Abstract
Background
Patients’ loss to follow-up (LTFU) from tuberculosis treatment and care is a growing worry in Ethiopia. But, available information is inadequate in assessing the time to tuberculosis patient loss to follow-up difference between health centers and a general hospital in Ethiopia. We aimed to assess time to LTFU difference between health centers and a general hospital in rural Ethiopia.
Methods
We conducted a retrospective cohort study from September 2008 to August 2015 and collected data from September 1 to October 02, 2016. A total of 1341 TB patients with known treatment outcomes were included into the study. Log rank test was used to compare the difference in time to TB patient loss to follow-up between health centers and a general hospital, whereas Cox proportional hazard model was used to assess factors associated with time to loss to follow-up in both settings.
Results
We reviewed a total of 1341 patient records, and the overall follow-up time was 3074.7 and 3974 person months of observation (PMOs) for TB patients followed at health centers and a general hospital, respectively. The incidence of loss to follow-up rate was 27.3 per 1000 PMOs and 9.6 per 1000 PMOs, at health centers and a general hospital, respectively. From the overall loss to follow-ups that occurred, 55 (65.5%) and 33 (86.8%) of LTFUs occurred during the intensive phase and grew to 78 (92.9%) and 38 (100%) at health center and a general hospital, respectively, at the end of 6-month observation period. Older age (AOR = 1.7, 95%CI, 1.2–2.5, P < 0.001), being a rural resident (AHR = 2.7, 95%CI, 1.6–4.6), HIV reactive (AHR = 2.2, 95%CI, 1.5–3.2), following treatment and care in health center (AHR = 3.38, 95%CI, 2.06–5.53), and living at more than 10 km away from the health facility (AHR = 3.4, 95%CI, 2.1–5.7) were predictors for time to loss to follow-up among TB patients on treatment and care.
Conclusion
Time to TB patient loss to follow-up between health centers and a general hospital was significant. Loss to follow-up was high in patients with older age, rural residence, sero positive for HIV, living further from the health facilities, and following treatment and care at health centers. Strengthening the DOTs program with special emphasis on health centers is highly recommended.
Similar content being viewed by others
Introduction
Although, highly efficacious treatment was available for decades, tuberculosis (TB) remains a major global public health problem [1]. In 2017, 10.5 million new cases and 1.5 million deaths were reported due to TB [1, 2]. Sub-Saharan Africa (SSA) accounts for the lion’s share of TB deaths, TB–HIV co-infections, and TB–HIV deaths. One underlying reason is the relatively low coverage and quality of TB prevention, treatment, and care programs in Africa [3, 4].
Ethiopia is among the 22 high burden TB countries and ranked tenth globally and fourth in Africa [2, 5]. Although there are remarkable achievements in the reduction of TB mortality from 2004 to 2014, there were an estimated 30,000 mortalities per annum and more than 80 TB associated deaths every day in Ethiopia during the same reporting period due to uncontrolled loss to follow-up [6, 7]. Loss to follow-up is believed to be the most significant indicator for a high burden of multidrug-resistant tuberculosis (MDR-TB). According to a recent systematic-review report, the pooled estimate of MDR-TB among new and previously treated cases attributable to loss to follow-up was 2% and 15%, respectively [8]. Even though about 85% of TB cases in Ethiopia occur in rural settings, the timing in loss to follow-up of TB patients following treatment and care in health facilities differing by their level of tiers remains undescribed [9]. Prior findings on TB patient loss to follow-up in Ethiopia did not consider the effect of health facilities differing in their level of service comprehensiveness on TB patients’ loss to follow-up, so it was difficult to inform the decision makers where and when to emphasize on TB patient monitoring and follow-up [10,11,12].
According to WHO, the directly observed treatment and short-course (DOTS) strategy is recommended to upgrade TB prevention and control [12] and Ethiopia has implemented this since 1991 [13, 14]. Consequently, about 92% of public hospitals and health centers in Ethiopia provide DOTS [15]. Loss to follow-up of TB patients is troubled with problems, primarily because of MDR-TB [16,17,18]. Non-adherence to complete treatment poses a substantial public health menace through disease recurrence, amplified transmission, and development of drug resistance [19, 20]. Besides the widespread expansion of DOTS services and the enormous participation of health extension workers (HEWs) in TB prevention and control activities, the patients still are failing to adhere to complete their treatment [21]. According to WHO 2017, substantial TB cases failed following some treatments and countless were relapsing and subjected to retreatment after completion of treatment [22]. The underlying reason for this was partly due to loss to follow-up of TB patients. Loss to follow-up from treatment and care is defined as a TB patient who did not start treatment or whose treatment was interrupted for two consecutive months or more [23]. In addition, the rate of loss to follow-up across different tiers of health systems is becoming recognizable currently. Understanding the time when TB patients’ loss to follow-up and the factors that predict the loss to follow-up are the bridges for developing time relevant intervention approaches. This study aimed at determining whether time to loss to follow-up differs between health centers and a general hospital; and associated factors among TB patients in Ethiopian rural health facilities.
Methods
Setting, design, and population
This is a retrospective study on TB patients registered for TB treatment from 21/09/2008 to 11/08/2015 in Sheka Zone, Ethiopia, which is located at 687 km away from the national capital, Addis Ababa. Sheka Zone is divided into three administrative districts, which are further divided into 66 kebeles, the smallest geographical administrative units in Ethiopia. Among the 66 kebeles, all six urban kebeles and 22 rural kebeles were located within 10 km distance from the nearest health facility (it applies for those only seven health centers and one general hospital included into this study) [24]. There are 14 health centers and one general hospital in the study catchment; even though during the study period only seven had health centers and one general hospital had functional laboratories for TB sputum smear microscopy and none had access to X-ray or culture facilities, only 7 health facilities were included into this study. Diagnosing and treating TB in Ethiopia is based on the Ethiopia’s national TB treatment guidelines [25]. Accordingly, a tuberculosis patient was classified as having smear-positive pulmonary TB (PTB+) if the patient has at least two initial sputum smear examinations positive for AFB by direct microscopy, or a patient has one initial smear examination positive for AFB by direct microscopy and culture positive, or a patient has one initial smear examination positive for AFB by direct microscope and radiographic abnormalities consistent with active TB as determined by a clinician. Similarly, a patient having symptoms suggestive of TB with at least three initial smear examinations negative for AFB by direct microscopy, and no response to a course of broad-spectrum antibiotics, and again three negative smear examinations by direct microscopy, and radiological abnormalities consistent with pulmonary tuberculosis, and decision by a clinician to treat with a full course of anti-tuberculosis or patient whose diagnosis is based on culture positive for M. tuberculosis but three initial smear examinations negative by direct microscopy was considered as smear-negative pulmonary TB (PTB−). Patients categorized as having extra-pulmonary tuberculosis (EPTB) were patients having TB in organs other than the lungs, proven by one culture-positive specimen from an extra-pulmonary site or histo-pathological evidence from a biopsy, or TB based on strong clinical evidence consistent with active EPTB and the decision by a physician to treat with a full course of anti-TB therapy. Patients with negative sputum smears who fail to respond to treatment with broad-spectrum antibiotics are considered to have smear-negative pulmonary TB [25, 26], although the diagnosis of smear-negative and extra-pulmonary cases also incorporates clinical judgment. Patients receive daily rifampicin, pyrazinamide, isoniazid, and ethambutol for 2 months (initial phase) followed by daily rifampicin and isoniazid for 4 months or more (continuation phase). Patients diagnosed with TB are registered in a TB unit register for DOTS at their presenting health facility; information on name, kebele of residence, age, sex, weight, type of facility (hospital or health center), sputum smear result, TB type, TB patient treatment category, HIV sero status, use of chemo prophylactic therapy (cotrimoxazole), anti-retroviral treatment (ART) status, anti-tuberculosis treatment regimen, treatment outcome, and dates of treatment initiation and treatment outcome are recorded.
All TB patients treated for TB according to the national TB treatment guidelines [25] from 2008 to 2015 and registered in the TB unit register log book with known treatment outcomes were included into the study. On the contrary, the patients with undocumented treatment outcome were excluded from the study. A total of 1504 patients had been registered in seven public health centers and one general hospital of which 161 patients were excluded from the study due to not documented status of outcomes and transferred out to other health facilities.
Sample size determination and sampling techniques
The sample size was calculated using the sample size calculations for survival analysis [27]. In this calculation, the number of event (NE) was primarily calculated followed by probability of the event (Pr(event). Accordingly, the number of events was calculated using the following formula.
Number of events (NE) = \( \frac{\left(\mathrm{Z}\frac{\alpha }{2}+\mathrm{Z}\upbeta \right)2}{\uppi 1\uppi 2\ \left(\log HR\right)2}=342 \), where \( \mathrm{Z}\frac{\alpha }{2} \) =1.96, Zβ =0.842, π1 and π2 are the proportions to be allocated to groups 1 and 2. For equal allocation, π1 = π2 = ½. Since similar studies that detect the survival time difference between health center and hospital were not available during the time this study was conducted, hazard ratio of 0.5 was considered. The probability of events was also calculated using the exponential survivorship function, S (t) = exp. {−λt}. Since, this study was a 7-year retrospective cohort (2008–2015); S (t) = exp. {− 0.7}. Accordingly, the probability of the event for group 1 was exp. {− 0.7}, which equals 0.5. Similarly, the probability of the event for group 2 was calculated using exp. {− 0.7*0.5}, since we used a hazard ratio of 0.5, and which becomes 0.7. Thus, the probability of the event becomes 1 − (0.4 + 0.7)/2 = 0.4. Thus, the final sample size was calculated using the following formula.
Sample size (n) = (\( \frac{number\ of\ events\ }{Probablity\ of\ events}\Big) \) = 855, approximately 428 for each groups. We have also applied Interim Analysis Adjustment (IAA) to make an adjustment for data incompleteness. Accordingly, deciding to use a Pocock boundary of 1.2 at power of 80 for three interim analyses plus final, we have made an adjustment as follows.
Number of adjusted events = 342*1.2 = 411 and number of adjusted sample size becomes 1028 (411/0.4). After adjusting for 10% loss to follow-up rate (1028/0.9), the final sample size becomes 1143, approximately 572 for each group. But, from the health center tuberculosis patient registration log book, we found a total of 614 eligible tuberculosis patients and 727 eligible tuberculosis patients from the general hospital tuberculosis patient follow-up log book. Accordingly, 614 patients from the health center and 727 from the general hospital were included into this study. Therefore, a total of 1341 tuberculosis patients enrolled for treatment and care from 21/09/2008 to 11/08/2015 in all public health facilities were included into the study. Since we included all available and eligible records, we did not use any sampling method.
Study variables and measurement
The dependent variable of interest was time to loss to follow-up, which was defined as patients who took treatment for at least 1 month and discontinue treatment for more than consecutive 8 weeks [24]. Records of patients registered to TB follow-up between 21/09/2008 and 11/08/2015 were reviewed to identify those who failed to keep scheduled appointments for more than 2 months. A list of these “loss to follow-up” patients was generated from medical register by observing the last treatment date. Death was considered as the death of TB patients on of follow-up in the reporting period due to any cause. A patient is cured who was initially smear-positive and who was smear-negative in the last month of treatment and on at least one previous occasion. Similarly, a patient who completed treatment but for whom smear results are not available at 7 months or 1 month prior to the completion of treatment is declared as treatment completed. Furthermore, if for whatever reason after 7 months of treatment the final sputum examination cannot be done and the sputum result at 5th month was negative or not done, the patient is declared treatment completed.
Treatment failure is declared when a patient whose sputum smear or culture is positive at 5 months or later during treatment or patients found to harbor a multidrug-resistant (MDR) strain at any point of time during the treatment, whether they are smear-negative or smear-positive. This definition applies to pulmonary smear-positive and smear-negative patients and to patients with extra-pulmonary disease. Transfer out is the official transferring of the patient to another TB clinic within or outside a catchment area [28, 29]. Independent variables included in this study were as follows: age of the TB patient, sex, distance from the nearest health facility (with in 10 km or more than 10 km distance), and residency (rural or urban) among the demographic information’s recorded for each patient. Baseline TB treatment category (new, relapse, failure, transferred in), HIV sero status (reactive, non-reactive), anti-retroviral treatment status (linked to ART, not linked to ART) status, anti-tuberculosis treatment regimen, treatment outcome, and dates of treatment initiation and treatment outcome are recorded. Data extraction tools were developed from WHO, national treatment guideline for TB treatment, and similar other studies [4, 6, 13, 24, 25, 28, 29]. To assure the data quality, the data were collected through structured data extraction formats developed from standard sources and data were collected by trained diploma nurses. Data collection process was supervised by two trained nurses holding Bachelor’s degree to assure the completeness of data.
Data processing and analysis
The completed questionnaire was cleaned, checked for completeness, coded, and edited before data entry. The data were entered to EpiData 3.1 and exported to SPSS 23 for analysis. Descriptive statistics including frequency, percentage, median, and standard deviation of the study variables were computed. Cox proportional hazard model was applied to estimate the effect of risk factors. Variables with the 푝 < 0.25 during the bivariate analysis were included into a multiple cox model. A 95% CI and 푝 value of < 0.05 for multiple cox model were considered statistically significant. Loss to follow-up rates were calculated by summing the number of patients’ who experienced the event loss to follow-up during a particular period of time divided by the total number of years of follow-up during this period. The difference in proportions of patients surviving at some time (t) was described using Kaplan-Meier survival plot. The proportional hazard assumption was checked by using log-log survival curves based on Schoenfeld residuals. We calculated incidence density for loss to follow-up using person months (PMOs) of contribution to the cohort. Patients who completed treatment or active at the end of observation period were considered censored. Kaplan-Meier method and log rank test were used to estimate survival probability and statistical significance for categorical covariates.
Result
Description of the cohort
A total of 1341 eligible tuberculosis patients were included into this study. The distribution of the study participants by sex was approximately the same in health center and general hospital. Accordingly, 356 (58%) from health centers and 431 (59.3%) from the general hospital were males by sex. With regard to residence of the study participants, 184 (30%) from health centers and nearly all 720 (99.0%) from general hospital were residents of a rural community. Concerning patients’ access to health facility, 429 (69.9%) of patients taking care in health centers and 715 (98.3%) on follow-up and care at general hospital had been residing within a 10-km distance. The baseline body weight was normally distributed for both TB patients receiving treatment care in both health centers and general hospital. Accordingly, the mean body weight was 48.7 kg with SD of 7.8 kg and 50.2 kg with SD of 7.8 kg for TB patients receiving treatment and care at health centers and hospital, respectively.
The smear negativity is almost the same for both patients diagnosed at health centers and general hospital. More than 85% of the study participants in both health centers and general hospital were new TB patients. From a total of TB patients who were tested for HIV, 90 (14.7%) and 144 (19.8%) were reactive for HIV at health centers and general hospital, respectively (Table 1).
With regard to TB patient loss to follow-up status by selected baseline socio demographic and clinical characteristics, majority of TB patient loss to follow-up (LTFU) occurred among males (83, 6.2%) and new TB patients at enrolment (106, 7.9%). In relation to type of health facility, majority of TB patient LTFU occurred among patients following treatment and care at health centers (Table 2).
Survival status and incidence of loss to follow-up
A total of 1341 patients were followed for a total of 7056.8 person months with median follow-up 5.3 months in TB treatment and care from 2008 to 2015. From this, tuberculosis patients were followed for a total of 3074.7 and 3974 person months of observations at health centers and general hospital, respectively. At the end of the observation period, of the total TB cases registered to TB treatment and care at heath facilities, 154(25.1%) were cured, 299 (48.7%) completed treatment, 24 (3.9%) died, three (0.5%) failed after treatment, 84(13.7%) were loss to follow-ups, and 50 (8.1%) were transferred into a health facility. Similarly, from the total TB cases registered to TB treatment and care at general hospital, 181 (24.9%) were cured, 441 (60.7%) completed treatment, 13 (1.8%) died, 38 (5.2%) were loss to follow-ups, and 54 (7.4%) were transferred into health facility.
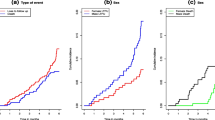
The incidence of tuberculosis patient LTFUs rate on the cohort was 27.3 per 1000 person months and 9.6 per 1000 person months (PMs), at health centers and general hospital, respectively. From the overall loss to follow-ups that occurred, 55 (65.5%) and 33 (86.8%) of LTFUs occurred during the intensive phase and grew to 78 (92.9%) and 38 (100%) at health center and general hospital, respectively, at the end of 6-month observation period (Figs. 1 and 2). The life table analysis for survival function of TB patients follow-up between general hospital and health centers also revealed that the cumulative proportion surviving at the end of the intensive phase was 87% at health center whereas it was 95% for patients followed at the general hospital (Table 3). The median estimate of loss to follow-up time for TB patients who were attending treatment and care in hospital was 6.1 (95%CI, 5.9–7.3) months whereas for TB cases who were attending follow-up and care in health centers was 5.6 (95%CI, 5.3–5.8) months. According to the log rank test, the observed survival time differences between these two categories of patients receiving treatment and care at health centers and general hospital was statistically significant (P < 0.001) (Fig. 3).
Equally, the median estimate of survival time for TB patients who reside within the catchment distance of less than or equal to 10 km was 5.3 (95% CI, 5.1–5.5) months whereas for patients who come from distant areas was 4.6 (95% CI, 4.3–4.9) months. The difference in survival time between the two different categories of patients with regard to their distance from the nearest health facility was also statistically significant (P < 0.001).
At enrolment, the cumulative proportions of retention for TB patients were higher for patients who lived within the 10 km from the nearest public health facilities, who were HIV sero status negative and who were rural residents when compared to their counter parts.
Independent predictors of loss to follow-up for TB patients
During bivariate analysis, age, sex, distance from the public health facility, residence, HIV sero status, and type of health facility were candidate variables for multiple cox model analysis of time to loss to follow-up of patients at health centers during TB treatment with a p value of < 0.25. Similarly, the age of the patient, sex, TB type, and HIV sero status were candidate variables during analysis of time to loss to follow-up of tuberculosis patients at general hospital.
During multiple Cox regression analysis, five variables were found to be significantly associated with time to loss to follow-up of TB patients. Variables significantly associated with time to loss to follow-up of TB patients included older ages (AOR = 1.7, 95%CI = [1.2–2.5], P < 0.001), being rural resident (AOR = 3.2, 95%CI = [1.9–5.3], P < 0.001), distance from the public health facility (AOR = 2.5, 95%CI = [1.5–4.2], P < 0.001), HIV sero status positive (AOR = 2.6, 95%CI = [1.6–4.2], P < 0.001), and following treatment and care at health center (AOR = 3.4, 95%CI = [2.1–5.5], P < 0.001) (Table 4).
Discussion
To attain WHO’s End TB strategy by 2030, TB patient treatment adherence is highly emphasized [2]. Thus, this study we aimed to determine the difference in time to LTFU of TB patients receiving treatment and care between health centers and general hospital, in rural Ethiopia. Accordingly, the incidence of tuberculosis patient loss to follow-up rate was 27.3 per 1000 PMOs and 9.6 per 1000 PMOs at health centers and general hospital, respectively. The time to loss to follow-up was significantly different between patients who were enrolled at health centers and general hospital. Although we did not get studies that compare the time to LTFU difference between TB patients receiving treatment and care at health centers and hospitals, we suggest that the significant time to LTFU difference might be explained from the fact that TB patient treatment and care service is more comprehensive in hospitals compared to health centers in Ethiopia [24, 26]. Thus, TB patients receiving treatment and care at facilities with low level of comprehensiveness might be forced to LTFU.
The incidence of TB patient LTFU recorded at health centers was lower when compared to finding from Morocco [28]. However, it was higher when compared to studies conducted across different parts of the world which includes Ethiopia [1], Ukraine [29], Ethiopia [30, 31], Dangila, Ethiopia [32], Cameroon [33], and South Africa [34]. The explanation for the variation could be explained from study setting and sample size. Unlike our study, which was conducted in rural and most peripheral part of Ethiopia, most studies mentioned in this comparison were conducted in areas adjacent to the main cities and their sample sizes were also much higher.
From the overall loss to follow-ups that occurred, 55 (65.5%) and 33 (86.8%) of LTFUs occurred during the intensive phase and grew to 78 (92.9%) and 38 (100%) at health center and general hospital respectively, at the end of 6-month observation period. This finding is greater compared to other studies, by 16% at the end of 6 months [1], by 13% (30% during intensive, 20% during continuous). This finding was lower in both intensive and continuous phase when compared to a study conducted in Pakistan [35] and Sudan [36]. This variation may be due to poor monitoring and tracking of loss to follow-up patients observed during early periods of treatment. The median estimate of loss to follow-up time for TB patients who were attending treatment and care in health centers was 5.6 (95%CI, 5.3–5.8) months, whereas for TB cases who were attending follow-up and care in hospital was 6.13 (95%CI, 5.9–7.3) months. The median time from treatment initiation to onset of loss to follow-up was short at health center compared to general hospital. This could be due to the delayed presentation and diagnosis of TB cases that lead to advancement of the disease, or it might be due to drug intolerance that resulted in early TB loss to follow-up. This finding is consistent with study in Thailand [37].
Over two thirds of TB/HIV co-infected patients were lost from care compared to TB patients whose HIV sero status was negative. The time to loss to follow-up for TB/HIV co-infected patients was nearly three-fold higher than those patients who were HIV sero status negative. Furthermore, the HIV non-reactive patients had much reduction in risk of loss to follow-up and improved survival time compared to those who were reactive for HIV, and the difference was statistically significant. This finding is consistent with other similar studies in that co-infected patients were more likely to loss to follow-up TB treatment relative to those whose HIV sero status was negative [38, 39]. Encouragingly, in TB–HIV co-infected patients, the receipt of ART was found to be protective against treatment loss to follow-up [40,41,42]. This could confirm the fact that TB is the most common opportunistic disease and the most common cause of burden in patients with HIV/AIDS infection in developing countries. But the mechanism by which the co-infection induces loss to follow-up from anti-TB treatment remains unclear [38].
In this study, TB patients with older age experienced loss to follow-up when compared to TB patients in younger age. This finding is consistent with a finding from Benin [38]. This could be plausible as this category of patients requests more social support from their communities. In addition, the duration of treatment, which is 6 to 8 months, is long in terms of separation from patients’ home towns and daily habits. Therefore, programs of DOTS at home or inside the community of origin could be a better TB surveillance strategy for the elder TB patients [38].
In this study, patients who lived > 10 km from the center had higher risk of early loss to follow-up when compared to those who lived within 10 km. This study is in line with finding from Uganda and Ethiopia [43, 44]. It was observed that the risk of non-adherence to treatment was higher among those who had lived > 10 km from the facility due to economic constrains to back transportation costs. Similarly a study from Philippines indicated that patients who lived near the service area were retained more on care as compared to those living abroad [44].
In this study, rural residence showed higher risk of TB patient loss to follow-up compared to their counter parts. This finding was consistent compared to other studies conducted in different settings. This may be explained by the fact that patients living in the rural area have little access to treatment centers in Ethiopia. Otherwise, they have to pay for public transport to reach treatment center, which could not be always possible as people in rural area have lower economic income.
Unlike other studies conducted so far, none of the variables did show significant association with time to patient loss to follow-up at general hospital in this study. Part of explanation for absence of significant association might be relatively small sample size as compared to other studies conducted on TB patient attrition at hospital level.
As part of limitation, the number of loss to follow-up might be overestimated or underestimated by exclusion of patients with undocumented outcomes. In addition, undetermined treatment outcomes of transfer out patients might have introduced bias too.
Conclusions
Time to loss to follow-up from treatment and care between health centers and hospitals was significant. Significant TB patient loss to follow-up occurred in the first 2 months of TB treatment. In addition, there was a substantially higher retention probability in TB patients that were sero–negative for HIV, being an urban resident and living near to the service area. Strengthening the DOTs program especially during the intensive phase of treatment and tracking of loss to follow-ups is highly recommended. Moreover, TB patients receiving treatment and care in health centers deserve special attention.
Abbreviations
- ART:
-
Anti-retroviral therapy
- CPT:
-
Cotrimoxazole prophylactic therapy
- HIV:
-
Human immunodeficiency virus
- LTFU:
-
Loss to follow-up
- PMOs:
-
Person months of observation
- SSA:
-
Sub-Saharan Africa
- TB:
-
Tuberculosis
References
Geremew MA, Mulualem T, Gemeda A. Survival analysis of loss to follow-up treatment among tuberculosis patients at Jimma University Specialized Hospital, Jimma, Southwest Ethiopia, vol. 2017. Geneva: Hindawi Publishing Corporation International Journal of Statistical Mechanics, WHO, global tuberculosis report; 2015, Article ID 923025,7.
WHO Africa. An epidemiological profile of HIV/AIDS, tuberculosis and malaria in sub-Saharan Africa. 2016. Accessed and cited in October 2019. Available from: https://aphrc.org/wp-content/uploads/2018/10/Epi-brief-V2.7-FINAL.pdf
Nájera-Ortiz JC, Sánchez-Pérez HJ, Ochoa-Díaz-López H, Leal-Fernández G, Navarro-Giné A. The poor survival among pulmonary tuberculosis patients in Chiapas, Mexico: the case of Los Altos Region: Hindawi Publishing Corporation Tuberculosis Research and Treatment; 2012, Article ID 708423. https://doi.org/10.1155/2012/708423.
WHO. Use of high burden country lists for TB by WHO in the post-2015. Geneva: WHO; 2015. Accessed and cited in November 2019. Available from: https://www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016-2020.pdf
FMOH/EPHI. Implementation Guideline for GeneXpert MTB/RIF Assay in Ethiopia. Addis Ababa: FMOH; 2014.
WHO. Global tuberculosis report 2015. Geneva: WHO; 2015.
Eshetie S, Gizachew M, Dagnew M, Kumera G, Woldie H, Ambaw F, et al. Multi drug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):219. https://doi.org/10.1186/s12879-017-2323-y. PMID:28320336
EPHI. First Ethiopian National Population Based Tuberculosis Prevalence survey. Addis Ababa: Ethiopian Health and Nutrition Research Institute; 2011.
Dangisso MH, Datiko DG, Lindtjorn B. Trends of tuberculosis case notification and treatment outcomes in the Sidama Zone, southern Ethiopia: ten-year retrospective trend analysis in urban-rural settings. PLoS One. 2014;9:e114225.
Dangisso MH, Datiko DG, Lindtjørn B. Accessibility to tuberculosis control services and tuberculosis programme performance in southern Ethiopia. Glob Health Action. 2015;8:29443.
Rogers JH, Jabateh L, Beste J, Wagenaar BH, McBain R, Palazuelos D, Wickett E, Oswald C, Napier HG, Toomey-Garbo J. Impact of community-based adherence support on treatment outcomes for tuberculosis, leprosy andHIV/AIDS-infected individuals in post-Ebola Liberia. Glob Health Action. 2018;11(1):1522150.
Medecins Sans Frontiers and Stop TB Partnership. Out of Step 2015 TB Policies in 24 Countries A survey of diagnostic and treatment practices. 2015; Available from:http://www.stoptb.org/assets/documents/news/report_out_of_step_2015_11_pdf_with_interactive_links.pdf
Eshetie S, Gizachew M, Alebel A, van Soolingen D. Tuberculosis treatment outcomes in Ethiopia from 2003 to 2016, and impact of HIV co-infection and prior drug exposure: a systematic review and meta-analysis. PLoS One. 2018;13(3):e0194675. https://doi.org/10.1371/journal.pone.0194675.
Shaweno D, Worku A. Tuberculosis treatment survival of HIV positive TB patients on directly observed treatment short course in Southern Ethiopia: a retrospective cohort study. BMC Res Notes. 2012;5(1):682.
World Health Organization. Global tuberculosis report: WHO; 2019. Accessed and cited on November 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1
Shao Y, Yang D, Xu W, et al. Epidemiology of anti-tuberculosis drug resistance in a Chinese population: current situation and challenges ahead. BMC Public Health. 2011;11(1):110.
Russell DG, Barry CE III, Flynn JL. Tuberculosis: what we don’t know can, and does, hurt us. Science. 2010;328(5980):852–6.
Akessa GM, Tadesse M, Abebe G. Survival analysis of loss to follow-up treatment among tuberculosis patients at Jimma University specialized hospital, Jimma, Southwest Ethiopia. Int J Stat Mech. 2015;2015:1–8.
Gezae KE, Abebe HT, Gebretsadik LG. Incidence and predictors of LTFU among adults with TB/HIV co-infection in two governmental hospitals, Mekelle, Ethiopia, 2009–2016: survival model approach. BMC Infect Dis. 2019;9(1):107.
USAID. USAID investments help improve testing, detection, and treatment of tuberculosis in Ethiopia. Accessed and cited on November 2019. Available from: https://www.usaid.gov/ethiopia/global-health/tuberculosis
World Health Organization. Global tuberculosis control report, WHO Report 2012. Geneva: World Health Organization; 2012.
World Health Organization. Definitions and reporting framework for tuberculosis 2013. Geneva: World Health Organization; 2013.
Shaweno D, Shaweno T, Trauer JM, Denholm JT, McBryde ES. Heterogeneity of distribution of tuberculosis in Sheka Zone, Ethiopia: drivers and temporal trends. Int J Tuberc Lung Dis. 2017;21(1):79–85. https://doi.org/10.5588/ijtld.16.0325.
Sheka Zonal Health Department. Facility based annual TB report. Masha: Sheka Zonal Health Department; 2016.
Federal Democratic Republic of Ethiopia Ministry of Health. Guidelines for clinical and programmatic management of TB, leprosy and TB/HIV in Ethiopia. Addis Ababa: MOH; 2012.
Ajagbe OB, Kabair Z, O’Connor T. Survival analysis of adult tuberculosis disease. PLoS One. 2014;9(11):e112838. https://doi.org/10.1371/journal.pone.0112838.
Thomas PR. Survival analysis and reliability. Sample size determination and power. Arlington, Evanston: Institute for Statistics Education, Virginia and Northwestern University; 2013. p. 315–25. ISBN 978-1-118-43760-5
Nabil T, Katia S, Mohamed B, Samira E, Mohammed CB, Karima ER, Chakib N. Determinants of tuberculosis treatment loss to follow-up in Morocco: results from a National Cohort Study. Pan Afr Med J. 2013;14:121. https://doi.org/10.11604/pamj.2013.14.121.2335.
Aibana O, Slavuckij A, Bachmaha M. Patient predictors of poor drug sensitive tuberculosis treatment. F1000Res, 2018;6:1873. https://doi.org/10.12688/f1000research.12687.3.
FMOH. Tuberculosis progress report. Addis Ababa: FMOH; 2011.
Reves R, Angelo S. CSIS, editor. As Ethiopia moves towards elimination, success requires higher investment. Washington DC: CSIS; 2016.
Birlie A, Tesfaw G, Dejene T, Woldemichael K. Time to death and associates factors among tuberculosis patients in Dangila Woreda, Northwest Ethiopia. PLoS One. 2015;10(12):e0144244. https://doi.org/10.1371/journal.pone.0144244.
Agbor AA, Bigna JJR, Billong SC, Tejiokem MC, Ekali GL, et al. Factors associated with death during tuberculosis treatment of patients co-infected with HIV at the Yaounde´ Central hospital, Cameroon: an 8-year hospital-based retrospective cohort study (2006– 2013). PLoS One. 2014;9(12):e115211. https://doi.org/10.1371/journal.pone.0115211.
Kigozi G, Heuni C, Chikobvu P, Botha S, van Rensburg D. Factors influencing treatment loss to follow-up among tuberculosis patients in a high burden province of South Africa. Int J Infect Dis. 2017;54:95–102.
Khan BJ, Kumar AMV, Stewart A, Khan NM, Selvaraj K, Fatima R. Alarming rates of attrition among tuberculosis patients in public-private facilities in Lahore, Pakistan. Public Health Action. 2018;7(2):127–33.
Elmuttalut M, Khidir M. Time and predicting factors of non-compliant TB patients to loss to follow-up treatment - a perspective from Sudan - 2016. Int J Contemp Med Res. 2017;4(1):225–8.
Moolphate S, Aung MN, Nampaisan O, Nedsuwan N, Kantipong P, Suriyon N, et al. Time of highest tuberculosis death risk and associated factors: an observation of 12 years in Northern Thailand. Int J Gen Med. 2011;4:10.
Sylvère TA. Loss to follow-up time from tuberculosis treatment in the Southern Republic of Benin using mixture cure model for survival analysis. Biom Biostat Int J. 2015;2(5):00039. https://doi.org/10.15406/bbij.2015.02.00039.
Amuha GM, Kutyabami P, Kitutu FE, OdoiAdome R, Kalyango JN. Non-adherence to anti-TB drugs among TB/HIV co-infected patients in Mbarara hospital Uganda: prevalence and associated factors. J Afr Health Sci. 2009;9(2):8–15.
Hailu H, TOL A, Shojaeizadeh D, Garmaroud G. Tuberculosis treatment non-adherence and lost to follow up among TB patients with or without HIV in developing countries: a systematic review. Iran J Public Health. 2015;44(1):1–11.
Yende-Zuma N, Naidoo K. The effect of timing of initiation of antiretroviral therapy on loss to follow-up in HIV-tuberculosis co-infected patients in South Africa: an open-label, randomized, controlled trial. J Acquir Immune Defic Syndr. 2016;72:430–6.
Belén M, Ramos S, Arrossi S. Determinants of non-adherence to tuberculosis treatment in Argentina: barriers related to access to treatment. Rev Bras Epidemiol. 2015;18(2):287–98.
Gebremariam KM, Bjune GA, Frich JC. Barriers and facilitators of adherence to TB treatment in patients on concomitant TB and HIV treatment: a qualitative study. BMC Public Health. 2010;10:651.
Thelma E, et al. Factors associated with loss to follow-up during treatment for multidrug-resistant tuberculosis, the Philippines, 2012–2014. Emerging Infect Dis. 2016;22:3. www.cdc.gov/eid
Acknowledgements
Our heartfelt thank goes to Sheka Zonal Health Department, Tepi General Hospital, and the health centers for the unlimited data access.
Ethical approval and consent to participate
The study was approved by the Melbourne University Health Sciences Human Ethics Subcommittee, Melbourne, VIC, Australia, and the Zonal Health Department, Sheka Zone, Ethiopia.
Funding
This study was privately funded.
Availability of data and materials
Available from the corresponding author upon reasonable request.
Author’s contributions
TS: conceived the study, formulated the research questions, and designed the study, TS: coordinated the data collection, analyzed the data, and drafted the paper. TS, MG and CF: did the analysis, reviewed the paper for intellectual content, and read and approved the final paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shaweno, T., Getnet, M. & Fikru, C. Does time to loss to follow-up differ among adult tuberculosis patients initiated on tuberculosis treatment and care between general hospital and health centers? A retrospective cohort study. Trop Med Health 48, 9 (2020). https://doi.org/10.1186/s41182-020-00198-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-020-00198-8