the external abdominal diameters (DRATIO) was also measured to assess correlation with radiation dose. Pearson correlation analysis and linear regression models were used.
Results
There was good correlation between DE and patient BMI (r = 0.88). An equation allowing calculation of DE from BMI was calculated by linear regression analysis as follows: DE = 0.76 (BMI) + 9.4. A weak correlation between radiation dose and DRATIO was demonstrated (r = 0.45).
Conclusions
Patient BMI can be used to accurately estimate DE, obviating the need to measure anteroposterior and lateral diameters in order to calculate a SSDE for abdominal CT.
Similar content being viewed by others
Key points
-
A strong correlation between BMI and DE was found
-
BMI can be used to estimate patient DE
-
Calculating patient BMI can facilitate individualised patient radiation dose estimation in abdominal CT
Background
The individual patient radiation dose from computed tomography (CT) is notoriously difficult to estimate. There is a growing interest in this topic, however, due to the ever-increasing CT use and general concerns about the risks associated with radiation exposure from medical imaging. This has precipitated the increased use of dose monitoring systems in clinical practice.
Current CT scanner radiation dose output following patient imaging is displayed in the CT dose report in terms of volume CT dose index (CTDIvol) and dose length product (DLP), standardised measurements deduced from homogenous phantoms under normalised conditions [1, 2]. These parameters do not provide a direct measure of the individualised patient radiation dose, a variable that is dependent on patient size.
The size-specific dose estimate (SSDE) is a dose-related metrics that incorporates patient size into its calculation. This metric has been advocated for the reporting of patient radiation dose in CT by the American Association of Physicists in Medicine (AAPM) task group 204 and has increasingly been applied and accepted [3, 4]. The SSDE is derived from the CTDIvol by applying a conversion factor determined from manually measured anteroposterior and lateral skin-to-skin patient diameters at the midslice level on CT-localiser images [3]. On a practical level this technique can be awkward and time-consuming, and open to interobserver measurement variability.
The objective of this study was to evaluate the potential for using body mass index (BMI) as an alternative size metrics, in lieu of measured body diameters, to estimate patient effective diameter (DE). Thus, estimation of SSDE at the time of CT scanning would be more user-friendly, contributing positively to patient radiation dose optimisation.
Methods
Subjects
This study was performed retrospectively on CT data acquired as part of a clinical trial protocol investigating the use of CT in inflammatory bowel disease (ClinicalTrials.gov Identifier: NCT 01244386) [5] with approval from the institution Clinical Research Ethics Committee. Fifty adult patients were included in this study and, as part of this trial protocol, all patients signed informed consent.
CT scan protocol
All patients underwent a CT scan of the abdomen and pelvis with a standardised protocol using the following parameters: scan range encompassing the lung bases to the pubic symphysis; 0.625-mm slice acquisition thickness; intravenously administered contrast (Iohexol, Omnipaque 300, General Electric Healthcare, Waukesha, WI, USA) delivered at 2.5 mL/s and imaged in the portal venous phase; 1.5 L of positive oral contrast (2% Gastrografin, Bracco Diagnostics Inc., Princeton, NJ, USA); tube voltage of 120 kVp; automated tube current modulation resulting in a variable current with a minimum of 50 mA and a maximum of 350 mA; gantry rotation time of 0.8 s; noise index 38. All CT images were acquired using a single 64-slice multi-detector row CT scanner (Lightspeed VCT-XTe, GE Healthcare, General Electric Medical Systems, Waukesha, WI, USA). The DLP and CTDIvol values, as well as the corresponding phantom size, were recorded from each CT dose report. CTDIvol and DLP tolerances were verified using a standard 32-cm Perspex phantom, a 10-cm ionisation chamber with a Victoreen NERO mAx unit (Fluke Biomedical, Solon, OH, USA).
The SSDEs were calculated by multiplying the CTDIvol of each patient by conversion factors corresponding to the effective patient diameters in the AAPM reference tables [3]. The imaging performance and assessment from CT patient dosimetry calculator (ImPACT version 0.99x, London, UK) was used to calculate the effective dose.
BMI measurement
Each patient had weight and height measurements performed and their BMI calculated immediately prior to CT scan using a dedicated calibrated measuring device (electronic measuring station Model 763, Seca Medical, Hamburg, Germany). BMI data were used to subdivide patient groups, where underweight referred to BMI < 18.5 kg/m2, normal weight referred to 18.5 ≤ BMI < 25 kg/m2, overweight referred to 25 ≤ BMI < 30 kg/m2 and obese referred to BMI ≥ 30 kg/m2.
Body diameter measurements
Images were reviewed on a picture-archiving and communication system (PACS) workstation (Impax 6.3.1, AGFA Healthcare, Morstel, Belgium) in a DICOM format. As per AAPM Report 204 guidelines, body diameters were measured at the midslice level (median image of the craniocaudal scanning length) on the CT-localiser images because, for larger patients, maximum skin-to-skin distance is often not included on transverse CT images [3, 6]. Diameter measurements were performed manually with the electronic callipers available on the PACS using a window width of 350 Hounsfield Units (HU) and window level of 50 HU. From personal experience at our institution, analysis of interoperator variability for PACS-based anthropometric measurements shows no statistically significant differences. Therefore, a single investigator carried out all measurements. A fixed window level and setting was used for each individual study.
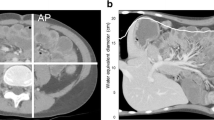
Maximum skin-to-skin anteroposterior diameter (DAP) and lateral diameter (DLAT) were measured in centimetres on lateral and anteroposterior localiser images, respectively. DAP is defined as the anteroposterior skin-to-skin diameter on the lateral localiser at the midslice level (Fig. 1a) while DLAT is defined as the lateral skin-to-skin diameter on the anteroposterior localiser image at the midslice level (Fig. 1b). The DE is defined as the diameter of the circle with area equivalent to the cross-sectional area of the patient at the particular z-axis level (i.e. the midslice level) and is calculated as the geometric mean of DAP and DLAT, as follows:
Measurement of lateral (DLAT) (a) anteroposterior (DAP) (b) skin-to-skin patient diameters at the midslice level on CT-localiser images. Measurement of the inner lateral and anteroposterior diameters on the axial midslice CT image excluding the subcutaneous adipose tissue (c) to allow calculation of the effect inner diameter (DIN) and the effective diameter ratio (DRATIO)
The outer DE (DOUT) equates to the conventional DE calculated using the AAPM method described above. The inner DE (DIN) is derived using the anteroposterior (DAP(IN)) and lateral diameters (DLAT(IN)) measured on an axial CT image at the midslice level, excluding the subcutaneous adipose tissue (Fig. 1c). The DIN is then calculated as the geometric mean of DAP(IN) and DLAT(IN), as follows:
The DE ratio (DRATIO), another patient size-related metrics proposed by Lamoureux et al. [7], was also calculated, as follows:
Statistical analysis
Data were collated using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) and statistical analyses were conducted by using Microsoft Excel 2010 and GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA). Descriptive statistics including means, standard deviations and ranges were calculated. BMI, dose indices (CTDIvol, DLP, SSDE, effective dose) and body diameters (DAP, DLAT, DAP+DLAT, DE, DRATIO) were recorded for each patient CT examination. The correlations between BMI, dose indices, and body diameter measurements were examined with Pearson correlation analysis (r). Linear regression models were used to assess the dependence of CTDI, DLP, SSDE, and effective dose on BMI. Linear regression models were also used to estimate the relationship of effective diameter (independent variable) with BMI (dependent variable). A p-value lower than 0.05 was taken to indicate statistical significance.
Results
Patient demographics
The study population (n = 50) comprised 19 men and 31 women with an age of 37.9 ± 14.4 years (mean ± standard deviation [SD], ranging from 17 to 73 years.
BMI and dose metrics
The overall BMI was 24.6 ± 4.8 kg/m2 (mean ± SD), ranging from 17.4 to 38.8 kg/m2. The mean CTDIvol, DLP, SSDE and effective dose measurements overall, and when stratified for BMI, are listed in Table 1.
Body diameter measurements
The overall DAP, DLAT, DAP + LAT and DE were 24.8 ± 4.5 cm, 31.5 ± 3.9 cm, 56.3 ± 7.9 cm, and 27.8 ± 4.1 cm (mean ± SD), respectively. The effective diameter ratio (DRATIO) was 1.23 ± 0.13 (mean ± SD). The correlations between BMI and diameter are shown in Fig. 2. Overall, the best correlation was found with DE (0.88), where correlations between mean BMI and mean body diameters were highly significant (p < 0.001).
Mean BMI and body diameters across BMI subgroups are shown in Table 2. Excluding DRATIO, the other body diameters strongly correlated with each other. The correlation coefficients (r) were 0.78 for DAP − DLAT, 0.96 for DAP − DE, and 0.92 for DLAT − DE, (p < 0.001 for all). There was a moderate correlation between DLAT and DRATIO with a correlation coefficient of 0.62 (p < 0.001) and weak but statistically significant correlations between DAP and DRATIO (r = 0.34, p = 0.016) and DE and DRATIO (r = 0.49, p < 0.001). A stronger correlation was found between DAP + LAT and DE (r = 0.99, p < 0.001) than with either DAP or DLAT alone and DE [5].
Both BMI and effective body diameter correlated strongly with all the dose metrics analysed (p < 0.001), with r values ranging from 0.84 to 0.90. A weaker but significant correlation of DRATIO with each dose metric was found: r values ranged from 0.45 to 0.48 (p ≤ 0.05). These data are summarised in Fig. 3.
Scatterplots show: volume-computed tomography dose index (CTDIvol) for body mass index (BMI) (a) and effective diameter (b) with correlation r values of 0.85 and 0.9, respectively; dose length product (DLP) for BMI (c) and effective diameter (d) with r correlation values of 0.84 and 0.89, respectively; size-specific dose estimate (SSDE) for BMI (e) and effective diameter (f) with r correlation values of 0.87 and 0.88, respectively; effective dose for BMI (g) and effective diameter (h) with r correlation values of 0.84 and 0.87, respectively
The equation of the linear regression trend-line plotting effective diameter as a function of BMI is as follows: y = 0.76(x) + 9.4 (Fig. 4). Utilising this equation, the patient’s BMI (x) can be used to calculate an estimate for the effective diameter (y), i.e. DE = 0.76(BMI) + 9.4. Table 3 demonstrates a list of conversion factors to calculate SSDE from CTDIvol based on the BMI of the patient.
Discussion
CTDIvol indicates the amount of radiation delivered by the scanner for a specific CT examination, calculated on the basis of a standardised and homogenous phantom study. It is a precisely defined metrics that is displayed on the dose protocol of every CT scanner and is a measure of scanner radiation output, indicating how much radiation is directed toward the patient rather than quantifying how much radiation a patient receives [2]. Increasing use of CT and concerns regarding radiation dose from medical imaging, however, increase the need for imaging providers to facilitate accurate estimation of radiation dose to patients.
SSDE is a dose parameter that takes into consideration corrections based on the size of the patient from linear dimensions measured on the patient images. SSDE is an estimate of the mean dose to the centre of the scan volume for an object having similar attenuation characteristics as a given patient; it is not a direct measurement of dose to a specific patient [8]. This metrics provides the ability to estimate the average radiation dose to a patient in a clinical setting, albeit with 10–20% variability of the dose estimate from the actual received dose, even when patient size is taken into account [3]. The AAPM report [3] recommends that the SSDE for each patient be estimated prior to CT scan using patient size parameters to best optimise the scanning parameters to achieve the diagnostic quality CT images with the lowest necessary radiation dose.
Khawaja et al. [6] demonstrated body weight to be a more simple and convenient measure than effective diameter to estimate SSDE in paediatric patients at the time of CT scanning. They argued that measurement of body diameters in clinical practice is awkward, inconvenient, time-consuming and open to interobserver variability, particularly in the absence of a automated methods of measurement. An overview of other attempts to estimate the radiation dose in CT is presented in Table 4 [9,10,11,12,13]. We hypothesised that BMI, being a composite measurement derived from both weight and height, may also represent an appropriate indicator of patient size to use in lieu of effective diameter in predicting SSDE. BMI is an easily, and often routinely, obtained measurement in clinical practice. It is an objective measure with limited bias or interobserver variability during calculation [14].
The present study assessed relevant size metrics and demonstrated a very strong correlation between effective diameter and SSDE (r = 0.88 or 0.87; p < 0.001) with BMI, indicating that BMI is an accurate alternative to effective diameter for SSDE estimation in abdominal CT.
Bias from other sources was minimised as all patients were scanned using a predefined and standardised CT abdomen and pelvis protocol on a single CT scanner by one of two radiographers. This paper demonstrates that effective diameter can be accurately estimated using an equation and patient BMI. SSDE can then be computed in a standard manner from CTDIvol by using AAPM look-up tables to derive conversion factors.
We found that in patients with a wide range of body habitus measurements (BMI range, 17.4–38.8; effective diameter range, 19–39.4), BMI measurements correlated strongly with diameters. The use of BMI to calculate SSDE has been shown to be a valid alternative to the traditional methods for manual measurement of anterior-posterior and lateral-lateral dimensions using the electronic callipers available on the PACS. A recent study by Babak Alikhani et al. [15] showed that in abdominal CT, the size-dependent conversion factor (f size) closely correlated with patient BMI, indicated by the exponentially decreasing f size values with increasing BMI. The current study echoes these results with the proposition that BMI can act as a surrogate for determining effective body diameter.
The effective diameter ratio (DRATIO) is a new metrics proposed by Lamoureux et al. [7] as a supplement to patient-specific size parameter data and is, as yet, not validated. DRATIO provides information about anatomical composition, particularly the volume of extra-abdominal adipose tissue but underestimates intra-abdominal adiposity. It proved to be a much weaker predictor of radiation dose to the patient in terms of CTDIvol, DLP and effective dose than either effective diameter or BMI (p < 0.001) in the present paper. Findings suggest that while this metrics is a good indicator of body fat distribution, it is suboptimal as a predictor of patient SSDE.
Our study has some limitations. Our sample size is relatively low (n = 50), with patients chosen because standardised CT imaging on this well-characterised cohort had already been performed and BMI measurements were available for all of them; CT images and data were readily available to test this hypothesis without the need to image further patients. A larger sample size may strengthen the assessment of the relationship between effective diameter and BMI. In addition, with a larger sample size, it could be possible to estimate trend lines stratified for BMI, which may lead to better estimates of diameter and hence of SSDE.
Due to the retrospective design of this study, BMI measurements only were available rather than the constituent height and weights. Statistical evaluation of the interplay of these parameters with BMI, body diameters and radiation dose may have yielded further supportive information. With the advent of automated body diameter and SSDE measurement technology, the applicability of our findings may not be relevant to the small proportion of centres that possess these technologies.
Dose optimisation is a key factor to current radiology practice, particularly for CT when the correct balance between radiation dose and image quality needs to be struck. To be useful and effective, any applied method of estimating patient radiation dose needs to be user-friendly and reproducible. The present paper indicates that patient BMI can be used to accurately estimate effective diameter, obviating the need to measure anteroposterior and lateral diameters in order to calculate SSDE at the time of CT.
Abbreviations
- AAPM:
-
American Association of Physicists in Medicine
- BMI:
-
Body mass index
- CT:
-
Computed tomography
- CTDIvol :
-
Volume CT dose index
- D AP :
-
Maximum skin-to-skin anteroposterior diameter
- D E :
-
Effective diameter
- D IN :
-
Inner DE
- D LAT :
-
Maximum skin-to-skin lateral diameter
- DLP:
-
Dose length product
- D OUT :
-
Outer DE
- D RATIO :
-
Ratio between the internal and the external abdominal diameters
- PACS:
-
Picture-archiving and communication system
- SD:
-
Standard deviation
- SSDE:
-
Size-specific dose estimate
References
Brenner DJ, McCollough C, Orton CG (2006) Is it time to retire the computed tomography dose index (CTDI) for CT quality assurance and dose optimisation? Med Phys 33:1189–1190
McCollough CH, Leng S, Yu L, Cody DD, Boone JM, McNitt-Gray MF (2011) CT dose index and patient dose: they are not the same thing. Radiology 259:311–316
American Association of Physicists in Medicine (2011) Size-specific dose estimates (SSDE) in pediatric and adult body CT examinations (Report 204). https://www.aapm.org/pubs/reports/RPT_204.pdf. Accessed Sept 22 2018
American Association of Physicists in Medicine (2014) Use of water equivalent diameter for calculating patient size and size-specific dose estimates (SSDE) in CT (Report 220). https://www.aapm.org/pubs/reports/RPT_220.pdf. Accessed Sept 22 2018
Craig O, O’ Neill S, O’Neill F et al (2012) Diagnostic accuracy of computed tomography using lower doses of radiation for patients with Crohn’s disease. Clin Gastroenterol Hepatol 10:886–892
Khawaja RD, Singh S, Vettiyil B et al (2015) Simplifying size-specific radiation dose estimates in pediatric CT. AJR Am J Roentgenol 204:167–176
Lamoureux R, Sinclair L, Mench A et al (2015) SU-E-I-28: introduction and investigation of effective diameter ratios as a new patient size metric for use in CT. Med Phys 42:3247–3248
Christner JA, Braun NN, Jacobsen MC, Carter RE, Kofler JM, McCollough CH (2012) Size-specific dose estimates for adult patients at CT of the torso. Radiology 265:841–847
Li X, Samei E, Segars WP et al (2011) Patient-specific radiation dose and cancer risk estimation in CT: part II. Application to patients. Med Phys 38:408–419
Cheng PM (2013) Automated estimation of abdominal effective diameter for body size normalization of CT dose. J Digit Imaging 26:406–411
Ikuta I, Warden GI, Andriole KP, Khorasani R, Sodickson A (2014) Estimating patient dose from x-ray tube output metrics: automated measurement of patient size from CT images enables large-scale size-specific dose estimates. Radiology 270:472–480
Groves AM, Owen KE, Courtney HM et al (2004) 16-detector multislice CT: dosimetry estimation by TLD measurement compared with Monte Carlo simulation. Br J Radiol 77:662–665
Li B, Behrman RH, Norbash AM (2012) Comparison of topogram-based body size indices for CT dose consideration and scan protocol optimization. Med Phys 39:3456–3465
Sebo P, Beer-Borst S, Haller DM, Bovier PA (2008) Reliability of doctors’ anthropometric measurements to detect obesity. Prev Med 47:389–393
Alikhani B, Getzin T, Kaireit TF et al (2018) Correlation of size-dependent conversion factor and body-mass-index using size-specific dose estimates formalism in CT examinations. Eur J Radiol 100:130–134
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
Professor Denis O’Sullivan Research Fellowship, University College Cork, Cork, Ireland.
Author information
Authors and Affiliations
Contributions
SON is involved with study design, data acquisition and analysis, manuscript drafting and revising. RGK is involved with data analysis, manuscript drafting and revising. BWC is involved with data analysis, manuscript drafting and revising. NM is involved with study design, data acquisition, manuscript drafting and revising. MM is involved with study design, manuscript drafting and revising. OJOC is involved with study design, data analysis, manuscript drafting and revising. All authors have given final approval to manuscript publication and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval for this study was granted by the Clinical Research Ethics Committee of the Cork Teaching Hospitals, Lancaster Hall, 6 Little Hanover Street, Cork, Ireland. Informed consent was obtained from each patient.
Consent for publication
The consent for publication is included in the informed consent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
O’Neill, S., Kavanagh, R.G., Carey, B.W. et al. Using body mass index to estimate individualised patient radiation dose in abdominal computed tomography. Eur Radiol Exp 2, 38 (2018). https://doi.org/10.1186/s41747-018-0070-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41747-018-0070-5