Abstract
Magnesium hydrogen phosphate (MHP, MgHPO4) recovered from swine manure was prepared as an alternative phosphorus (P) source. Conventional P additives, monocalcium phosphate (MCP), dicalcium phosphate (DCP), and tricalcium phosphate (TCP) were compared with the MHP in terms of growth and P availability by juvenile carp Cyprinus carpio. A basal diet as a negative control was prepared using practical feed ingredients without P supplementation to which four supplemental P sources were added at the level of 2%. Five groups of 450 fish having mean body weight of 6.5 g following 24 h fasting after 2 weeks of adaptation period were randomly distributed into each of 15 tanks (30 fish/tank). Fish were hand-fed to apparent satiety twice a day for 9 weeks. Fish fed the MHP had weight gain (WG), feed conversion ratio (FCR), protein efficiency ratio (PER), and specific growth rate (SGR) comparable to those fed the MCP. Those values of both the MHP and MCP groups were significantly different (p < 0.05) from the other groups. Fish groups fed control and the TCP showed the lowest WG, PER, and SGR and the highest FCR among treatments. No fish were died among treatments during the experimental period. Fish fed control and the TCP showed hematocrit and hemoglobin significantly lower (p < 0.05) than fish fed the MHP. The lowest inorganic P (Pi) in plasma was found in the control group. Even though Pi was not significantly different (p > 0.05) from other phosphate groups, fish fed the MCP and MHP retained higher P in whole body than the other groups. P availability was determined to be 93.2, 62.4, 6.1, and 98.0% for MCP, DCP, TCP, and MHP, respectively. The present results suggested that the MHP recovered from wastewater stream could be used as an alternative P source in carp diet.
Similar content being viewed by others
Background
Phosphorus (P) is a component of organic phosphate in diverse forms, such as nucleotides, phospholipids, coenzymes, deoxyribonucleic acid, and ribonucleic acid. Inorganic phosphates also serve as important buffers to maintain normal pH of intra- and extra-cellular fluids (Zubay 1983). Fish have the dietary requirement of P like other animals for growth. The requirement was reported to range from 0.3 to 0.6% for channel catfish (Wilson et al. 1982), rainbow trout (Rodehutscord and Pfeffer 1995), striped bass (Brown et al. 1993; Dougall et al. 1996), and white fish (Vilema et al. 2002). However, somewhat higher requirement value of 0.7 to 0.9% was estimated for haddock (Roy and Lall 2003), red tilapia (Phromkunthong and Udom 2008), African catfish (Nwanna et al. 2009), red drum (Davis and Robinson 1987), yellow croaker (Ma et al. 2006), and carp (Kim et al. 1998). On the other hand, P discharged into water stream as a major nutrient for eutrophication is known to play a vital role in promoting algal growth (Beveridge 1984; Auer et al. 1986).
Theoretically, diet for fish should contain all essential nutrients including P above the needs and maintain total P levels as low as its available requirements in order to achieve both maximal growth and minimum P discharge. However, the practical diet contains an excessive P originating from animal and plant sources, which is excreted to the cultural water body due to the low availability by stomachless species like carp (Kim and Ahn 1993). Therefore, soluble P sources like monocalcium phosphate (MCP) and dicalcium phosphate (DCP) are being supplemented to the diet to meet the requirement of P for maximum growth. Based on this point of views, some significant discharge of P is inevitable from fish farming. Kim et al. (1998) reported dietary available P of 0.7% with 2% MCP exerted both maximal growth and minimum P loss in juvenile carp. An adequate combination of low-P protein meals supplemented with 0.5% MCP significantly reduced the P loading on rainbow trout without compromising the growth (Satoh et al. 2003; Hernandez et al. 2004, 2005).
Such an important source for living creatures as one of non-renewable elements in the nature is mostly produced from phosphate rock of which production in the world has constantly increased from 198 million metric tons in 2011 to 210 million metric tons in 2012 according to U.S. Geological Survey (USGS 2013). Shu et al. (2006) expected all reserves of phosphate rock would be depleted by 2090, assuming an annual increase of 1.5% in its demand. Considering this estimation, it is necessary to recover phosphorous from diverse downstream of agricultural and industrial fields. One of resources to retrieve phosphorous could be swine manure which contains high levels of phosphorous and nitrogen. Swine manure becomes a source of pollution on surface waters and induces eutrophication near the site when it is under improper treatment. Thus, the control of wastewater stream must be achieved and struvite precipitation could be an effective way to control phosphorus from wastewater stream with the addition of magnesium (Liu et al. 2011). If this alternative phosphate recovered from swine manure could be effectively employed as an available P source for animals including fish, it would not only substitute for import of several phosphates but also protect our environment through recycling of the waste source.
Cyprinid is the species the most cultured worldwide. In 2012, China produced more than 90% of the world’s carp which relies on formulated feed with the exception of filter-feeding species (Cao et al. 2015). Considering the low availability of P in both animal and plant feedstuffs for carp (NRC 1983), the amount of dietary P supplemented through its additive would be substantial. Nevertheless, the potential use of dietary alternative phosphate as an available P source for growth of the fish was not examined until now. This study was therefore carried out to investigate the supplemental effect of various conventional phosphate sources and the alternative (MHP) on growth, feed utilization, plasma inorganic P, whole body P, and the availability of juvenile carp.
Methods
The protocol for the present experiment was approved by the Institutional Animal Care and Use Committee of Kangwon National University, Chuncheon, Republic of Korea. Commercially selling phosphate additives (MCP, BIOFOS®, USA; DCP, SICHUAN MIANJHUSANJIA FEED Co., China; tricalcium phosphate (TCP), FOODCHEM, Shandong, China) were obtained from fish feed companies in Korea, and the testing P additive, magnesium hydrogen phosphate (MHP, MgHPO4), was prepared from P-recovery process in swine farm operated by Kangwon National University.
A P-recovery process was a pilot scale with the effective volume of 400 L, and the operational condition was set with hydraulic retention time of 3 h and pH 8 to 9 controlled by aeration (33 L air/m3 reactor.min) resulting in CO2 stripping. Such a condition leads to precipitate crystal called struvite which is composed of magnesium, ammonium, and phosphate in equimolar ratio. In order to provide magnesium source that is a deficient source in swine manure, magnesium chloride (MgCl2) was added to meet Mg to P ratio of approximately 1.2.
The precipitates collected was struvite, which was identified by X-ray diffractometer (XRD) (Rigaku, Model D/Max-2500 V, Japan). The MHP was obtained by removing ammonium-N through incineration of the recovered struvite at 550 °C for 30 min. It was finely ground to use as phosphate additive.
Preparation of diets
The diet was prepared with MCP, DCP, TCP, and MHP at the level of 2% but the control diet did not contain phosphate source of 2% in lieu of cellulose. The major ingredients were constituted with fish meal (25%), soybean meal (40%), wheat flour (27%), fish oil (2%), and soy oil (2%) as a basal diet containing 42.5% protein and 6.5% lipid (Table 1). Prior to diet formulation, chemical composition of fish meal, soybean meal, wheat flour, and four phosphate sources were determined. All the ingredients were weighed following the formula and ground to 100-mesh size by a hammer mill and thoroughly mixed for 10 min using a V-mixer (Hangjin Co., Korea) in order to make a mixture of 500 kg per diet. Then, the mixture was transferred to a twin extruder (Model ATX-2, Fesco Precision Co., Korea) and manufactured to the sinking pellets with two sizes of 1.5 and 2.5 mm, respectively, for feeding during the first 6- and second 3-week growth trials, respectively. Operating conditions of extrusion were as follows: feeder speed, 16~18 rpm; conditioner temperature, 80~90 °C; main screw speed, 250~320 rpm; temperature of the second and the third barrel compartment, 105~135 °C; steam heater pressure, 4~6 kgf/cm2 and temperature of the fourth barrel compartment, 80~90 °C. Extruded pellets were dried at 60 °C for 6 h using a drying oven which resulted in the moderate moisture content of 5 to 8%.
For the measurement of P digestibility, chromic oxide of 1.0% was mixed with an aliquot of 10 kg of each extruded diet fully ground. Then, each diet mixture was added with 20% distilled water, and the mixture was pelletized using a meat chopper and dried for 12 h in a ventilated oven at 60 °C. The diets were stored in a freezer at −20 °C for P digestibility measurement until the growth trials.
Growth trial
Carp fry of 5000 with around body weight of 5 g were kindly provided from Kyeongnam freshwater fish institute and acclimated to the experimental conditions for 3 weeks with a control diet. Following a 24-h fasting, five groups (triplicates/group) of 450 fish of a mean body weight of 6.6 g were randomly allotted to each of 15 tanks (0.4 × 0.6 × 0.36 cm, water volume of 66 L). The feeding experiment lasted 9 weeks during which each diet was hand-fed to apparent satiety twice a day (08:30 and 17:30) at the 4% of body weight in every 6 days per week. A recirculation freshwater system was employed where water temperatures for first, second, and third weeks were maintained at 26 ± 1.2, 22 ± 1.2, and 18 ± 1.5 °C, respectively, and dissolved oxygen 5.5~6.4 mg O2/L for the whole experimental periods. The flow rate of 5 L/min was constantly set. The extruded pellet of 2.5 mm size was fed for the last feeding of 3 weeks. Fish were bulk-weighed at the beginning of the experiment in every 3 weeks. Daily feed intake (DFI, %/av. body weight/d), weight gain (WG, %), feed conversion ratio (FCR), protein efficiency ratio (PER), specific growth rate (SGR, %), and survival rate (SR, %) were calculated as follows:
and
Digestibility trial
At the end of the growth trial, digestibility measurement was conducted to calculate the available P of the experimental diets. Following a 24-h fasting, 300 fish (mean body weight, 22.2 g) were randomly distributed into each of five 130 L capacity tanks (60 fish/tank) with a fecal collection column. Following 1 week of feeding, fecal collections were made for three consecutive weeks as described by Kim et al. (2006). Each diet was fed by hand to apparent satiety twice a day (08:30 and 16:30). One hour after final feeding of the day, the drain pipes and fecal collection columns were thoroughly cleaned with a brush to remove feed residues and feces from the system. The settled feces and surrounding water were carefully collected into 250-ml centrifuge bottles each morning (08:00). Apparent digestibility coefficient (ADC) of P in experimental diets was calculated according to Maynard and Loosli (1969). P availability of phosphate additives was calculated by dividing the difference of total P into that of available P between control and each phosphate containing diet:
where ID is % indicator in the diet, PF represents % P in the feces, IF indicates % indicator in the feces, and PD is % P in the diet.
P availability of the phosphorus additives was calculated according to the following equation:
where APDP indicates % available P in the diet containing P source, APCD is % available P in control diet, TPDP shows % total P in the diet containing P source, and TPCD is % total P in control diet.
Sample collection and analysis
At the end of the experimental period, fish were anesthetized with AQUI-S (New Zealand Ltd., Lower Hutt, NZ) and bulk-weighed and counted for calculation of WG, FCR, SGR, PER, and SR. Blood samples were obtained from the caudal vessels with a heparinized syringe from two fish of each tank after fish were starved for 24 h and anesthetized with AQUI-S. Feces collected in the same bottle from each tank for 6 days a week were used as one replicate for the treatment. After collection of three replicate samples of each diet during 3 weeks, fecal samples were lyophilized, finely ground, and frozen at −20 °C until analysis.
Chemical analyses of feed ingredient, diets, and feces were performed by the standard procedure of AOAC (1990) for moisture, crude protein, crude fat, and crude ash. Moisture content was obtained after drying in an oven at 105 °C for 24 h. Crude protein (N × 6.25) was determined by Kjeldahl method after acid digestion. Crude fat was determined by the soxhlet extraction method by using Soxtec system 1046 (Foss, Hoganas, Sweden) and crude ash from incineration in a muffle furnace at 550 °C for 12 h. Chromium in diets and feces for P digestibility measurement was analyzed using a spectrophotometer (Shimadzu, UV-120-12) at a wavelength of 440 nm after perchloric acid digestion (Bolin et al. 1952). Ca in diets and P in diets and the whole body of the final fish (five fish per replicate) were measured using inductively coupled plasma mass spectrometer (ICP-MS) (Perkin-Elmer, NexION 300D, Waltham, MA, USA) after the pretreatment of test materials following the method from US Environmental protection agency (USEPA 1996). Hematocrit (PCV, %) and hemoglobin (Hb, g/dL) were measured with the same fish (two fish per replicate) by the microhematocrit method (Brown 1980) and the cyanmethemoglobin procedure using Drabkin’s solution, respectively. Hb standard prepared from human blood (Sigma Chemical, St. Louis, MO) was employed. Blood plasma from two fish per replicate was obtained after blood centrifugation (3500×g, 5 min, 4 °C) and stored at −80 °C until inorganic P (Pi) was analyzed. The plasma Pi was measured using a blood chemical analyzer (HITACHI 7600-210, Hitachi High-Technologies Co., Ltd., Japan) with commercial clinical investigation reagent (Clinimate IP, Sekisui medical Co., Ltd., Tokyo, Japan).
Statistical analysis
Data of growth trial (initial and final fish, DFI, WG, FE, PER, SGR, and SR), P availability among both replications and treatments, whole body P, and hematological and serological parameters were analyzed using one-way analysis of variance (ANOVA), and significant differences among treatment means were compared using Duncan’s multiple range test (Duncan 1955). Prior to the analysis, homogeneity of variance of all data was verified using Cochran’s test (Sokal and Rohlf 1994). All statistical analyses were carried out using the SPSS Version 10 (SPSS 1999). Statistical significance of the differences was determined by a significant level of 5% (p < 0.05).
Results
Growth performance, whole body P, and P digestibility
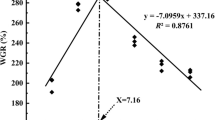
As shown in Table 1, crude protein level of dry diets was determined to be 42.2 (DCP) to 42.9% (MCP) and crude fat to be 6.6 (DCP) to 6.9% (control). At the end of the 9-week growth trial, daily feed intake (DFI, %) per averaged weight of fish ranged from 1.88 (MHP) to 2.17 (control and TCP). Fish fed the MHP showed the highest WG of 278%, which was not significantly different (p > 0.05) from that (270%) of fish fed the MCP, while fish fed the control and TCP showed lowest WG among the treatment groups (p < 0.05). FCR ranged from 0.99 (MHP) to 1.29 (control). The highest value of PER and SGR were found in fish fed the MCP and MHP, while fish fed the control and TCP showed the lowest level (Table 2). Relative weight gain (RWG) was significantly higher in DCP, MCP, and MHP compared to that in control and TCP (Fig. 1). Fish fed the control showed the lowest P content (0.36%) in whole body, while those fed the MHP the highest (0.46%) after 9-week feeding trial. (Fig. 3). The significant difference in P availability of repetition was not shown from manure analyses every week. Apparent availability of P in the experimental diets varied from 24.2 to 41.9% for TCP and MHP, respectively. The values of P were found to be 93.2, 62.4, 6.1, and 97.8% for MCP, DCP, TCP, and MHP, respectively (Table 3). The available P level in diets (Table 1) was calculated based on determined P digestibility, which ranged from 0.32 (control) to 0.66% (MCP).
Hematological parameters and plasma inorganic P
Hematological parameters and inorganic P in plasma of fish fed the experimental diets are shown in Figs. 2 and 3, respectively. PCV (%) of fish fed TCP (24.2) and control (25.5) showed a significant difference (p < 0.05) from that of fish fed MHP (28.8). Hb (g/dL) of fish ranged from 7.5 (TCP) to 8.8 (MHP), which were significantly different (p < 0.05). Significantly lower P (mg/dL) in plasma was found in fish fed the control (4.3), while the other groups did not show any significant difference (p > 0.05) each other.
Discussion
As the environmental risks has been increased by releasing animal fecal wastes including fisheries, the P-recovery from swine manure has been introduced in the form of struvite and widely studied (Yoon et al. 2015). Its application has been only limited as a fertilizer but it is much desirable to apply P recovered to new applications after appropriate manufacturing and securing safety. Magnesium hydrogen phosphate (MHP) was newly manufactured from struvite which was to recycle P from swine manure. It is first attempt to investigate the effect of the MHP as dietary P source on growth and feed utilization of carp. Based on the earlier study (Kim et al. 1998), who found that 2% of MCP in diet for carp improved growth and decreased P loads, the same level of various P sources was incorporated into the experimental diets (Table 1). Fish fed the MHP showed the best WG at the end of growth trial, although it was not significantly different (p > 0.05) from that of fish fed the MCP. Same tendencies were found in FCR, PER, and SGR (Table 2). The result suggests that juvenile carp could utilize P from MHP as effectively as MCP. From the results, it was evident that MHP was a good P source competitive with MCP in terms of WG and FCR in juvenile carp. As shown in Fig. 1, RWG of MCP, DCP, TCP, and MHP to control at the level of 2% in each diet were 135, 119, 103, and 139% on WG, respectively. Such relative differences among various P sources might be due to the difference in availability of P, by which the requirement could be met or not. Available P requirement is known to be 0.6~0.7% (Ogino and Takeda 1976; Kim et al. 1998) for carp. The present study confirmed such a requirement to be optimal.
Ogino et al. (1979) extensively studied the P availability of inorganic P sources and various feed ingredients by fish. They reported that the availability of MCP, DCP, and TCP was 94, 46, and 13 by carp, respectively, using egg albumin-based diet. Though somewhat higher values were obtained for DCP and TCP in the present study, the value for MCP corresponds well with their result. On the other hand, MHP had the higher P availability than MCP, suggesting its potential use for carp. Similar results were reported in the previous study conducted using juvenile catfish (Yoon et al. 2014). They found that MHP (90.9%) had P availability comparable to MCP (88.1%) at 2% incorporation level in diet. Sarker et al. (2009) determined P availability of different P sources by yellowtail using albumin-based diet. They incorporated MCP (2.7%), DCP (3.7%), and TCP (3.6%) into the respective diets and obtained P availabilities of 92.4, 59.2, and 48.8% for MCP, DCP, and TCP, respectively. Lovell (1978) reported that the availability was found to be 94 and 65% for reagent grade MCP and DCP, respectively, in channel catfish. On the other hand, Eya and Lovell (1997) determined the net absorption of P from various P sources using all-plant basal diet in channel catfish. They obtained the values of 81.2, 74.8, and 54.8% for MCP, DCP, and TCP, respectively.
To our knowledge, this is the first time P availability of feed grade MCP, DCP, and TCP as well as MHP was determined for juvenile carp. In the present study, P availabilities of both MCP and DCP were found to be high while that of TCP was very low (Table 3). P availability of MCP seems to be comparable to those reported by Ogino et al. (1979), Lovell (1978), Kim et al. (1997), and Sarker et al. (2009). However, it was higher than those reported by Pimentel-Rodrigues and Oliva-Teles (2007) and Eya and Lovell (1997). The availability of DCP was comparable to those obtained in yellowtail (Sarker et al. 2009) and European sea bass (Pimentel-Rodrigues and Oliva-Teles 2007), while it was lower than those obtained in channel catfish (Lovell 1978), rainbow trout (Ogino et al. 1979), and far eastern channel catfish (Yoon et al. 2014). On the other hand, reported P availability of TCP by stomach fish ranged from 42% (Pimentel-Rodrigues and Oliva-Teles 2007) to 64% (Ogino et al. 1979). In contrast, the value from the present study was as low as that obtained by carp, stomachless species (Ogino et al. 1979). Bioavailability of dietary P is influenced by several factors including chemical form, digestibility of diet, particle size, interaction with other nutrients, feed processing, and water chemistry (Lall 1991). It remains to be explained whether such discrepancies in P availability of various P sources, especially TCP, are due to the differences in species and diet composition employed for the digestibility measurement as well as methodological approach in fecal collection (Kim et al. 1996). On the other hand, P availability of MHP was higher than those of MCP obtained from carp and rainbow trout by Ogino et al. (1979) and from yellowtail by Sarker et al. (2009), suggesting that MHP could be a potential P source for fish.
Hematological and serological parameters are useful in monitoring the physiological status of fish and as indicators of the health of the aquatic environment, although they are not routinely used in fish disease diagnosis (El-Sayed et al. 2007). Hematocrit (PCV, %) provides an indirect measurement of the body’s oxygen carrying ability, while Hb (g/dL) a direct measurement of the oxygen carrying capacity of the blood (McClatchey 2002). It was reported that hematological parameters could be influenced by nutritional status (Spannhof et al. 1979), infectious disease (Barham et al. 1980; Iwama et al. 1986), environmental changes (Giles et al. 1984), and stress (Ellsaesser and Clem 1986). The normal ranges of healthy adult Atlantic salmon were reported 44 to 49 and 8.9 to 10.4 for PCV and Hb, respectively (Sandnes et al. 1988). Somewhat lower values for cichlid fish were reported by V´azquez and Guerrero (2007), which were 22.5 to 39.2 for PCV and 5.2 to 8.3 for Hb. Recently, Rahimnejad and Lee (2013) reported 30.7 to 34.3 for PCV and 4.4 to 5.4 for Hb of red sea bream fed various dietary valine levels. Our findings for the parameters are in good agreement with those obtained from tilapia (Hrubec et al. 2000) and striped bass (Hrubec et al. 2001), although there is no available information on the effect of dietary available P on the parameters of fish. In the present study (Fig. 2), PCV and Hb of fish groups fed low available P in diet (control and TCP) were significantly lower than the MHP group (p < 0.05). Differently from the present results, Yoon et al. (2014) observed that dietary low P resulted in PCV significantly lower but did not affect Hb. An increase in available P in diet resulted in an increase in plasma Pi (Vielma and Lall 1998; Bureau and Cho 1999; Avila et al. 2000). A clear evidence was observed by Yoon et al. (2014) that plasma Pi increased in fish fed MCP and MHP with higher available P, resulting in the significant improvement in WG by meeting dietary P need. However, any significant difference in plasma Pi was not found in fish groups fed diets with various P sources (p > 0.05) in the present study. Nevertheless, P in whole body was greatly affected by dietary available P levels (Fig. 3), suggesting successive P intake through diet maintains plasma Pi constant then deposition in skeletal tissues occurs when P requirement is met. More researches remain to be clarify the difference in plasma Pi level observed in previous (Yoon et al. 2014) and present studies.
Conclusions
In conclusion, the potential use of MHP recovered from swine manure was proven sufficiently to replace MCP as an alternative P source with respect to WG and FCR as well as P availability. “Such a re-use of P from swine manure could positively influence on the development of other useful sources from livestock manure.”
Abbreviations
- DCP:
-
Dicalcium phosphate
- DF:
-
Daily feed intake
- FCR:
-
Feed conversion ratio
- Hb:
-
Hemoglobin
- MCP:
-
Monocalcium phosphate
- MHP:
-
Magnesium hydrogen phosphate
- P:
-
Phosphorus
- PCV:
-
Hematocrit
- PER:
-
Protein efficiency ratio
- Pi:
-
Inorganic P
- RWG:
-
Relative weight gain
- SGR:
-
Specific growth rate
- SR:
-
Survival rate
- TCP:
-
Tricalcium phosphate
- USGS:
-
U.S. Geological Survey
- WG:
-
Weight gain
- XRD:
-
X-ray diffractometer
References
AOAC. Official Methods of Analysis. Association of Official Analytical Chemists:Arlington, VA, USA;1990. p. 1298.
Auer MT, Kiesser MS, Canale RP. Identification of critical nutrient levels through field verification of models for phosphorus and phytoplankton growth. Can J Fish Aquat Sci. 1986;43:379–88.
Avila EM, Tu H, Basantes S, Ferraris RP. Dietary phosphate regulates intestinal transport and plasma concentrations of phosphate in rainbow trout. J Comp Physiol. 2000;170B:210–09.
Barham WT, Smit GL, Schoonbee HJ. The haematological assessment of bacterial infection in rainbow trout, Salmo gairdneri Richardson. J Fish Biol. 1980;17:275–81.
Beveridge MCM. Cage and pen fish farming: carrying capacity models and environmental impact. FAO Fish Tech Pap. 1984;255:131.
Bolin DW, King RP, Klosrerman EW. A simplified method for the determination of chromic oxide (Cr2O3) when used as an inert substance. Science. 1952;116(3023):634–5.
Brown BA. Routine hematology procedures, Hematology: Principles and Procedures. Philadelphia: Lea and Febiger; 1980. p. 71–112.
Brown ML, Jaramillo Jr F, Gatlin III DM. Dietary phosphorus requirement of juvenile sunshine bass, Morone chrysops × M. saxatilis. Aquaculture. 1993;113:355–63.
Bureau DP, Cho CY. Phosphorus utilization by rainbow trout (Oncorhynchus mykiss): estimation of dissolved phosphorus waste output. Aquaculture. 1999;179:127–40.
Cao L, Naylor R, Henriksson P, Leadbitter D, Metian M, Troell M, Zhang W. China’s aquaculture and the world’s wild fisheries. Science. 2015;347:133–5.
Davis DA, Robinson EH. Dietary phosphorus requirement of juvenile red drum Sciaenops ocellatus. J World Aquacult Soc. 1987;18:129–36.
Dougall DS, Wood III LC, Douglass LW, Soares JH. Dietary phosphorus requirement of juvenile striped bass Morone saxatilis. J World Aquacult Soc. 1996;27:82–91.
Duncan DB. Multiple range and multiple ‘F’ tests. Biometrics. 1955;11:1–42.
Ellsaesser CF, Clem LW. Haematological and immunological changes in channel catfish stressed by handling and transport. J Fish Biol. 1986;28:511–21.
El-Sayed YS, Saad TT, El-Bahr SM. Acute intoxication of deltamethrin in monosex Nile tilapia, Oreochromis niloticus with special reference to the clinical, biochemical and haematological effects. Environ Toxicol Phamacol. 2007;24:212–7.
Eya JC, Lovell RT. Net absorption of dietary phosphorus from various inorganic sources and effect of fungal phytase on net absorption of plant phosphorus by channel catfish Ictalurus punctatus. J World Aquacult Soc. 1997;28:386–91.
Giles MA, Majewski HS, Hobden B. Osmoregulatory and haematological responses of rainbow trout (Salmo gairdneri) to extended environmental acidification. Can J Fish Aquat Sci. 1984;41:1686–94.
Hernandez A, Satoh S, Kiron V, Watanabe T. Phosphorus retention efficiency in rainbow trout fed diets with low fish meal and alternative protein ingredients. Fish Sci. 2004;70:580–6.
Hernandez A, Satoh S, Kiron V. Effect of monocalcium phosphate supplementation in a low fish meal diet for rainbow trout based on growth, feed utilization, and total phosphorus loading. Fish Sci. 2005;71:817–22.
Hrubec TC, Cardinale JL, Smith SA. Hematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Vet Clin Pathol. 2000;29:7–12.
Hrubec TC, Smith SA, Robertson JL. Age related changes in haematology and chemistry values of hybrid striped bass chrysops Morone saxatilis. Vet Clin Pathol. 2001;30:8–15.
Iwama GK, Greer GL, Randall DJ. Changes in selected haematological parameters in juvenile Chinook salmon subjected to a bacterial challenge and a toxicant. J Fish Biol. 1986;28:563–72.
Kim JD, Ahn KH. Effects of monocalcium phosphate supplementation on phosphorus discharge and growth of carp (Cyprinus carpio) grower. Asian Australas J Anim Sci. 1993;6:521–6.
Kim JD, Kim KS, Song JS, Lee JY, Jeong KS. Optimum level of dietary monocalcium phosphate based on growth and phosphorus excretion of mirror carp, Cyprinus carpio. Aquaculture. 1998;161:337–44.
Kim JD, Kim KS, Song JS, Jeong KS, Woo YB, Choi NJ, Lee JY. Comparison of feces collection methods for determining apparent phosphorus digestibility of feed ingredients in growing mirror carp (Cyprinus carpio). Korean J Anim Nutr Feed. 1996;20:201–6.
Kim JD, Kim KS, Lee SB, Jeong KS. Nutrient and energy digestibilities of various feedstuffs fed to Israeli strain of growing common carp (Cyprinus carpio). J Aquacult. 1997;10:327–34.
Kim JD, Tibbets SM, Milley JE, Lall SP. Effect of the incorporation level of herring meal into test diet on apparent digestibility coefficients for protein and energy by juvenile haddock, Melanogrammus aeglefinus L. Aquaculture. 2006;258:479–86.
Lall SP. Digestibility, metabolism and excretion of dietary phosphorus in fish. In: Cowey CB, Cho CY, editors. Nutritional Strategies and Aquaculture Wastes. Ontario: University of Guelph; 1991. p. 21–36.
Liu YH, Kumar S, Kwag JH, Kim JH, Kim JD, Ra CS. Recycle of electronically dissolved struvite as an alternative to enhance phosphate and nitrogen recovery from swine wastewater. J Hazard Mater. 2011;195:175–81.
Lovell RT. Dietary phosphorus requirements of channel catfish. Trans Am Fish Soc. 1978;107:617–21.
Ma K, Zhang C, Ai Q, Duan Q, Xu W, Zhang L, Luifu Z, Tan B. Dietary phosphorus requirement of large yellow croaker, Pseudosciaena crocea R. Aquaculture. 2006;251:346–53.
Maynard LA, Loosli JK. Animal nutrition. 6th ed. New York: McGraw-Hill; 1969. p. pp 613.
McClatchey KD. Clinical laboratory medicine. Baltimore: Williams & Wilkins; 2002. p. 833–9.
NRC. Nutrient requirements of warmwater fishes and shellfishes. Washington: National Academy Press; 1983.
Nwanna LC, Adebayo IA, Omitoyin BO. Phosphorus requirements of African catfish, Clarias gariepinus, based on broken-line regression analysis methods. Science Asia. 2009;35:227–33.
Ogino C, Takeda H. Mineral requirements in fish-III calcium and phosphorus requirements in carp. Bull Jap Soc Sci Fish. 1976;42:793–9.
Ogino C, Takeuchi L, Takeda H, Watanabe T. Availability of dietary phosphorus in carp and rainbow trout. Bull Jap Soc Sci Fish. 1979;45:1527–32.
Phromkunthong W, Udom U. Available phosphorus requirement of sex-reversed red tilapia fed all-plant diets. Songklanakarin J Sci Technol. 2008;30:7–16.
Pimentel-Rodrigues A, Oliva-Teles A. Phosphorus availability of inorganic phosphates and fish meal in European sea bass (Dicentrarchus labrax L.) juveniles. Aquaculture. 2007;267:300–7.
Rahimnejad S, Lee KJ. Dietary valine requirement of juvenile red sea bream Pagrus major. Aquaculture. 2013;416–417:212–8.
Rodehutscord M, Pfeffer E. Requirement for phosphorus in rainbow trout (Oncorhynchus mykiss) growing from 50 to 200 g. Water Sci Technol. 1995;31:137–41.
Roy PK, Lall SP. Dietary phosphorus requirement of juvenile haddock (Melanogrammus aeglefinus L.). Aquaculture. 2003;221:451–68.
Sandnes K, Lie O, Waagbo R. Normal ranges of some blood chemistry parameters in adult farmed Atlantic salmon, Salmo salar. J Fish Biol. 1988;32:129–36.
Sarker PK, Fukada H, Masumoto T. Phosphorus availability from inorganic phosphorus sources in yellowtail (Seriola quinqueradiata Termminck and Schlegel). Aquaculture. 2009;289:113–7.
Satoh S, Hernandez A, Tokoro T, Morishita Y, Kiron V, Watanabe T. Comparison of phosphorus retention efficiency between rainbow trout (Oncorhynchus mykiss) fed a commercial diet and a low fish meal based diet. Aquaculture. 2003;224:271–82.
Shu L, Schneider P, Jegatheesan V, Johson J. An economical evaluation of phosphorus recovery as struvite from digester supernatant. Bioresour Technol. 2006;97:2211–6.
Sokal RR, Rohlf FJ. Nested analysis of variance, Biometry. New York: Freeman W. H; 1994. p. 272–342.
Spannhof L, Nasev D, Kreutzmann HL. Early recognition of metabolic disturbances in trout (Salmo gairdneri Rich.) stocks. Aquaculture. 1979;18:317–23.
SPSS Inc. SPSS Base 10.0 for Windows User's Guide. Chicago: SPSS Inc; 1999.
USEPA (US Environmental protection agency). 1996. Acid digestion of sediments, sludge, and solids, Method 3050B, Revision 2 (December 1996). Available at: https://www.epa.gov/sites/production/files/2015-06/documents/3050b.pdf.
USGS (US Geological survey). 2013. Mineral commodity summaries. Digital Data Series. Available at: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf.
V´azquez GR, Guerrero GA. Characterization of blood cells and hematological parameters in Cichlasoma dimerus (Teleostei, Perciformes). Tissue Cell. 2007;39:151–60.
Vilema J, Koskela J, Ruohonen K. Growth, bone mineralization, and heat and low oxygen tolerance in European whitefish (Coregonnus lavaretus L.) fed with graded levels of phosphorus. Aquaculture. 2002;212:321–33.
Vielma J, Lall SP. Control of phosphorus homeostasis of Atlantic salmon (Salmo sala) in fresh water. Fish Physiol Biochem. 1998;19:83–93.
Wilson RP, Robinson EH, Gatlin III DM, Poe WE. Dietary phosphorus requirement of channel catfish. J Nutr. 1982;112:1197–202.
Yoon TH, Lee DH, Won SG, Ra CS, Kim JD. Effects of dietary supplementation of magnesium hydrogen phosphate (MgHPO4) as an alternative phosphorus source on growth and feed utilization of juvenile far eastern catfish (Silurus asotus). Asian Australas J Anim Sci. 2014;27:1141–9.
Yoon TH, Lee DH, Won SG, Ra CS, Kim JD. Optimal incorporation level of dietary alternative phosphate (MgHPO4) and requirement for phosphorus in Juvenile far eastern Catfish (Silurus asotus). Asian Australas J Anim Sci. 2015;28:111–9.
Zubay GL. Biochemistry. Reading: MA, Addison-Wesley Publishing Company, Inc.; 1983. p. pp 388.
Acknowledgements
We are grateful to the funding by Rural Development Administration, Korea. Also, this study was partially supported by 2015 Research Grant from Kangwon National University.
Funding
This study was supported by the grant from Rural Development Administration, Korea (PJ008390) for the design of study, experimental procedures, and interpretation of data derived from this study. In addition, this study was partially supported by 2015 Research Grant from Kangwon National University (No. 520150143) for the experiments in this manuscript.
Availability of data and materials
We authors do not think that there is not any further additional supporting data that will be shared. All relevant data have been used and presented in the tables and figures in this manuscript.
Authors’ contributions
JDK designed this study, led in analyzing data derived from this study, and wrote this manuscript. THY mainly analyzed the data from this study and wrote this manuscript. SW, DHL, JWC, and CR participated in analyzing and writing this manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yoon, TH., Won, S., Lee, DH. et al. Effect of a new phosphorus source, magnesium hydrogen phosphate (MHP) on growth, utilization of phosphorus, and physiological responses in carp Cyprinus carpio . Fish Aquatic Sci 19, 39 (2016). https://doi.org/10.1186/s41240-016-0038-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41240-016-0038-4