Abstract
Background
The monitoring of pathogens of fishery auction markets is important to obtain safe fishery products regarding hygiene and sanitation. In this study, aerobic, coliform, Escherichia coli, and Vibrio cholerae were monitored in the fishery products and environmental samples obtained from fishery auction markets.
Methods
The fishery products (flounder, octopus, skate, rock cod, sea bass, snail, monkfish, flatfish, comb pen shell, corb shell, conger eel, hairtail, croaker, and pilchard) were placed in filter bags, and the environmental samples (samples from the water tanks at the fishery auction markets, seawater from the fishery distribution vehicles, ice from wooden or plastic boxes, and surface samples from wooden and plastic boxes used for fish storage) were collected. Aerobic bacteria, E. coli, and coliform in the samples were enumerated on aerobic count plates and E. coli/coliform count plates, respectively. For V. cholerae O1 and V. cholerae non-O1 quantification, most probable number (MPN)-PCR analysis was performed.
Results
Aerobic and coliform bacteria were detected in most samples, but E. coli was not detected. Wooden boxes were contaminated with high levels of aerobic and coliform bacteria in all seasons (spring, summer, and fall). During fall, V. cholerae non-O1 were detected in snails, hairtails, croakers, flatfishes, pilchards, plastic boxes, and water samples.
Conclusions
These results indicate an increased prevalence of V. cholerae contamination in fishery products in fall, including food contact samples, which can be vehicles for cross-contamination.
Similar content being viewed by others
Background
Global fish production increased to 171 million tons in 2016, and the amount of fish consumed has been growing continually (20.5 kg/person/year in 2017) (FAO 2018). A considerably dynamic import and export of fishery products has been evidenced between countries (FAO 2019). More fish and fishery products were consumed in S. Korea in 2016 (59.9 kg/person/year) than meat (56.0 kg/person/year). The degree of self-sufficiency in S. Korea was 67.3% in 2016 (KREI 2017). Fishery products arrive at the auction market directly after harvesting. Sanitation from collection to distribution is essential for obtaining safe fishery products that are protected from cross-contamination (Ahmed 1991). S. Korea is surrounded by the East, West, and South Sea (Chough et al. 2000). Especially, the West Sea is comprised of mudflats and has high tides and estuary waters (Cho et al. 1999; Koh and Shin 1988). Therefore, fishery products from the West Sea may become cross-contaminated from these environments. In particular, Di et al. (2017) detected V. cholerae (0.1%) in the tidal water collected from the southern coast in June and V. cholerae (0.5%) in the tidal water in September 2013. Therefore, the microbial contamination of products from the West Sea should be monitored.
Foodborne illness occurring through the consumption of fish (17%) is common, followed by dairy (11%) and chicken (10%) in the USA from 2009 to 2015 (Dewey-Mattia et al. 2018). Vibrio spp. are gram-negative bacilli and major pathogens which present in coastal or estuarine environments (Horseman and Surani 2011; Reidl and Klose 2002). V. cholerae is a causative agent for cholera in humans which grows in 0–3% NaCl and relatively low salinity. There was a foodborne outbreak, caused by V. cholerae in 2016 through domestic sea water (KCDC 2017). For the case of 2016, raw seafoods (sea bass, sea squirt, abalone, crab, mackerel, flatfish, rockfish, shrimp, sea cucumber, octopus, and squid) were assumed as causative foods for three patients in the outbreak (Kim et al. 2018). The V. cholerae O1 isolated from the South Sea seawater and the fecal samples collected from three patients were Ogawa serotype, El Tor biotype, and contained cholera toxin (ctx) (KCDC 2017). The O1 serotype of V. cholerae is known as exhibiting explosive growth (Maheshwari et al. 2011; Labbé and García 2013). Since 2016, monitoring of V. cholerae has been ongoing, and the importance of tracking V. cholerae has been emphasized in S. Korea.
The monitoring of fishery auction markets for pathogens is essential for obtaining safe fishery products with regard to hygiene and sanitation. Therefore, the fishery auction markets in the West Sea, S. Korea were monitored in this study. Microbial contamination was evaluated by detecting V. cholerae and other hygiene indicator microorganisms in environmental samples from the fishery auction markets and the fishery products harvested in the West Sea.
Methods
Sample collection and preparation
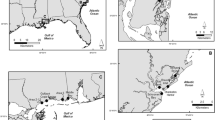
Seventy-eight fishery products (N = 41) and environmental samples (N = 37) were collected at two fishery auction markets in the West Sea, S. Korea from March to September 2017. Of the 78 samples, 29, 24, and 25 were collected in spring (March–April), summer (July–August), and fall (September), respectively. Because of season and daily circumstances in each market, types of fishery products samples were different between markets (Table 1). The fishery products that were harvested in each season were collected before, during, and after the auction. The environmental samples of the fishery auction markets were collected from water in tanks, seawater in fishery distribution vehicles, ice in wooden or plastic boxes, and from the wooden and plastic boxes used for fish storage. The surfaces (10 × 10 cm2) of the wooden and plastic boxes were swabbed using a swab-sampler (3 M, St. Paul, MN, USA). All samples were transported in an ice cooler to a laboratory. Twenty-five-gram samples were removed from the gills of the fish and the edible portion of the shellfish for microbial analysis.
Quantification of aerobic, coliform, and E. coli bacteria
The fishery product samples were placed aseptically into filter bags (3 M) and 50 mL of 0.1% alkaline peptone water (APW; Becton, Dickinson and Company, Sparks, MD, USA) was added. After shaking 30 times, a 1-mL aliquot of the homogenate was serially diluted with 9 mL APW. The diluents were plated on an Aerobic Count Plate (Petrifilm™; 3 M) and an E. coli/Coliform Count Plate (Petrifilm™; 3 M). One milliliter of collected water, seawater, ice, and suspension was taken from swab-samples of wooden and plastic boxes were also diluted, and the diluents were plated on both plates of the environmental samples. All plates were incubated at 35 °C for 24 h. The red aerobic bacteria, blue with gas E. coli, and red and blue coliform colonies were manually counted.
Quantification of V. cholerae by MPN-PCR analysis
The suspensions (10, 1, and 0.1 mL) from filter bags contained 25 g or 25 mL samples with 225 mL APW were inoculated in five test tubes containing 10 mL APW to target 1 × APW final concentration. All test tubes were incubated at 35 °C for 14 h. For PCR analysis, 1 mL aliquots of the cultures were centrifuged at 13,475×g for 2 min, and the supernatants were removed. The pellets were suspended with 0.1 mL distilled water then heated at 100 °C for 10 min. After centrifuging at 13,475×g for 2 min, the supernatants were used as a DNA template. The primers for V. cholerae (F: 5′-CACCAAGAAGGTGACTTTATTGTG-3′, R: 5′-GAACTTATAACCACCCGCG-3′; 586 bp) and V. cholerae O1 (F: 5′-CTCAGACGGGATTTGTTAGGCACG-3′, R: 5′-TCTATCTCTGTAGCCCCTATTACG-3′; 302 bp) were used (Kim et al. 2015; Rajpara et al. 2013; Nandi et al. 2000). PCR amplification was performed using a FastMix kit (Intron Bio, Gyeonggi, Korea) composed of dNTP, DNA polymerase, reaction buffer, and MgCl2. For the amplification of V. cholerae and V. cholerae O1, the following steps were performed: initial denaturation at 94 °C for 4 min, 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 90 s, extension at 72 °C for 90 s, and final extension at 72 °C for 10 min. The results of amplification were electrophoresed on 1.5% agarose gel for 20 min and visualized using UV light. The number of positive test tube samples per five test tubes that were analyzed by PCR analysis was counted for each dilution, and the most probable number (MPN) of V. cholerae and V. cholerae O1 was determined using an MPN table (FDA 2010).
Results and discussions
From March to September 2017, 41 fishery products (March–April, 14; June–July, 16; and September, 11) and 37 environmental samples (March–April, 15; June–July, 8; and September, 14), which were collected from two fishery auction markets located in the West Sea of S. Korea, were analyzed for microbial contamination.
At fishery auction market A, aerobic bacteria were detected in the fishery products (1.5 × 102–2.2 × 104 CFU/g) and the environmental samples (2.7 × 10–2.2 × 106 CFU/mL or /100 cm2), and coliform were detected in the fishery products (7.2 × 10–1.9 × 102 CFU/g) and the environmental samples (6.0 × 10–1.6 × 102 CFU/mL or /100 cm2) in spring (Table 2). E. coli and V. cholerae were below the limit of detection in all samples. Aerobic bacteria and coliform were detected in flounder irrespective of the period of the fishery auction (before, during, and after the auction). Of the environmental samples, the wooden boxes for fish storage were the most contaminated with aerobic bacteria (1.7 × 104–2.2 × 106 CFU/100 cm2), followed by the plastic fish boxes (5.8 × 103–8.0 × 103 CFU/100 cm2), and even seawater in the fishery distribution vehicle (4.3 × 103 CFU/mL) and water in the tanks of the fishery auction market (2.7 × 10–3.0 × 10 CFU/mL). In particular, coliform was detected in the wooden (6.0 × 10–1.6 × 102 CFU/100 cm2) and plastic boxes (1.5 × 102 CFU/100 cm2) (Table 2). In summer (June–July), aerobic bacteria were detected in the fishery products (7.5 × 102–2.0 × 104 CFU/g) and the environmental samples (1.6 × 103–1.3 × 107 CFU/mL or /100 cm2). Coliform was detected in the fishery products (1.4 × 102–2.6 × 103 CFU/g) and the environmental samples (5.7 × 102–2.5 × 104 CFU/mL or /100 cm2). However, E. coli and V. cholerae were below the limit of detection in all samples. In addition, there was no difference between aerobic and coliform bacteria respective to the period of the auction (before, during, and after the auction) and in the fishery products (flounder, rock cod, and sea bass). Among the environmental samples, wooden boxes were the most contaminated with aerobic (1.3 × 107 CFU/100 cm2) and coliform bacteria (2.5 × 104 CFU/100 cm2), compared to other environmental samples (Table 3). In fall (September), V. cholerae non-O1 were detected only in snails (20−5,400 MPN/100 g). Aerobic bacteria were detected in the snails (2.6 × 10–8.4 × 103 CFU/g) and the environmental samples (1.3 × 103–5.8 × 107 CFU/g). Similar to the results of contamination in spring and summer, the wooden boxes were the most contaminated with aerobic (1.8 × 107–5.8 × 107 CFU/100 cm2) and coliform bacteria (3.6 × 105–5.4 × 105 CFU/100 cm2) (Table 4).
For fishery auction market B, aerobic bacteria were detected in the majority of fishery products (3.9 × 10–1.3 × 103 CFU/g) and environmental samples (1.5 × 102–5.2 × 107 CFU/mL or /100 cm2) in spring (March–April). Among the environmental samples, aerobic bacteria were at the highest levels in the wooden boxes (1.1 × 106–5.2 × 107 CFU/100 cm2), followed by the plastic boxes (5.8 × 103–1.1 × 104 CFU/100 cm2), ice in the boxes (1.4 × 103 CFU/mL), and water in the tanks (1.5 × 102–1.1 × 103 CFU/mL) at the fishery auction market. In addition, aerobic bacteria were detected in the monkfish (9.0 × 10–1.2 × 103 CFU/g) and flatfish (3.9 × 10–1.3 × 103 CFU/g). Coliform were detected only in the monkfish (1.1 × 102 CFU/g) and the wooden box for fish storage (2.5 × 102 CFU/100 cm2). However, E. coli and V. cholerae were below the limit of detection in all fishery products and environmental samples (Table 2). In summer (June–July), aerobic (fishery products: 1.4 × 102–1.1 × 106 CFU/g, environmental samples: 1.4 × 102–1.3 × 106 CFU/mL or /100 cm2) and coliform bacteria (fishery products: 4.2 × 10–1.2 × 105 CFU/g, environmental samples: 1.4 × 102–4.0 × 105 CFU/mL or /100 cm2) were detected in higher quantities, compared to the samples in spring. E. coli and V. cholerae were below the limit of detection (Table 3). In fall (September), aerobic (fishery products: 2.3 × 104–2.7 × 105 CFU/g, environmental samples: 9.8 × 102–1.3 × 108 CFU/mL or /100 cm2) and coliform bacteria (fishery products: 3.7 × 102–5.2 × 104 CFU/g, environmental samples: 3.3 × 102–3.4 × 104 CFU/mL or /100 cm2) were similar to the samples from summer. E. coli were below the limit of detection (Table 4). Meanwhile, V. cholerae non-O1 were detected in the hairtail (200 MPN/100 g), croaker (40–110 MPN/100 g), flatfish (20 MPN/100 g), large-eyed herring (45 MPN/100 g), water in the tanks at the fishery auction market (20 MPN/100 mL), and the plastic boxes (20 MPN/100 cm2) in fall, which was little bit higher than market A sample numbers for V. cholerae presence (Table 4).
The seasonal differences in microbial contamination for fishery products and environmental samples at two fishery auction markets were observed. Aerobic bacteria were detected in most fishery products and environmental samples in all seasons (spring, summer, and fall). Coliform was detected in most samples in fall and summer, followed by spring. E. coli and V. cholerae O1 were not detected in any sample collected in all seasons (spring, summer, and fall). Meanwhile, V. cholerae non-O1 of the fishery products (20−5,400 MPN/100 g in the snail, hairtail, croaker, flatfish, and pilchard) and the environmental samples (20 MPN/100 mL or /100 cm2 in water and plastic boxes) were detected only in fall (Tables 2, 3, and 4). V. cholerae detected in the fishery products may have been contaminated by seawater, as cross-contamination between these products and environmental samples in fishery auction markets can occur. Aerobic, coliform, and E. coli bacteria are hygiene indicator microorganisms for sanitary quality. Vibrio spp. are a cause of foodborne illness caused by the consumption of fishery products. V. cholerae is a pathogen in marine environments which causes cholera by producing the cholera toxin (CT), a vital virulence factor. V. cholerae O1 and O139 are representative serotypes (Halpern and Izhaki 2017). Although the isolates in this study were identified as V. cholerae non-O1, and most V. cholerae non-O1 do not produce this toxin, it has been reported as the third most common group of Vibrio bacteria that causes diarrheal disease (CDC 2019). The prevalence of Vibrio in fishery products may be affected as the sea surface temperature of S. Korea continues to increase, having increased by 1.1 °C over the last 50 years (East Sea 1.7 °C, West Sea 0.3 °C, and South Sea 1.4 °C increase) (NIFS 2019). Chávez et al. (2005) and Singleton et al. (1982) suggest that warm temperatures may influence the occurrence of V. cholerae O1 and non-O1. Thus, a detection rate of V. cholerae in fishery products will be gradually increased.
Little increase was observed in the bacterial cell counts (aerobic and coliform bacteria) of the fishery products (flounder, monkfish, flatfish, rock cod, sea bass, snail, hairtail, croaker, and pilchard), as the time period of the fishery auction (before, during, and after auction) progressed (Tables 2, 3, and 4). The bacterial cell counts in the fishery products may increase as temperature increases, and fishery products can be cross-contaminated by storage facilities (wooden or plastic boxes) that have not been decontaminated. Coliform in the wooden boxes were detected in spring (6.0 × 10–2.5 × 102 CFU/100 cm2), summer (2.5 × 104–4.0 × 105 CFU/100 cm2), and fall (9.6 × 103–4.3 × 105 CFU/100 cm2) (Tables 2, 3, and 4). Therefore, the replacement or decontamination of storage facilities at fishery auction markets is required to prevent cross-contamination. In particular, the bacteria in wooden boxes could accumulate if the boxes are not decontaminated to be microbiologically safe.
Conclusions
In conclusion, V. cholerae can be detected in fall and can cross-contaminate between the fishery products and environmental factors such as water and storage boxes in the fishery auction markets. Therefore, food safety practices at fishery auction markets such as the frequent replacement and decontamination of storage facilities and tools should be performed to prevent foodborne disease outbreaks. Overall, the results of this study may be useful in establishing food safety practices for fishery auction markets in S. Korea.
Availability of data and materials
All datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APW:
-
Alkaline peptone water
- MPN:
-
Most probable number
References
Ahmed FE. Seafood safety. Institute of medicine (US) committee on evaluation of the safety of fishery products. Washington (DC): National Academies Press (US); 1991.
CDC (Centers for Disease Control and Prevention) (2019) Non-O1 and Non-O139 Vibrio cholerae infections. Available at: https://www.cdc.gov/cholera/non-01-0139-infections.html. Accessed 17 Apr 2019.
Chávez MDRC, Sedas VP, Borunda EO, Reynoso FL. Influence of water temperature and salinity on seasonal occurrences of Vibrio cholerae and enteric bacteria in oyster-producing areas of Veracruz, México. Mar Pollut Bull. 2005;50:1641–8.
Cho YG, Lee CB, Choi MS. Geochemistry of surface sediments off the southern and western coasts of Korea. Mar Geol. 1999;159:111–29.
Chough SK, Lee HJ, Yoon SH. Marine geology of Korean seas. 2nd ed. Amsterdam: Elsevier Science; 2000. p. 328.
Dewey-Mattia D, Manikonda K, Hall AJ, Wise ME, Crowe SJ. Surveillance for foodborne disease outbreaks-United States, 2009–2015. MMWR Surveill Summ. 2018;67:1–11. https://doi.org/10.15585/mmwr.ss6710a1.
Di DY, Lee A, Jang J, Han D, Hur HG. Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Appl Environ Microbiol. 2017;83:e02680–16.
FAO (Food and Agriculture Organization of the United Nations). The state of world fisheries and aquaculture 2018-Meeting the sustainable development goals. Rome: Licence: CC BY-NC-SA 3.0 IGO; 2018.
FAO (Food and Agriculture Organization of the United Nations). Globefish-Information and analysis on world fish trade. Republic of Korea; 2019. Available at: http://www.fao.org/in-action/globefish/countries/countries/kor/republic-of-korea-trade/en/. Accessed 17 Apr 2019
FDA (U.S. Food and Drug Administration) (2010) Bacteriological analytical manual (BAM) appendix 2: most probable number from serial dilutions. Available at: https://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm109656.htm#references. Accessed 19 Jan 2019.
Halpern M, Izhaki I. Fish as hosts of Vibrio cholerae. Front Microbiol. 2017;8:282.
Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:e157–66.
KCDC (Korea Centers for Disease Control and Prevention). (2017) Epidemiological investigation of infectious diseases in Korea. Annual report 2016.
Kim HJ, Ryu JO, Lee SY, Kim ES, Kim HY. Multiplex PCR for detection of the Vibrio genus and five pathogenic Vibrio species with primer sets designed using comparative genomics. BMC Microbiol. 2015;15:239.
Kim JH, Lee J, Hong S, Lee S, Na HY, Jeong YI, Choi EJ, Kim J, Kawk HS, Cho E. Cholera outbreak due to raw seafood consumption in South Korea, 2016. Am J Trop Med Hyg. 2018;99:168–70.
Koh CH, Shin HC. Environmental characteristics and distribution of macrobenthos in a mudflat of the west coast of Korea (Yellow Sea). Neth J Sea Res. 1988;22:279–90.
KREI (Korea Rural Economic Institute). (2017) 2016 Food balance sheet. Available at: http://library.krei.re.kr/pyxis-api/1/digital-files/5251e7c7-2191-4284-8f7c-5fe8eb93d0c5. Accessed 18 Apr 2019.
Labbé RG, García S. Guide to foodborne pathogens. 2nd ed. Hoboken: Wiley; 2013. p. 488.
Maheshwari M, Nelapati K, Kiranmayi B. Vibrio cholerae-a review. Vet World. 2011;4:423–8.
Nandi B, Nandy RK, Mukhopadhyay S, Nair GB, Shimada T, Ghose AC. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J Clin Microbiol. 2000;38:4145–51.
NIFS (National Institute of Fisheries Science). (2019) Statistics for sea temperature of South Korea. Available at: http://www.nifs.go.kr/femo/data_statistics.femo. Accessed 26 Mar 2019.
Rajpara N, Vinothkumar K, Mohanty P, Singh AK, Singh R, Sinha R, Nag D, Koley H, Bhardwaj AK. Synergistic effect of various virulence factors leading to high toxicity of environmental V. cholerae non-O1/non-O139 isolates lacking ctx gene: comparative study with clinical strains. PloS One. 2013;8:e76200.
Reidl J, Klose KE. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev. 2002;26:125–39.
Singleton FL, Attwell R, Jangi S, Colwell RR. Effects of temperature and salinity on Vibrio cholerae growth. Appl Environ Microbiol. 1982;44:1047–58.
Acknowledgements
This research was supported by a grant (17162MFDS652) from the Ministry of Food and Drug Safety in 2017.
Funding
This research was supported by a grant (17162MFDS652) from the Ministry of Food and Drug Safety in 2017.
Author information
Authors and Affiliations
Contributions
ISS and YY participated in the design of this study. YC and YL carried out the sample collection and data analysis. JL, JH, and HO helped to analyze the data, and SL and SK helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Choi, Y., Lee, Y., Lee, S. et al. Microbial contamination including Vibrio cholerae in fishery auction markets in West Sea, South Korea. Fish Aquatic Sci 22, 26 (2019). https://doi.org/10.1186/s41240-019-0140-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41240-019-0140-5