Abstract
Older adults frequently suffer from sleep disturbances. In addition, with increasing age such disturbances may accompany mild cognitive changes that are symptomatic of a range of neurodegenerative conditions. There is increasing evidence that sleep may represent a prodromal symptom, risk factor or agitator of further decline in cognitive functioning of the older adult. Current research is focused on understanding the impact that effective sleep treatments have on a range of psychological and cognitive variables.
Similar content being viewed by others
Background
Given the challenges posed by changing life circumstances and the accumulation of threats to physical health in later life, it is no surprise that almost 40% of those age 65 and older report experiencing some form of sleep disruption and as many as 70% of older adults suffer from four or more comorbid illnesses (Foley et al. 2004). The U.S. population of those age 65 and older is currently estimated at 40 million and this population is projected to double in the next 30 years underscoring the importance of attention and further research on geriatric sleep and treatments for common disturbances.
There are age dependent changes that occur and involve changes in the continuity, depth, and distribution of sleep. Age in itself does not necessarily equate to sleep disturbance but rather the interplay between health and psychosocial status in conjunction with the developmental changes combine to impact an older adult’s sleep. There is a shift towards advance phase sleep leading to earlier bedtimes and wake times that starts during middle age (Buysse et al. 2005). Additional developmental changes in sleep include an increase in sleep fragmentation related to increase in N1 sleep architecture, a lighter sleep stage, at the expense of a decrease in N3 deep sleep (Dijk & Czeisler 1995). In the sleep regulatory system, N3 sleep is related to an increase in homeostatic pressure that builds throughout the day and in younger adults leads to shorter sleep onset and deep sleep the first half of the night of sleep (Cajochen et al. 2006). Following adolescence we experience a progressive decline in our amount of deep sleep with each passing decade. For those 65 and older there is very little deep sleep, contributing to an increase in sleep latency, increased awakenings after sleep onset, and decrease in sleep efficiency.
These changes, in combination with decreases in daytime activity levels, declines in health status and an increase in prescriptions to treat emerging health issues, can ultimately result in significant sleep disruptions and daytime symptoms, as well as compensatory behaviors, such as daytime napping (Bliwise et al. 2005). The prevalence rate of sleep disorders in older adults, such as obstructive sleep apnea is estimated 7% in women and 18% in men, periodic limb movement disorder at 3%, restless legs syndrome at 8%, can impact the quality and quantity of sleep and leave the older adult at risk for the development of insomnia (Bixler et al. 1998; Ohayon & Roth 2002). Insomnia is the most common sleep disorder across the lifespan and is estimated to be 35–40% in those age 65 and older (Foley et al. 1999). Insomnia is associated with negative consequences including depressive symptoms, decreased quality of life, increase in healthcare utilization and daytime impairment (Foley et al. 2004; Hatoum et al. 1998). There is cross sectional evidence that insomnia is related to an increased risk for dementia (de Almondes et al. 2016). McKinnon, et al. (McKinnon et al. 2014) and colleagues have found that depression symptoms, impaired cognition, antidepressant use, alcohol consumption, in addition to age and education, were all significant predictors of poor sleep quality. Not only does poor sleep negatively impact one’s quality of life, studies have revealed that older individuals with both long and short sleep duration have increased rates of all-cause mortality (da Silva et al. 2016).
Mild cognitive impairment- what is it?
Mild cognitive impairment (MCI) is the transitional state between intact cognitive functioning and dementia. The most common diagnostic criteria was proposed by Petersen (Petersen 2004), and includes those with a decline in cognitive functioning, while controlling for their particular age and education level, combined with a relatively intact performance in activities of daily living. Further subtypes have also been delineated, to specify impairment that is primarily due to memory impairment (amnestic MCI; aMCI) and impairment in cognitive domains other than memory (nonamnestic MCI; naMCI) (Petersen 2004).
Rates of conversion from MCI to dementia is at about 10–15% per year (Petersen et al. 2001). Because the identification of those in this transitional stage (between normal cognition and mild cognitive impairment) can be difficult, clinical symptoms and biomarkers that characterize this stage, including a range of neuropsychiatric symptoms such as sleep disturbance, depression and apathy, may be helpful in identifying patients that may be at an increased risk for continued decline. Sleep disturbances are one of the most common neuropsychiatric symptoms and appear to signal individuals at greater risk of conversion to dementia. This is particularly relevant given the high prevalence of sleep issues in older adults with mild cognitive impairment, with studies detecting rates that range from 14 to 59% (Beaulieu-Bonneau & Hudon 2009), and some as high as 63% (McKinnon et al. 2014), who report experiencing poor sleep quality.
Relationship between sleep and cognitive functioning
Although some sleep parameters change with normal aging as stated above, such changes are more prevalent and severe in individuals suffering from cognitive impairment, including those with preclinical signs and symptoms and those with detectable impairment, such as MCI (Lyketsos et al. 2002; Muangpaisan et al. 2008; Ramakers et al. 2010), and there is growing evidence for a shared neurodegenerative etiology. Sleep disturbances in quality and quantity of sleep, as well as disruption of the sleep-wake rhythm, occur frequently in older adults with cognitive impairment and there is growing support for a bidirectional relationship (Guarnieri & Sorbi 2015). Studies looking at cognitive functioning have linked sleep disturbance prospectively to the emergence of cognitive deficits (Beaulieu-Bonneau & Hudon 2009), and have identified sleep issues as part of a prodromal syndrome of various neurodegenerative diseases (Postuma et al. 2009). Sleep disturbance may also represent an early marker or a risk factor for cognitive decline. For example, a study by Diem, et al. (Diem et al. 2016) found that lower sleep efficiency and longer sleep latencies in older women were associated with a 1.5 and 1.4 greater odds of developing MCI or dementia within five years. In addition, worsening sleep problems are often observed to occur in conjunction with deteriorating cognitive symptoms (Moe et al. 1995), raising the question of whether they each play a part in fueling the other.
Studies on sleep and cognitive functioning have concluded that there is a strong relationship between disturbed sleep and an increased risk for cognitive impairment and dementia (Lim et al. 2013). Contributing to this idea, alterations in sleep architecture have been found in those with MCI and appear to negatively impact memory consolidation (Westerberg et al. 2012), which is believed to contribute to declines in memory functioning. Further support for this hypothesis comes from a study that tested the impact of artificially improving slow-wave sleep in older adults to determine if it would improve their subsequent performance on memory tests, when compared to a sham control group. Initial findings have found that the use of transcranial current oscillating at a slow frequency in the treatment group during sleep resulted in an improved performance on memory tests before and after the sleep period (Westerberg et al. 2015).
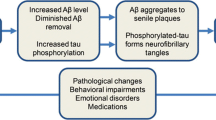
Sleep has been found to have strong ties to changes in the brain associated with neurodegenerative diseases. A recent study by Varga, et al. (Varga et al. 2016) found higher levels of amyloid beta in the brain, the deposition of which remains a hallmark of Alzheimer’s Disease (AD), which are associated with reduced and fragmented slow-wave sleep. Further, the daytime sleepiness and nighttime experience of parasomnias, such a rapid eye movement (REM) behavior disorder (RBD), observed in association with Lewy Body and Parkinson’s related dementia, provide yet another example of disturbed sleep’s connection to cognitive impairment. Naismith, et al. (Naismith et al. 2014) found that circadian misalignment, and specifically advanced timing of melatonin secretion, were evident in MCI patients and also associated with disturbed sleep and poor performance on memory tests.
Clinically, neuropsychiatric symptoms, also referred to as behavioral and psychological symptoms of dementia (BPSD), are more prevalent in individuals with MCI than their counterparts with normal cognition, specifically, depression, sleep disturbances and apathy (Kohler et al. 2016), and often impact individuals quality of life. A study by Yu, et al. (Yu et al. 2016) studied individuals with MCI and found that the sleep parameters measured (e.g., sleep latency, disturbances, duration and efficiency) were worse in those with MCI, even when analyses controlled for clinical levels of anxiety and depression.
Sleep plays a crucial role in learning and memory across the lifespan (Poe et al. 2010), and thus is it not surprising that it is also associated with the decline in cognitive functioning. Several prospective studies have linked several sleep disturbances to a range of cognitive deficits and impaired performance, including measures assessing areas such as memory, attention, executive functioning and problems solving skills [see excellent review of studies in Yaffe, et al. (Yaffe et al. 2014)]. It appears that this connection between sleep disturbance and MCI is strong, regardless of MCI subtype. Naismith, et al. (Naismith et al. 2010) looked at a small group of individuals with the nonamnestic subtype of MCI and found that objectively assessed disturbed sleep was associated with impaired attention and executive functioning, while increased arousals during rest were related to poorer nonverbal learning and problem solving.
Questions under study in this area
Assessing sleep in older adults could help in the identification of those at risk for cognitive decline. In addition, sleep may be a target that, when treated, could alter the trajectory of cognitive decline, thereby providing a relatively efficient and cost-effective treatment that would positively impact outcomes of a large number of patients.
It may also be that targeting other factors that impact sleep may ultimately impact cognitive functioning as well. For example, given the connection of depression and antidepressant use to sleep quality in MCI (McKinnon et al. 2014), researchers have made the case that treating depression may also have a broader impact on individual functioning.
Our research
One key question, under investigation by our group, is whether established treatments can be successfully used with individuals who have both impaired sleep and MCI. Due to concerns of polypharmacy, cognitive impairment effects, and risk of falls with the use of sleep medications in older adults the use of nonpharmacological treatments, specifically cognitive behavioral therapy for insomnia (CBT-I), is an effective and optimal treatment choice for those with late life insomnia. CBT-I treatment outcomes have not been shown to be age related and are comparable to younger and middle aged adults (Morin et al. 1994; Morin et al. 2006). Significant improvements with CBT-I treatment have been seen in sleep quality, time to sleep onset, time awake after sleep onset, and sleep efficiency for those 55 and older (Irwin et al. 2006). Older adults with insomnia found CBT-I to be a more acceptable form of treatment, have less side effects, more beneficial for daytime functioning, and long-term effectiveness over pharmacotherapy for insomnia (Morin et al. 1992).
A CBT-I treatment was disseminated by a clinical psychologist to older adults with insomnia and MCI in two residential care facilities for the elderly (RCFEs). The study involved a two-arm individual randomization to 6 sessions of CBT-I intervention or six sessions of active control nutrition class. It was hypothesized that those that received the CBT-I intervention would experience improvement in assessed sleep parameters and, secondarily, positive changes in their performance on cognitive testing.
Participants were 28 older adults (mean age, 89.36 years), meeting the key inclusion criteria of meeting diagnostic criteria for insomnia according to the DSM-IV (APA 1994), and the core clinical criteria for MCI used by healthcare workers without access to advanced imaging techniques (Albert et al. 2011) (i.e., subject memory complaints, preservation of independence in functional abilities, performance on at least one of the cognitive tests at 1.5SD below published age/educational matched normative means, and not demented). In addition to having a positive effect on subjective (i.e., Insomnia Severity Index ratings) and objective ratings of several sleep parameters (i.e., improved sleep latency, wake after sleep onset, and sleep efficiency), we also found a positive improvement in the treatment group on an outcome measure of executive functioning (D-KEF Color-Word Interference Test) (Cassidy-Eagle et al. 2015). Deficits in executive processes (e.g., planning, problem solving), memory and language functions are, more frequently observed in MCI and are particularly important to assess in light of their potential impact on treatment given the cognitive processing involved in CBT. Nonpharmacological interventions such as CBT-I may be beneficial for people with MCI. Further study of CBT-I in people with MCI is warranted. Such research should include pre- and post-intervention measures of cognitive functions. Targeting of sleep has the potential to have broad public health impact, including in people with MCI.
Future opportunities
Over the entire trajectory of cognitive decline, does sleep play a role in causing or exacerbating cognitive impairment and/or is it the result of cognitive decline? In addition to studies that will further elucidate the neuronal overlap and causal mechanisms in these conditions, more work in this area is needed as the results could be incredibly useful in designing effective, personalized treatments.
There are many possible targets for intervention that merit further study and consideration for their potential role in preventing or slowing cognitive decline experienced by older adults. Given the impact of various medications, non-pharmacological interventions are of great interest. Exercise, increased socialization, bright-light therapy, and CBT-I all show promise and merit additional study. Naismith, et al. (Naismith et al. 2014) and colleagues have called for more focused studies on the potential use of supplemental melatonin as a potentially effective treatment of sleep-wake disorders in those with MCI. Treating older patients with sleep apnea with continuous positive airway pressure (CPAP) devices could also slow down or delay further the deterioration in cognitive functioning observed in MCI and AD (Yaffe et al. 2011; Canessa & Ferini-Strambi 2011).
Access to effective treatments is also a growing concern that needs to be addressed. Sleep disturbances in older adults are commonly addressed by sleep specialists. Given the shortage of clinicians specializing in assessing and treating sleep difficulties, it is imperative that sleep be evaluated routinely in other clinical settings and that alternative implementation models, such as using sleep coaches (Alessi et al. 2016), be considered to reach more patients in need of effective treatments. Given the high prevalence of sleep disturbances, assessments need to be incorporated in regular health checks and referrals made for a thorough diagnostic workup when disturbances are reported, particularly given the evidence that it may represent a prodromal state of dementia. Future work also needs to include prospective studies that utilize both subjective and objective measures of sleep parameters, in order to confirm or challenge studies to date that support a relationship between self-reported sleep disturbances (e.g., sleep quality or quantity measures).
Conclusions
Although challenging, sleep represents a clear, easily identified and measured, modifiable risk factor that plays a significant role in neurodegenerative conditions and has the potential to significantly impact the consequences of several major public health problems.
References
Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. doi:10.1016/j.jalz.2011.03.008 [published Online First: Epub Date].
Alessi C, Martin JL, Fiorentino L, et al. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J Am Geriatr Soc. 2016;64(9):1830–8. doi:10.1111/jgs.14304 [published Online First: Epub Date].
APA APA. Diagnostic and statistical manual of mental of mental disorders, Fourth Edition (DSM-IV). Washington, D.C.: American Psychiatric Association; 1994.
Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr. 2009;21(4):654–66. doi:10.1017/S1041610209009120 [published Online First: Epub Date].
Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–8. doi:10.1164/ajrccm.157.1.9706079 [published Online First: Epub Date].
Bliwise DL, Ansari FP, Straight LB, Parker KP. Age changes in timing and 24-hour distribution of self-reported sleep. Am J Geriatr Psychiatry. 2005;13(12):1077–82. doi:10.1176/appi.ajgp.13.12.1077 [published Online First: Epub Date].
Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28(11):1365–76.
Cajochen C, Munch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23(1–2):461–74. doi:10.1080/07420520500545813 [published Online First: Epub Date].
Canessa N, Ferini-Strambi L. Sleep-disordered breathing and cognitive decline in older adults. JAMA. 2011;306(6):654–5. doi:10.1001/jama.2011.1124 [published Online First: Epub Date].
Cassidy-Eagle E, Siebern A, Unti L, Glassman J, O’Hara R. Treating insomnia in older adults with mild cognitive impairment in residential care settings. Seattle: Associated Professional Sleep Societies; 2015.
da Silva AA, de Mello RG, Schaan CW, Fuchs FD, Redline S, Fuchs SC. Sleep duration and mortality in the elderly: a systematic review with meta-analysis. BMJ Open. 2016;6(2):e008119. doi:10.1136/bmjopen-2015-008119 [published Online First: Epub Date].
de Almondes KM, Costa MV, Malloy-Diniz LF, Diniz BS. Insomnia and risk of dementia in older adults: systematic review and meta-analysis. J Psychiatr Res. 2016;77:109–15. doi:10.1016/j.jpsychires.2016.02.021 [published Online First: Epub Date].
Diem SJ, Blackwell TL, Stone KL, et al. Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. Am J Geriatr Psychiatry. 2016;24(3):248–58. doi:10.1016/j.jagp.2015.12.002 [published Online First: Epub Date].
Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15(5 Pt 1):3526–38.
Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22 Suppl 2:S366–72.
Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. doi:10.1016/j.jpsychores.2004.02.010. published Online First: Epub Date.
Guarnieri B, Sorbi S. Sleep and cognitive decline: a strong bidirectional relationship. It is time for specific recommendations on routine assessment and the management of sleep disorders in patients with mild cognitive impairment and dementia. Eur Neurol. 2015;74(1-2):43–8. doi:10.1159/000434629 [published Online First: Epub Date].
Hatoum HT, Kong SX, Kania CM, Wong JM, Mendelson WB. Insomnia, health-related quality of life and healthcare resource consumption. A study of managed-care organisation enrollees. Pharmacoeconomics. 1998;14(6):629–37.
Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14. doi:10.1037/0278-6133.25.1.3 [published Online First: Epub Date].
Kohler CA, Magalhaes TF, Oliveira JM, et al. Neuropsychiatric disturbances in Mild Cognitive Impairment (MCI): a systematic review of population-based studies. Curr Alzheimer Res. 2016;13(10):1066–82.
Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–32. doi:10.5665/sleep.2802 [published Online First: Epub Date].
Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–83.
McKinnon A, Terpening Z, Hickie IB, et al. Prevalence and predictors of poor sleep quality in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2014;27(3):204–11. doi:10.1177/0891988714527516 [published Online First: Epub Date].
Moe KE, Vitiello MV, Larsen LH, Prinz PN. Symposium: cognitive processes and sleep disturbances: sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J Sleep Res. 1995;4(1):15–20.
Morin CM, Gaulier B, Barry T, Kowatch RA. Patients’ acceptance of psychological and pharmacological therapies for insomnia. Sleep. 1992;15(4):302–5.
Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151(8):1172–80. doi:10.1176/ajp.151.8.1172 [published Online First: Epub Date].
Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998-2004). Sleep. 2006;29(11):1398–414.
Muangpaisan W, Intalapaporn S, Assantachai P. Neuropsychiatric symptoms in the community-based patients with mild cognitive impairment and the influence of demographic factors. Int J Geriatr Psychiatry. 2008;23(7):699–703. doi:10.1002/gps.1963 [published Online First: Epub Date].
Naismith SL, Rogers NL, Hickie IB, Mackenzie J, Norrie LM, Lewis SJ. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23(2):123–30. doi:10.1177/0891988710363710 [published Online First: Epub Date].
Naismith SL, Hickie IB, Terpening Z, et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38(4):857–66. doi:10.3233/JAD-131217 [published Online First: Epub Date].
Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53(1):547–54.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. doi:10.1111/j.1365-2796.2004.01388.x [published Online First: Epub Date].
Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–92.
Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi:10.1016/B978-0-444-53702-7.00001-4 [published Online First: Epub Date].
Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72(15):1296–300. doi:10.1212/01.wnl.0000340980.19702.6e [published Online First: Epub Date].
Ramakers IH, Visser PJ, Aalten P, Kester A, Jolles J, Verhey FR. Affective symptoms as predictors of Alzheimer’s disease in subjects with mild cognitive impairment: a 10-year follow-up study. Psychol Med. 2010;40(7):1193–201. doi:10.1017/S0033291709991577 [published Online First: Epub Date].
Varga AW, Wohlleber ME, Gimenez S, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Abeta42 levels in cognitively normal elderly. Sleep. 2016;39(11):2041–8.
Westerberg CE, Mander BA, Florczak SM, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18(3):490–500. doi:10.1017/S135561771200001X [published Online First: Epub Date].
Westerberg CE, Florczak SM, Weintraub S, et al. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol Aging. 2015;36(9):2577–86. doi:10.1016/j.neurobiolaging.2015.05.014 [published Online First: Epub Date].
Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–9. doi:10.1001/jama.2011.1115 [published Online First: Epub Date].
Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–28. doi:10.1016/S1474-4422(14)70172-3 [published Online First: Epub Date].
Yu J, Mahendran R, Rawtaer I, Kua EH, Feng L. Poor sleep quality is observed in mild cognitive impairment and is largely unrelated to depression and anxiety. Aging Ment Health. 2016;1–6. doi:10.1080/13607863.2016.1161007 [published Online First: Epub Date].
Acknowledgements
Not applicable.
Funding
The research summarized in this manuscript, including the support needed to carry out the interventions and analyze the findings, was supported by Grant #2012-199 from the Retirement Research Foundation.
Availability of data and materials
Not applicable.
Authors’ contributions
Both ECE and AS contributed to the conceptualization, writing and research summarized in this review/manuscript. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cassidy-Eagle, E.L., Siebern, A. Sleep and mild cognitive impairment. Sleep Science Practice 1, 15 (2017). https://doi.org/10.1186/s41606-017-0016-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41606-017-0016-5