Abstract
Background
Geniposidic acid is a natural compound affiliated with iridoid glycoside which is contained in various plants such as Gardenia seeds, Eucommia bark, and Psyllium, and it is known that it has such effects as anti-inflammation, anti-virus, anti-cancer, anti-oxidation, suppression of stress, improvement of immune system, and relief of liver injury. However, there is a lack of research on action of geniposidic acid in dermal cells, so this research verifies effects of geniposidic acid on preservation of human keratinocytes, anti-oxidation, and DNA repair and finds out its mechanism.
Methods
This study demonstrated feasibility of geniposidic acid on cosmeceutical application via ultraviolet (UV) B-induced damage protective effects such as oxidative stress reduction, DNA repair, and decrease of apoptotic cell death. To investigate cytotoxicity of angelic acid and proliferation influence, researchers used formazan detection by water-soluble tetrazolium salts (WST). To examine cell cycle distribution and sub-G1 portion, fluorescence-activated cell sorting (FACS) analysis was used to the experiment. For further investigation, cleaved caspase-3 levels were determined in apoptotic cell death analysis. And we conducted comet assay, in vitro anti-oxidant effects using radical scavenging assay, lipid peroxidation analysis, and mRNA expression analysis.
Results
Geniposidic acid protects UVB-induced cytotoxic damage and reduces apoptotic cell death caused by UVB in human keratinocytes (HaCaT). Also, this research finds out that geniposidic acid decreases formation of cleaved caspase-3 increase and reduces both tailed DNA and cyclobutane pyrimidine dimer (CPD) which is increased by UVB. Through radical scavenging assay, we demonstrate in vitro anti-oxidant effect of geniposidic acid and, via quantitative real-time polymerase chain reaction (qRT-PCR), find out that gene expression of superoxide dismutase 1 and 2 (SOD1 and SOD2) which are known as anti-oxidant gene increases dependently on concentration of geniposidic acid. Furthermore, geniposidic acid reduces lipid peroxidation to lead anti-oxidation effect. This research finds out that gene expression of XPC (XPC complex subunit, DNA damage recognition, and repair factor) and PCNA (proliferating cell nuclear antigen) which are DNA repair gene increases dependently on concentration of geniposidic acid.
Conclusions
Through this research, we verify that geniposidic acid has effects on anti-oxidation and DNA repair in human HaCaT damaged by UVB and suggest that geniposidic acid as a cosmetic material is fully worthy to use to delay dermal cellular senescence by UVB effectively.
Similar content being viewed by others
Background
Dermal senescence is a complex phenomenon which is classified into intrinsic aging caused by the heredity and extrinsic aging through environmental exposure by UV (Jenkins 2002), and intrinsic aging is occurred by reactive oxygen species (ROS) which is formed in the course of cellular metabolism (Lee et al. 2012), and extrinsic aging occurs due to reactive oxygen which is formed by dermal permeation of UVB although there are several causes for extrinsic senescence (Kulms et al. 2002). Intracellular ROS causes damages on DNA and mitochondria and results in such intracellular damages as heterology in the course of energy metabolism as well as protein oxidation. The intracellular damages caused by oxidative stress occur cell cycle arrest, cellular senescence, and apoptosis which are shown on dermal keratinocyte to lead to dermal senescence (Cerella et al. 2009). DNA damage caused by UV activates ATM (ataxia-telangiectasia mutated) and ATR (ATM- and Rad3-related) proteins, which activate p53. When DNA gets damaged, activation of p53 which is a transcription factor induces expression of p21 to stop cell cycle and hinder cell growth (Smits and Medema 2001). So in this experiment, to examine preservation effect on HaCaT against UVB, as object, I selected geniposidic acid which is anti-cancer, anti-oxidation, and anti-inflammatory properties.
Geniposidic acid as corresponding to (1S,4aS,7aS)-1-(β-D-glucopyranosyloxy)-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylic acid is a natural compound affiliated with iridoid glycoside which is contained in various plants such as Gardenia seeds, Eucommia bark, and Psyllium, and research on senescence-related insulin resistance improvement of genipin (Guan et al. 2013) and also research on airway inflammation and induction suppression of geniposide have been reported (Deng et al. 2013) with regard to biological action of Gardenia seeds. Also, the research on anti-oxidation and anti-inflammation action of manufactured Gardenia seeds and crocetin is known (Hong and Yang 2013). In the research on cancer cell proliferation suppression effect of Eucommia extract (Choi et al. 2003), geniposide and geniposidic acid were separated and refined respectively, and at the case of oral administration of these compounds, it significantly reduced tumor size of mouse in which cancer cell was transplanted (Hsu et al. 1997), and at in vitro experiment targeting C6 glioma cell, it induced apoptosis effectively (Chang et al. 2002). Also, in the experimental study depending on manufacturing of eucommia, as indicator substances, I selected geniposidic acid and geniposide which are mental stability factors in iridoid glycoside and examined its content depending on manufacturing, and through pharmacological experiment on catecholamine content within brain and plasma under the condition of pain, lipid metabolism, and restraint stress, it found out that it is significantly effective (Park and Kim 1992). Since the recent research trends on geniposidic acid, there has been a process of research on anti-cancer, anti-inflammation, and anti-oxidation, but nothing about research on application of geniposidic acid as cosmetic materials, and also, there have been no researches on mechanism in dermal cells. Also, researches on skin preservation against UV and about natural substances which delay cellular senescence have been increasing continuously in these days. So it is necessary to investigate various effects of geniposidic acid on usual action of keratinocyte in the course of dermal senescence. Therefore, this research intends to study effects of geniposidic acid such as cell preservation, anti-oxidation, and DNA repair and examine its effects to find out possibility for application of geniposidic acid as natural cosmetic materials which can help delaying dermal cellular senescence.
Methods
Cell culture
HaCaT cell line and HaCaT keratinocytes (ATCC, Manassas, VA, USA) were cultured using Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) which contains 10% of fetal bovine serum (FBS; Hyclone) and 1% of penicillin/streptomycin (penicillin 100 IU/mL, streptomycin 100 μg/mL; Invitrogen/Life Techmologies, Carlsbad, CA, USA) for HaCaT culture and cultured it within cell incubator kept under the condition of 5% of CO2 with the temperature at 37 °C.
Sample treatment
I purchased geniposidic acid which is refined in the form of powder from Santa Cruz Biotech (Santa Cruz, CA, USA) and dissolved it in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) in a proper density to use it in the experiment. After I cultured HaCaT cell (1 × 106 cells/well) in the cell medium for 24 h, I added geniposidic acid in a proper density to culture medium and pretreated for 3 h, and for ultraviolet B test on cells, I used UV-B lamp (UVP, Upland, CA, USA) to examine. I used fiber optic Spectrometer System USB2000 (Ocean Optics, Dunedin, FL, USA) to estimate UVB wavelength, and at the UVB test on HaCaT, I washed it twice with phosphate-buffered saline (PBS; (Thermo Fisher Scientific, USA) in a pH of 7.4 to remove culture medium of cell culture dish. To prevent drying of cell, I opened cell culture dish lid for UVB test after adding 1 mL of PBS to cell culture dish, removed PBS after UVB testing, added culture medium again to culture in the culture incubator for 24 h, and then used in the experiment.
Cell viability estimation
I used the principle of WST assay to estimate cell viability. I used EZ-Cytox cell viability assay kit (Itsbio, Seoul, Korea), and after inoculating HaCaT (3 × 103 cells/well) in 96-well plate and cultured for 24 h, I treated HaCaT with geniposidic acid of each density of 5, 10, 20, 30, and 40 μM respectively, tested 40 mJ/cm2 of UVB, and then cultured for 24 h. After I added 10 μL of EZ-Cytox cell viability assay kit reagent (ItsBio) into cultured well and cultured for 1 h, I used microplate reader (Bio-Rad, Hercules, CA, USA). I estimated the absorbance in the scale of 490 nm, repeated each experiments three times separately, and derived average value and standard deviation of cell viability.
Cell cycle analysis
I used an equipment of BD FACS Calibur Flow Cytometer (BD Biosciences, San Jose, CA, USA). I estimated the number of cells in sub-G1, G1, S, and G2/M cell cycle to analyze cell cycle. After I inoculated 2 × 105 cells/well of HaCaT in 60 mm of culture dish, I cultured it for 24 h, and then, I pretreated it with geniposidic acid for 3 h. After testing UVB, I cultured it for 24 h more and obtained cultured cells, and then, I centrifuged it at 5000 rpm with temperature at 4 °C for 5 min and precipitated cells. After I removed the supernatant and dissolved the cell pellet with 300 μL of PBS, I slowly added 700 μL of absolute ethanol (Biopure, Canada) while vortexing, and I refrigerated it with temperature at 4 °C for 3 h to fix cells. I added 1 mL of PBS, centrifuged at 5000 rpm with temperature at 4 °C for 5 min to remove supernatant, dissolved the pellet with 200 μL of propidium iodide (PI) staining buffer (PI 50 μg/mL, RNase 0.1 μg/mL, 0.05% Triton X-100; Sigma Aldrich), and then settled with temperature at 37 °C for 1 h. Afterward, I centrifuged HaCaT cells at 5000 rpm with temperature at 4 °C for 5 min to remove supernatant and washed with PBS, and then, I dissolved the pellet with 1 mL of PBS and through Flow Cytometer estimated the number of cells in each cell cycle.
qRT-PCR analysis
To analyze and find out gene expression occurred within HaCaT by geniposidic acid quantitatively, I used qRT-PCR method. I mixed 0.2 μM of primers, 20 mM of Tris/HCl in a pH of 8.4, 50 mM KCl, 0.8 mM of dNTP, 3 mM of MgCl2, 0.5 U Extaq DNA polymerase, and 1× SYBR green (Invitrogen) in PCR tube to manufacture reaction solution and used Linegene K (BioER, Zhejiang, China). After denaturation with temperature at 94 °C for 3 min, I performed denaturation (94 °C, 30 s), annealing (58 °C, 30 s), and polymerization (72 °C, 30 s) for 40 cycles and proceeded PCR. With melting curve, I verified the effectiveness of PCR, and by standardizing expression of β-actin, I performed the comparative analysis for each gene expression, and the primer used in the experiment is shown as Table 1.
DPPH radical scavenging activity assay
I injected the geniposidic acid diluent of each density into 96-well plate, added 50 μL of 0.2 mM of 1,1-diphenyl-2-picrylhydrazyl (DPPH), and settled it for 30 min. Using a microplate reader (Bio-Rad), I estimated the absorbance in the scale of 514 nm and repeated this estimation three times. I derived the average value and standard deviation of absorbance.
ABTS+ radical scavenging assay
I mixed 7.4 nm of 2,2-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) and 2.6 mM of potassium persulfate in the final density, reacted in dark room at room temperature for 12 h, and formed ABTS+, and then, I made the value of the absorbance to be 0.706 (± 0.01) in the scale of 732 nm. After adding 100 μL of ABTS+ into 100 μL geniposidic acid in 96-well plate and neglecting for 7 min, I estimated the absorbance in the scale of 732 nm. I repeated this estimation three times and derived the average value and standard deviation of the absorbance.
Caspase-3 activity
Caspase-3 which induces apoptosis is affiliated with cysteine proteinase family, and I used Apoalert caspase-3 colorimetric assay kit (Clontech, Mountain View, CA, USA) as method to estimate pNA (p-nitroanilide) action using its character to decompose Ac-DEVD-pNA (N-acetyl-Asp-Glu-Val-Asp p-nitroanilide) which is a substrate complex. I separated the cells treated by sample from culture dish, centrifuged it at 1500 rpm for 5 min, and added cold cell lysis buffer into cell pellet whose culture medium was removed, and then, I settled it under the condition of the ice for 10 min and centrifuged it at 15000 rpm with temperature at 4 °C for 3 min to separate supernatant only. With regard to the separated supernatant, I used bradford assay to quantify only total 50 μg of protein and apply to caspase-3 activity reaction. I put suspension which I finished protein quantification into 96-well plate, added reaction buffer, and cultured it with temperature at 37 °C for 30 min. Then, I added caspase-3 substrate, cultured with temperature at 37 °C for 1 h, and used microplate reader (Bio-Rad) to estimate the absorbance in the scale of 405 nm.
Comet assay (single-cell gel electrophoresis)
I used CometAssay® Reagent Kits (Trevigen, Gaithesburg, MD, USA) and melted LMAgarose (low melting agarose) completely for 5 min, and then, I covered it with cab, cooled in water bath whose temperature was at 37 °C for 20 min, and mixed 1 × 105 cells/mL of cell with melted LMAgarose (at 37 °C) at the ratio of 1 to 10 (v/v). Afterward, I poured 50 μL of mixed liquor onto Comet slide and kept in slide in the refrigerator with temperature at 4 °C for 10 min, and then, I immersed the slide in alkaline solution at room temperature for 20 min. After I immersed the slide in alkaline electrophoresis solution and covered, I electrophoresed it with voltages at 21 V for 30 min, and after finishing electrophoresis, I immersed it twice in distilled water for 4 min to remove electrophoresis solution and immersed in 70% ethanol for 5 min. I dried it with temperature at 37 °C, put dried agarose gel into 100 μL of SYBR® gold nuclei acid gel stain (Invitrogen), and dyed it in the dark room for 30 min. I rinsed it with water simply to remove dyeing reagent and observed agarose gel which I dried it completely with temperature at 37 °C through fluorescence microscope.
MDA assay
I used colorimetric method to estimate malondialdehyde (MDA) level and used Lipid Peroxidation (MDA) Assay Kit (Abcam, Cambridge, UK). First, I inoculated 1 × 107 cells/well of HaCaT in 6-well plate and pretreated it with reagent, and then, I tested the UV and cultured it for 48 h. I used a microplate reader (Bio-Rad) to estimate in the scale of 586 nm and used the principle that oxidated lipid (MDA) reacts with TBA (thiobarbituric acid) within kit and detects through colorimetric method.
Statistical process
In this research, I performed every experiment more than three times separately under the same condition and obtained experimental result, and used Student’s t test for each experiment and found p value. When p value in every experimental result is less than 0.05, I analyzed that it is statistically significant.
Results
Cell viability analysis
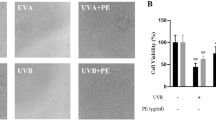
To find out cytotoxicity which geniposidic acid influences on HaCaT, I performed the cell viability assay. I treated the HaCaT keratinocytes with geniposidic acid of each density of 5, 10, 20, 30, and 40 μM respectively. As the result of testing cytotoxicity, it did not influence on viability till 20 μM. When geniposidic acid was 20 μM, the viability was 87%, and when geniposidic acid was 30 μM, it was 73%, and when geniposidic acid was 40 μM, it was 66%, so the cell viability appeared as decreasing (Fig. 1a). To find out whether geniposidic acid has an effect to preserve HaCaT keratinocytes against UVB irradiation, I pretreated the HaCaT keratinocytes with geniposidic acid of each density of 10, 20, and 30 μM respectively and tested UVB of 40 mJ/cm2 to find out changing of cell viability. As the result, when I did not treat HaCaT keratinocytes with geniposidic acid but tested UVB only, cell viability decreased to 72%, but at the case of pretreatment with geniposidic acid of 10 μM, it was 75%; at the case of pretreatment with 20 μM, it was 81%; at the case of pretreatment with 30 μM, it was 87%, and 88% at 40 μM pretreatment. Therefore, I found out that cell viability of HaCaT keratinocytes restores dependently on density of geniposidic acid (Fig. 1b).
Analysis of cell cycle distribution and apoptotic cell death
To find out through what mechanism geniposidic acid works with regard to the effect to preserve against cytotoxicity caused by UVB dependently on density, I performed cell cycle analysis. I dyed HaCaT with PI and used flow cytometer for untreated control group, treatment group with UVB of 40 mJ/cm2, and treatment group with both UVB 40 mJ/cm2 and geniposidic acid of each density of 10, 20, and 30 μM respectively to estimate cell cycle progress. As the result of examining changing of cell cycle distribution, it was shown as Fig. 2a in which G1 value of treatment group with UVB of 40 mJ/cm2 reduces 39.2 to 34.4% while G1 value of control group is 73.6%, so it was shown that UVB causes G1 cell cycle arrest to suppress cell growth. When with geniposidic acid of each density of 10, 20, and 30 μM, I treated the treatment group with UVB of 40 mJ/cm2 respectively, G1 value increased to 41.8, 53.4, and 64.9% individually. It is considered that G1 cell cycle arrest caused by UVB is suppressed by geniposidic acid (Fig. 2a).
Cell cycle and sub-G1 analysis of geniposidic acid-treated HaCaT, damaged by UVB. a Cell cycle distribution of geniposidic acid-treated HaCaT, damaged by UVB. b Effect of geniposidic acid on UVB-induced sub-G1 population in HaCaT. c Effect of geniposidic acid on UVB-induced cleaved caspase-3 level in HaCaT (*p < 0.05)
I found out the effect of geniposidic acid on sub-G1 of HaCaT irradiated with UVB. Sub-G1 value of untreated control group increased from 2.4 to 15.9% by UVB; however, it was shown that the value decreases dependently on density of geniposidic acid, so at the case of treatment with 10 μM of geniposidic acid, the value was 12.7%; at the case of 20 μM, it was 7.9%; and at the case of 30 μM, it was 4.8%. It is considered that UVB causes increasement in cell population of sub-G1 and apoptosis increases, but geniposidic acid reduces sub-G1 similar to control group to suppress apoptosis (Fig. 2b).
As the result of searching relative value of cleaved caspase-3 of HaCaT cell treated with geniposidic acid, the amount of cleaved caspase-3 increased to 9.1 at the case of treatment with UVB 40 mJ/cm2, when the amount of cleaved caspase-3 of untreated control group was 1. It is found that UVB induces apoptosis, and when with geniposidic acid of each density of 10, 20, and 30 μM, I treated the treatment group with UVB 40 mJ/cm2 respectively, the amount of cleaved caspase-3 decreased to 7.8, 5.3, and 2.2 individually. It is considered that UVB causes an increase in the amount of cleaved caspase-3 and apoptosis increases, but geniposidic acid suppresses apoptosis, and so geniposidic acid has an effect to preserve cells (Fig. 2c).
Incitation of DNA damage protection and repair mechanism
I found out cell preservation effect of geniposidic acid on DNA damage through comet assay. As the result about changing of DNA damage in HaCaT which was treated with UVB and again with geniposidic acid, DNA tail increased to 67% after treating with UVB of 40 mJ/cm2, while it was 3% at the case of untreated control group of HaCaT cells. It is considered that UVB causes DNA damage, and when with geniposidic acid of each density of 10, 20, and 30 μM, I treated the treatment group with UVB 40 mJ/cm2 respectively, DNA tail decreased to 61, 44, and 19% individually. It is considered that UVB causes increase of tailed DNA and DNA damage, but geniposidic acid reduces it to preserve DNA, and that geniposidic acid has an effect to preserve cells (Fig. 3a). To find out what effects on damaged DNA caused by UV geniposidic acid have, I studied changing of CPD, and the result showed that CPD increases to 100% after treating with UVB 40 mJ/cm2, while it is 1% at the case of untreated control group of HaCaT. It is considered that UVB causes DNA damage and CPD increases, and when with geniposidic acid of each density of 10, 20, and 30 μM, I treated the treatment group with UVB 40 mJ/cm2 respectively, CPD decreased to 77, 31, and 12% individually. It is considered that UVB causes increasement in CPD and DNA damage increases, but geniposidic acid preserves cells, and that geniposidic acid has an effect to preserve cells (Fig. 3b). To examine changing of XPC expression, the DNA repair gene within HaCaT, caused by geniposidic acid, I performed qRT-PCR. XPC expression decreased to 0.37 at the case of treatment with 40 mJ/cm2 of UVB as compared with the value of untreated control group which was 1. It is expected that UVB causes DNA damage, and I found that when with geniposidic acid of each density of 10, 20, and 30 μM, I treated the treatment group with UVB of 40 mJ/cm2 respectively, XPC expression which was decreased due to UVB irradiation increased to 0.41, 0.54, and 0.82 individually as dose-dependent manner of geniposidic acid (Fig. 3c). It is considered that geniposidic acid has an effect on DNA damage caused by UVB to protect DNA. PCNA is a nucleoprotein of 36 kDa which is called as cyclin, and it increases all over the cell cycle from the end of G1 to S period. This is a secondary protein of DNA polymerase delta, and it has been reported that it has an important role in the beginning of cell proliferation (Jung and Jeong 1997). To examine changing of expression of PCNA, the DNA repair gene within HaCaT, I performed qRT-PCR. PCNA expression decreased to 0.29 at the case of treatment with UVB of 40 mJ/cm2 as compared with the value of untreated control group which was 1. It is expected that UVB causes DNA damage, and I found that when with geniposidic acid of each density of 10, 20, and 30 μM with irradiation of UVB at 40 mJ/cm2 respectively, the expression which has decreased by UVB increased significantly to 0.39, 0.77, and 0.91 individually and dependently on density (Fig. 3d). It is considered that geniposidic acid has an effect on DNA damage repair caused by UVB.
Analysis of DNA damage protection activity of geniposidic acid on UVB-irradiated HaCaT. a Effect of geniposidic acid on UVB-induced DNA damage in HaCaT. b Effect of geniposidic acid on UVB-induced CPD formation in HaCaT. c Effect of geniposidic acid on UVB-induced XPC gene expression in HaCaT. d Effect of geniposidic acid on UVB-induced PCNA gene expression in HaCaT (*p < 0.05)
Anti-oxidant properties analysis
DPPH is a water-soluble substance which has chemically stable free radical and has its maximum absorbance at the scale of 515–520 nm. When it meets with substances which show anti-oxidant action, it gives an electron and its radical becomes extinct to change its color. DPPH radical scavenging ability is a method to estimate the degree of anti-oxidation through the course of reacting with anti-oxidant substance to accept hydrogen atom and suppress oxidation. As the result of examining the anti-oxidation effect of geniposidic acid, I found that when I treated it with geniposidic acid of each density of 10, 20, 30, and 40 μM, its anti-oxidation effect was respectively about 18, 25, 65, and 80% dependently on density and similar to L-ascorbic acid, the positive control group (Fig. 4a).
Anti-oxidant effect of geniposidic acid on HaCaT. a Effect of geniposidic acid on DPPH radical scavenging activity. b Effect of geniposidic acid on ABTS radical scavenging activity. c Effect of geniposidic acid on UVB-induced SOD1 gene expression in HaCaT. d Effect of geniposidic acid on UVB-induced SOD2 gene expression in HaCaT. e Effect of geniposidic acid on UVB-induced lipid peroxidation in HaCaT (*p < 0.05)
ABTS reacts with potassium persulfate to form ABTS+ of blue/green. It is the method to evaluate anti-oxidation action through analysis on the degree of decoloration by anti-oxidant. As the result of examining the anti-oxidation effect of geniposidic acid, I found that when I treated it with geniposidic acid of each density of 10, 20, 30, and 40 μM respectively, the anti-oxidation effect was about 8, 20, 80, and 95% dependently on density and similar to L-ascorbic acid, the positive control group (Fig. 4b). Within the human body, there is a representative anti-oxidation enzyme, SOD which can protect itself against damages by reactive oxygen and has a role to transform the reactive oxygen radical (O2−) into H2O and H2O2 to reduce the density of reactive oxygen in the body (McCord and Fridovich 1969; Fridovich 1995). Among this SOD enzyme, there are SOD1 existing in the cytoplasm, SOD2 existing in mitochondria, and SOD3 existing in the outside of cell (Huang et al. 1999), and SOD1 is the representative anti-oxidation enzyme of all SOD enzymes, contains cytoplasm, and has a role to decrease ROS with regard to all tissues in which oxygen exists (Crapo et al. 1992).
To examine what effects on changing of SOD1 expression geniposidic acid have, I performed qRT-PCR. It was shown that while SOD1 expression in HaCaT decreases 0.37 times by irradiation with UVB of 40 mJ/cm2, the amount of expression increases dependently on density of geniposidic acid, so at the case of treatment with 10 μM, the amount increases 0.41 times; at the case of 20 μM, it increases 0.54 times; and at the case of 30 μM, it increases 0.82 times (Fig. 4c). It is considered that geniposidic acid induces increase in SOD1 expression to reduce reactive oxygen and suppress cellular senescence. To examine what effects on changing of mRNA expression of SOD2 which is anti-oxidant enzyme existing within mitochondria geniposidic acid have, I used qRT-PCR. It was shown that while SOD2 expression in HaCaT decreases 0.44 times by treatment with UVB of 40 mJ/cm2, the amount of expression increases dependently on density of geniposidic acid, so at the case of treatment with 10 μM, the amount increases 0.49 times; at the case of 20 μM, it increases 0.63 times; and at the case of 30 μM, it increases 0.79 times (Fig. 4d). Next, I searched what effects on changing of lipid peroxidation geniposidic acid have. When I treated it with UVB of 20 mJ/cm2, lipid peroxidation increased to 184%, and at the case of treatment with UVB of 40 mJ/cm2, it increased to 347%. It is considered that UVB causes oxidative stress and lipid peroxidation increases. With geniposidic acid of 30 μM, I treated both treatment group with UVB 20 and 40 mJ/cm2 respectively, and I found that lipid peroxidation decreases to 122% at the case of treatment group with UVB 20 mJ/cm2 and decreases to 153% at the case of treatment group with UVB 40 mJ/cm2 (Fig. 4e).
Discussion
Cell preservation effects of geniposidic acid on HaCaT against UVB
DNA damage caused by UV results in increase in p53 gene expression, and p53, the transcriptional factor, controls more than 100 genes which are induced by DNA damage (Latonen et al. 2001; Jin et al. 2000). p53 is a representative transcriptional factor which induces cell cycle arrest, suppression of cell growth, and promotion of apoptosis (Yoon 2013), and cell cycle arrest occurs in G1/S or G2 period of cell cycle depending on the condition of p53 action. Also, when UV causes DNA damage, p53 induces p21 gene expression, and expressed p21 causes cell cycle arrest through activity of cyclin-dependent kinase (CDK) to hinder cell growth (Smits and Medema 2001; Yoon 2013; Gewirtz et al. 2008). According to recent papers, it has been reported that p53 expression is suppressed in order to stop the promotion of senescence of normal tissue in adult mouse as the result of analysis on p53 mouse model (Gannon et al. 2011).
In this research, I found out what effects on cell damage caused by UVB geniposidic acid have, when I treated human keratinocytes with geniposidic acid. When I pretreated HaCaT keratinocytes with geniposidic acid of each density of 10, 20, and 30 μM respectively and tested with UVB of 40 mJ/cm2, I found that the cell viability restores dependently on density (Fig. 1b). I could know that geniposidic acid increases in the number of cells during G1 period although UVB causes G1 cell cycle arrest (Fig. 2a), and I could find that sub-G1 decreases as the density of geniposidic acid increases (Fig. 2b). Therefore, it is considered that geniposidic acid has effects on cell damage caused by UVB to suppress apoptosis and preserve cells.
Caspase-3 amplifies initial signal to caspase-8 and caspase-9, and it is known that it is directly related with apoptosis (Kennedy et al. 2001), and it has been recognized that it induces DNA fragmentation and pycnosis to cause apoptosis (Soldani and Scovassi 2002).
As the result of examining changing of caspase-3 action in this research, the amount of cleaved caspase-3 expression increased by UVB, but I could find that the amount of cleaved caspase-3 expression decreases by treatment with geniposidic acid of each density (Fig. 2c). Therefore, it is judged that geniposidic acid has effects on cell damage caused by UVB to suppress apoptosis and preserve cells.
DNA repair effect of geniposidic acid on HaCaT against UVB
As the result of examining cell preservation effects of geniposidic acid on DNA damage, I could know that UVB increases in tailed DNA, but it reduces by treatment with geniposidic acid as indicated concentration (Fig. 3a). Also, I studied changing of CPD formation to find what effects on damaged DNA by UVB have, and I could know that UVB causes increase in CPD and DNA damage and find that CPD reduces as the density of geniposidic acid increases (Fig. 3b). Therefore, it is judged that geniposidic acid has effects to protect DNA and preserve cells. Finally, it is considered that geniposidic acid would have important roles to control expression of hypostatic genes of p53 caused by UVB, to normalize cell cycle, and to suppress dermal cellular senescence.
P53 is activated by UV, oxidative stress or DNA damage, etc. (Miliani de Marval and Zhang 2011; Lee et al. 2016), and it controls expression of genes related with various senescence such as p21 (cyclin-dependent kinase inhibitor 1A), 14–3-σ and GADD45ɑ (growth arrest and DNA-damage-inducible, alpha) which are subordinate targets (Brown et al. 1997; Kortlever et al. 2006). P21 arrests cell cycle and hinders cell growth through the activity of CDK (Smits and Medema 2001; Gewirtz et al. 2008). GADD45ɑ controls superordinate stage of transmission system of Cdc2 protein kinase, PCNA, p21Waf1/Cip1 protein, core histone protein, MTK/MEKK4, and JNK/SAPK, and it is concerned with suppression of cell cycle and DNA repair through protein and signal transmission system within various cells, and it has an important role to prevent DNA damage. XPC is known that it reacts first for recognition of damaged DNA. As the result of examining changing of expression of XPC which is one of DNA repair genes, I found that XPC expression significantly increases dependently on density of geniposidic acid while it reduces by UVB (Fig. 3c). Therefore, it is considered that geniposidic acid has an effect on DNA damage to repair DNA. It has been reported that PCNA is a nucleoprotein of 36 kDa which is called as cyclin and increases all over the cell cycle from the end of G1 to S period, and it is also secondary protein of DNA polymerase delta and has an important role in the beginning of cell proliferation (Jung and Jeong 1997). As the result of examining changing of expression of PCNA, the DNA repair gene, I could know that PCNA expression increases dependently on density of geniposidic acid while it reduces by UVB (Fig. 3d). Therefore, it is judged that geniposidic acid has an effect on damaged DNA by UVB to repair DNA.
Anti-oxidation effects of geniposidic acid on HaCaT against UVB
ROS which has been increased within cells causes intracellular damages such as DNA damage, mitochondria damage, protein oxidation, and disorder of energy metabolism, and intracellular damage caused by oxidative stress can result in cell cycle arrest, cellular senescence, and apoptosis (Cerella et al. 2009).
As shown in Fig. 4a, b, with regard to anti-oxidation effects, I found that geniposidic acid has an anti-oxidation effect similar to L-ascorbic acid which is known as anti-oxidation standard substance through DPPH assay and ABTS+ assay with treatment of geniposidic acid. Its function to remove reactive oxygen works effectively at normal time to continue homeostasis in our human body, but it can have harmful effects on cells when it loses its balance to incline toward promotion of oxidation, and this harmful activity is called as oxidative stress. To examine what effects on changing of SOD1 and SOD2 expression geniposidic acid have, I performed qRT-PCR in this research. As shown in Fig. 4c, d, I could know that the amount of SOD1 and SOD2 expression increases dependently on density of geniposidic acid while it decreases by UVB which is one of oxidative stress. Therefore, it is considered that geniposidic acid increases SOD1 and SOD2 expression to reduce reactive oxygen and suppress cellular senescence, and it is judged that geniposidic acid has an anti-oxidation effect. To examine anti-oxidation effects of geniposidic acid, I used lipid peroxidation which is an indicator to estimate oxidative stress and observed its changing. As the result of this experiment, I found that lipid peroxidation reduces at the case of treatment with geniposidic acid of 30 μM while the oxidative stress caused by UVB increases in lipid peroxidation (Fig. 4e). It is considered that with particular density of 30 μM, geniposidic acid has an anti-oxidation effect.
Finally, it is considered that the study on the effectiveness of geniposidic acid on the mechanism controlling expression of factors which promote senescence through damage of dermal cells caused by UVB can help evaluating whether geniposidic acid is a natural functional cosmetic material which is helpful to preserve dermal cells and delay their senescence against UVB.
Conclusions
This paper used a natural compound, geniposidic acid, affiliated with iridoid glycoside which is known as effective for anti-oxidation, anti-inflammation, anticancer, anti-stress, and improvement of immune system to study about such effects as cell preservation, anti-oxidation, and DNA repair on human keratinocyte damaged by UVB.
I found that at the case of testing HaCaT with UVB of 40 mJ/cm2, cell viability reduces to 26% and cell proliferation is suppressed, but at the case of testing with the same UVB of 40 mJ/cm2 after pretreating with geniposidic acid of each density of 10, 20, and 30 μM respectively for 3 h, the cell viability restores dependently on density (Fig. 1b).
I performed cell cycle analysis and found that geniposidic acid has an effect to increase in G1 to prevent G1 cell cycle arrest caused by UVB (Fig. 2a) and that UVB causes increasement in cell population of sub-G1 and apoptosis increases, but sub-G1 reduces as the density of geniposidic acid increases. From these results, I could know that geniposidic acid has effects to suppress apoptosis and preserve cells (Fig. 2b). As the result of examining changing of caspase-3 action, I found that the amount of cleaved caspase-3 decreases to 7.8, 5.3, and 2.2 respectively when with geniposidic acid of each density of 10, 20, and 30 μM, I treated treatment group with UVB of 40 mJ/cm2 individually. I could know that UVB causes increasement in the amount of cleaved caspase-3 expression and apoptosis increases, and it decreases at the case of treatment with geniposidic acid of each density, and so geniposidic acid has effects to suppress apoptosis and preserve cells (Fig. 2c). Through single-cell gel electrophoresis, I found cell preservation effect of geniposidic acid on DNA damage. I found that when with geniposidic acid of each density of 10, 20, and 30 μM, I treated treatment group with UVB of 40 mJ/cm2 respectively, DNA tail decreases to 61, 44, and 19% individually, and I could know that UVB causes increasement in DNA tail and DNA damage increases, and it decreases as I treated it with geniposidic acid of each density, and so geniposidic acid has a cell preservation effect (Fig. 3a). To find out what effects on damaged DNA by UVB geniposidic acid have, I studied changing of CPD. As the result of this experiment, I found that CPD increases to 100% when I treated HaCaT with UVB of 40 mJ/cm2, and at the case of treatment with geniposidic acid of each density of 10, 20, and 30 μM respectively, CPD decreases to 77, 31, and 12% individually. I found that UVB causes increasement in CPD and DNA damage increases, and I could know that CPD decreases at the case of treatment with geniposidic acid of each density, and so geniposidic acid has effects to protect DNA and preserve cells (Fig. 3b).
By using qRT-PCR, I analyzed the effect of geniposidic acid on gene expression in HaCaT which was treated with UVB. I found that XPC, the DNA repair gene, decreases in its expression by UVB, but XPC expression significantly increases dependently on density of geniposidic acid, and I could find that geniposidic acid has a DNA repair effect on DNA damage caused by UVB (Fig. 3c). I found that PCNA, the DNA repair gene, decreases in its expression by UVB, but PCNA expression significantly increases dependently on density of geniposidic acid, and I could find that geniposidic acid has a DNA repair effect on DNA damage caused by UVB (Fig. 3d).
To examine an anti-oxidation effect of geniposidic acid, I used DPPH radical scavenging activity. As the result of analysis, I found that when I treated it with geniposidic acid of each density of 10, 20, 30 and 40 μM, its anti-oxidation effect appears as about 18, 25, 65, and 80% dependently on density, and so geniposidic acid has a similar anti-oxidation effect to L-ascorbic acid which is the positive control group (Fig. 4a). Also, as the result of examining the anti-oxidation effect of geniposidic acid through ABTS radical scavenging activity, I found that when I treated it with geniposidic acid of each density of 10, 20, 30, and 40 μM respectively, its anti-oxidation effect appears as about 8, 20, 80, and 95% dependently on density, and so geniposidic acid has a similar anti-oxidation effect to L-ascorbic acid which is the positive control group (Fig. 4b). To find out what effects on changing of expression of SOD which is an anti-oxidation enzyme existing in our human body have, I performed qRT-PCR. Among SOD enzyme, I found that SOD1 which exists in cytoplasm increases in its amount of expression dependently on density of geniposidic acid and that this increase in SOD1 expression reduces reactive oxygen within cytoplasm, and so geniposidic acid has an anti-oxidation effect (Fig. 4c). Also, I found that SOD2 which exists in mitochondria increases in its expression dependently on density of geniposidic acid and that increase in SOD2 expression reduces reactive oxygen within mitochondria, and so geniposidic acid has an anti-oxidation effect and cellular senescence suppression effect (Fig. 4d). Finally, I examined what effects on changing of lipid peroxidation geniposidic acid have. As the result, I found that lipid peroxidation increases in accordance with the amount of UVB but decreases as I treated it with geniposidic acid of particular density, and from this, I could find that geniposidic acid shows an effective anti-oxidative activity against the oxidative stress caused by UVB (Fig. 4e).
In this research, I found that geniposidic acid has such effects as cell preservation, anti-oxidation, and DNA repair in HaCaT damaged by UVB and I assure that this result proves sufficient value of geniposidic acid as a natural cosmetic material which effectively preserves cells and delays dermal cellular senescence against damage caused by UVB.
Abbreviations
- ABTS:
-
2,2-Azino-bis-3-ethylbenzoline-6-sulphonic acid
- Ac-DEVD-pNA:
-
N-Acetyl-Asp-Glu-Val-Asp p-nitroanilide
- ATM:
-
Ataxia-telangiectasia mutated
- ATR:
-
ATM- and Rad3-related
- CDK:
-
Cyclin-dependent kinase
- CPD:
-
Cyclobutane pyrimidine dimer
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- FACS:
-
Fluorescence-activated cell sorting
- FBS:
-
Fetal bovine serum
- G.A:
-
Geniposidic acid
- GADD45α:
-
Growth arrest and DNA-damage-inducible, alpha
- HaCaT:
-
Human keratinocyte
- LMAgarose:
-
Low melting agarose
- MDA:
-
Malondialdehyde
- P21:
-
Cyclin-dependent kinase inhibitor 1A
- PBS:
-
Phosphate-buffered saline
- PCNA:
-
Proliferating cell nuclear antigen
- PI:
-
Propidium iodide
- pNA:
-
p-nitroanilide
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- ROS:
-
Reactive oxygen species
- SOD1:
-
Superoxide dismutase 1
- SOD2:
-
Superoxide dismutase 2
- TBA:
-
Thiobarbituric acid
- UV:
-
Ultraviolet
- WST:
-
Water-soluble tetrazolium salts
- XPC:
-
XPC complex subunit, DNA damage recognition, and repair factor
References
Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–4.
Cerella C, Coppola S, Maresca V, De Nicola M, Radogna F, Ghibelli L. Multiple mechanisms for hydrogen peroxide-induced apoptosis. Ann N Y Acad Sci. 2009;1171:559–63.
Chang YC, Tseng TH, Lee MJ, Hsu JD, Wang CJ. Induction of apoptosis by penta-acetyl geniposide in rat C6 glioma cells. Chem Biol Interact. 2002;141:243–57.
Choi YH, Seo JH, Kim JS, Heor J, Kim SK, Choi SU, et al. Inhibitory effects of the stem bark extract of Eucommia ulmoides on the proliferation of human tumor cell lines. Kor J Pharmacogn. 2003;34:308–13.
Crapo JD, Qury T, Rabouille C, Slot JW, Chang LY. Cooper, zinc superoxide dismutase is primarily a cytosolic protein human cells. Pro Natl Acad Sci U S A. 1992;89:10405–9.
Deng Y, Guan M, Xie X, Yang X, Xiang H, Li H, et al. Geniposide inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. Int Immunopharmacol. 2013;17:561–7.
Fridovich I. Superoxide radical and superoxide dismutase. Annu Rev Biochem. 1995;64:97–112.
Gannon HS, Donehower LA, Lyle S, Jones SN. Mdm2-p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Dev Biol. 2011;353:1–9.
Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76:947–57.
Guan L, Feng H, Gong D, Zhao X, Cai L, Wu Q, et al. Genipin ameliorates age-related insulin resistance through inhibiting hepatic oxidative stress and mitochondrial dysfunction. Exp Gerontol. 2013;48:1387–94.
Hong YJ, Yang KS. Anti-inflammatory activities of crocetin derivatives from processed Gardenia jasminoides. Arch Pharm Res. 2013;36:933–40.
Hsu HY, Yang JJ, Lin SY, Lin CC. Comparisons of geniposidic acid and geniposide on antitumor and radioprotection after sublethal irradiation. Cancer Lett. 1997;113:31–7.
Huang TT, Carlson EJ, Raineri I, Grillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann N Y Acad Sci. 1999;893:95–112.
Jenkins G. Molecular mechanisms of skin aging. Mech Ageing Dev. 2002;123:801–10.
Jin S, Antinore MJ, Lung FD, Dong X, Zhao H, Fan F, et al. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem. 2000;275:16602–8.
Jung SI, Jeong GB. P53 and PCNA protein expression on the colorectal cancer tissue. Chungbuk Med J. 1997;7:223–36.
Kennedy DO, Kojima A, Yano Y, Hasuma T, Otani S, Matsui-Yuasa I. Growth inhibitory effect of green tea extract in Ehrlich ascites tumor cells involves cytochrome c release and caspase activation. Cancer Lett. 2001;166:9–15.
Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–84.
Kulms D, Zeise E, Pöppelmann B, Schwarz T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene. 2002;21:5844–51.
Latonen L, Taya Y, Laiho M. UV-radiation induces dose-dependent regulation of p53 response and modulates p53-HDM2 interaction in human fibroblasts. Oncogene. 2001;20:6784–93.
Lee S, Han HS, An IS, Ahn KJ. Effects of amentoflavone on anti-inflammation and cytoprotection. Asian J Beauty Cosmetol. 2016;14:201–11.
Lee YR, Noh EM, Han JH, Kim JM, Hwang JK, Hwang BM, et al. Brazilin inhibits UVB-induced MMP-1/3 expressions and secretions by suppressing the NF-κB pathway in human dermal fibroblasts. Eur J Pharmacol. 2012;674:80–6.
McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide and oxygen. J Biol Chem. 1969;244:6056–63.
Miliani de Marval PL, Zhang Y. The RP-Mdm2-p53 pathway and tumorigenesis. Oncotarget. 2011;2:234–8.
Park SD, Kim GW. Experimental studies of eucommiae cortex according to processing. The Journal of Dong Guk Oriental Medicine. 1992;1:81–107.
Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta. 2001;1519:1–12.
Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–8.
Yoon YM. Gene expression profiling in protection mechanism of silibinin against damage to human dermal fibroblasts caused by UVB. Asian J Beauty Cosmetol. 2013;11:93–102.
Acknowledgements
Not applicable
Funding
Not applicable
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The author declares no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, N.K. Preservation effects of geniposidic acid on human keratinocytes (HaCaT) against UVB. biomed dermatol 2, 5 (2018). https://doi.org/10.1186/s41702-017-0015-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41702-017-0015-2