Abstract
Background
Vascular access (VA) intervention therapy (VAIVT) has been increasingly used for treating VA failure (VAF) in patients undergoing hemodialysis; however, clinical evidence demonstrating the efficacy of prevention of VAF after VAIVT is limited. Therefore, we aimed to assess characteristics of patients developing VAF after VAIVT and analyze risk factors for VAF after VAIVT.
Methods
This retrospective study included 96 patients with VAF who underwent ultrasound-guided VAIVT by interventional nephrologists between January 2013 and March 2018 at the Department of Nephrology, University of Tokyo Hospital, Japan. Patient information included age, sex, medication history, and comorbidities that could potentially affect VAF onset. Patients were categorized into two groups based on antiplatelet treatment. Multivariate Cox regression analysis was performed for evaluating effect of various factors on VAF after VAIVT.
Results
Median age of patients at the time of VAIVT was 71 years (interquartile range 63–79); the most prevalent etiology underlying end-stage renal disease was diabetic nephropathy (40.7%). Comparison between the antiplatelet and non-antiplatelet groups revealed that the incidence of VAF was significantly lower in the antiplatelet group. Multivariate analysis revealed that antiplatelet treatment was associated with a lower risk of VAF after VAIVT.
Conclusion
Administration of antiplatelet agents was associated with a significant reduction in VAF risk after VAIVT.
Similar content being viewed by others
Background
Vascular access (VA) is a lifeline for patients undergoing hemodialysis (HD). According to the Japanese Society for Dialysis Therapy (JSDT) guidelines, complications, such as stenosis and thrombosis, cannot be avoided because of prolonged VA use [1]. Moreover, VA failure (VAF) due to stenosis or thrombosis of the VA site hinders dialysis continuation, with a serious impact on patients undergoing HD and increase in medical expenses that are incurred during restoring VA. Therefore, VA stability is critical for maintaining the quality of life of patients undergoing HD and for reducing their medical expenses. For instance, the annual expenditure associated with VA is more than one billion US dollars in the USA [2, 3]. Therefore, several studies investigating approaches for preventing VAF that often occurs after VA construction reported that treatment with antiplatelet agents reduced the risk of stenosis and improved the duration of primary unassisted patency of newly created VA [4, 5].
Recently, vascular access intervention therapy (VAIVT) has become one of the established therapy options for resolving VAF [1, 6,7,8,9,10,11]. Although VAIVT is less invasive than surgical reconstruction [7, 12,13,14], information on prevention of VAF after VAIVT is limited.
To clarify these issues, we conducted a retrospective analysis of 96 patients with VAF at a single institute, with the definition of VAF based on the JSDT guidelines [1]. Our findings indicated that antiplatelet treatment might prevent VAF due to frequent restenosis after VAIVT.
Methods
Study design
This retrospective study included data from 96 patients with VAF who underwent ultrasound-guided VAIVT by intervention nephrologists at the University of Tokyo Hospital between January 2013 and March 2018. These enrolled patients were hospitalized. All procedures during this study were performed by the same team.
Indications for VAIVT were defined according to the JSDT guidelines [1] and included a stenosis rate of ≥ 50% in addition to ≥ 1 of the following clinical medical abnormalities: (i) decreased blood flow, (ii) increased venous pressure, (iii) an abnormally high blood urea nitrogen level, and (iv) unexplained reduction in dialysis efficiency [1].
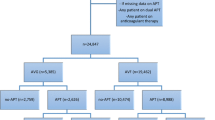
Among the 96 adults who underwent ultrasound-guided VAIVT included in this study, six with primary failure of intervention and 31 with VA construction within 180 days before intervention were excluded. Therefore, 59 patients with a primary VAF episode were eligible for the final analysis (Fig. 1).
The primary outcome was 1-year VA patency after VAIVT; the secondary outcome was frequency of any complication after VAIVT.
Definitions
Primary success was defined according to the reporting standards of the American Society of Interventional Radiology [15]. Clinical success was defined as recovery of palpable continuous thrill perception, loss of initial clinical abnormalities associated with VAF, or at least one successfully performed dialysis session. According to the JSDT guidelines, VAF was defined as HD discontinuation because of stenosis or acute thrombus occlusion [1].
Patients who continuously received any of the antiplatelet agents, such as aspirin, clopidogrel, and cilostazol, before VAIVT were categorized in the antiplatelet group, whereas those who did not receive any antiplatelet agent were categorized in the non-antiplatelet group. The antiplatelet group was further divided into two groups according to the type of antiplatelet agent as monotherapy (aspirin or clopidogrel) and dual antiplatelet therapy (DAPT).
Older age has been reported as an independent risk factor for VAF after VA construction. Therefore, to examine the influence of age on vascular access outcome, we defined individuals age older than the median age in this study group as “the older age” [16, 17].
Ultrasound-guided VAIVT
VA was accessed with a single-entry needle under ultrasound guidance using a diagnostic Noblus ultrasound scanner (Hitachi Healthcare, Tokyo, Japan). All procedures were completed via a 4- or 5-Fr sheath introducer, with a 0.018–0.035-in. curved or straight-tip guidewire (Radifocus guidewire M, GT wire angle, or straight type; Terumo, Tokyo, Japan) and a balloon with a diameter ranging from 4 to 6 mm and a length of 40 mm (SABER®, Cardinal Health Japan, Japan, Mustang™ and Sterling™ Boston Scientific, USA). Brachial artery blood flow volume was measured by using ultrasound scanner before and after VAIVT.
Statistical analysis
All data were presented as means ± standard deviation or median (interquartile range). Categorical variables, including age, sex, medical history, and etiology of chronic kidney disease G5D (CKDG5D), were compared using Pearson’s chi-squared or Fisher’s exact test. Significance of associations among categorical variables was assessed using the chi-squared test and Spearman’s rank correlation coefficient, and, to evaluate the relative risk to VA patency after VAIVT, univariate and multivariate Cox regression analyses were performed; results were reported as hazard ratios and 95% confidence intervals. Before performing Cox regression analysis, we tested the proportional hazards assumption using the Schoenfeld residual test. Covariates assessed in univariate analyses included the older age; sex; prevalence of smoking history; prevalence of diabetes mellitus (DM); prevalence of diabetic nephropathy (DN); cardiovascular disease (CVD); dyslipidemia; peripheral artery disease (PAD); medications, including renin–angiotensin–aldosterone system (RAAS) inhibitors, statins, and antiplatelet agents; and arteriovenous fistula (AVF) anastomosis type. According to the Japan Atherosclerosis Society 2012 guidelines, dyslipidemia was defined as low-density lipoprotein cholesterol level ≥ 140 mg/dl, high-density lipoprotein cholesterol level < 40 mg/dl, or triglyceride level ≥ 150 mg/dl [18]. Dyslipidemia also included patients who received statin or ezetimibe. The primary patency rate after VAIVT was analyzed using the Kaplan–Meier test; patency curves were compared using the log-rank and Wilcoxon tests.
Statistical significance was defined as a p value < 0.05. Statistical analyses were performed using JMP® software (version 8.0; SAS Institute, Cary, NC, USA) or EZR software (version 1.37; Saitama Medical Center, Jichi Medical University, Saitama, Japan).
This study was approved by the institutional review board of the University of Tokyo [IRB number 2879-(6)] and was conducted in accordance with guidelines of the Declaration of Helsinki. Informed consent was obtained from all patients at the time of hospital admission for VAIVT.
Results
Patient characteristics
Patient characteristics are shown in Table 1. The study group comprised 59 patients (45 men and 14 women) who were followed up for 266.6 ± 326.2 days. Median age was 71 years; therefore, we defined individuals aged > 71 years as “the older age” (Additional file 1: Figure S1). The most prevalent etiology underlying CKDG5D in the current study was DN (40.7%). The median duration of dialysis was approximately 28.6 months. The most common AVF type was radiocephalic fistula (n = 48), followed by brachiocephalic (n = 10) and radiobasilic (n = 1) fistula. The median flow volume before VAIVT was 418.7 ml/min. More than half of the patients received any of antiplatelet agents; aspirin, clopidogrel, and cilostazol were administered to 28 (47.5%), 14 (23.7%), and 1 (1.7%) patients, respectively. Of these, 20 patients received DAPT. Antiplatelet agents were administrated in 31 patients. At the end of the observation period, although the administration conditions were unknown without data in three (9.7%) patients, 28 (90.3%) had continuously received these antiplatelet agents.
Initial success and primary patency rates
During the entire study period, the initial VAIVT success rate was 93.8%, similar to that reported by previous studies [19,20,21,22,23,24,25]. In addition, the 1-year patency rate was 39.7% (Fig. 2).
Characterization of patients in the antiplatelet and non-antiplatelet group
Clinical characteristics of patients in the antiplatelet and non-antiplatelet group are summarized in Table 2. Rates of patients with smoking history (p = 0.008), dyslipidemia (p = 0.009), and CVD (p < 0.001) were significantly higher in the antiplatelet group than in the non-antiplatelet group. However, there were no statistical differences in rates of DN, DM, or PAD between the two groups. Adverse events were similar between the two study groups. There were no serious bleeding events, such as intracranial or gastrointestinal hemorrhage, and no significant differences in CVD events occurred during the follow-up period.
Primary patency in the antiplatelet group
Next, we assessed the association between antiplatelet agent treatment and cumulative primary patency rates by the Kaplan–Meier test (Fig. 3). The 1-year patency rate after VAIVT was significantly higher in the antiplatelet group than in the non-antiplatelet group (p = 0.035). We also assessed the number of antiplatelet agents used to examine whether there was a difference in 1-year patency rate between monotherapy and DAPT (Fig. 4). There was a significant difference in the 1-year patency rate after VAIVT among the three groups (p = 0.048). However, we did not find a significant difference between monotherapy and DAPT.
Analysis of independent risk factor for VAF
To identify variables that were significantly associated with VAF after VAIVT, we conducted a univariate Cox regression analysis after confirming the proportional hazards assumption using a Schoenfeld residuals test. The result of the proportional hazards assumption is shown in Additional file 2: Table S1 and Additional file 3: Figure S2. We excluded age from covariates because the proportional assumption of age was violated. As shown in Table 3, only treatment with antiplatelet agents was significantly associated with VAF. Conversely, the older age, sex, DN, DM, dyslipidemia, CVD, PAD, smoking history, or AVF anastomosis type were not found to be risk factors for primary patency.
In several studies, old age, female sex, smoking history, dyslipidemia, and CVD were reported as independent risk factors for VAF after VA construction [12, 16, 17, 26,27,28,29]. Therefore, we conducted a multivariate Cox regression analysis using these factors to determine if they were independent risk factors for VAF after VAIVT (Table 4). Treatment with antiplatelet agents was found to be independently associated with patency after VAIVT (hazard ratio, 0.28; 95% confidence interval, 0.09–0.82; p = 0.02).
Overall, these findings supported an association between antiplatelet agent treatment and primary patency.
Discussion
The current study investigating the association between primary patency after VAIVT and antiplatelet agent treatment using a medical record review determined that the long-term patency rate was good, with a cumulative patency rate of 39.7% for 1 year after VAIVT (Fig. 2), and that the cumulative patency rate was higher in patients treated with antiplatelet agents than in those not treated with an antiplatelet agent. The frequency of bleeding or other serious adverse events was not higher in the antiplatelet group. Our results showed that administration of antiplatelet agents may contribute to the improvement of 1-year patency rate after VAIVT (Table 4). To the best of our knowledge, this is the first study to demonstrate the protective effect of antiplatelet agents against VAF after VAIVT.
Previous studies and systematic reviews have reported that antiplatelet agents are effective in preventing VAF, including those resulting from thrombosis [4, 30,31,32,33]. For example, a randomized control study showed that VAF risk after arteriovenous graft construction was reduced by 5% following administration of antiplatelet agents [4]. A recent systematic review demonstrated that antiplatelet agents were effective in preventing AVF and central venous thrombosis [32]. The same systematic review also reported that VAF was significantly reduced by 57% in the antiplatelet group compared with that in the placebo or non-treatment groups (relative risk, 0.43; 95% confidence interval, 0.26–0.73; I2 = 25%) [31]. However, these studies investigated the efficacy of antiplatelet agents against primary patency after VA construction. Conversely, results of the current study suggest that antiplatelet agents are useful for VA patency after VAIVT. Based on a previous study [34], we excluded patients with VA construction within 180 days before intervention because our study aim was to examine the effect of antiplatelet agents on secondary patency and not primary patency.
This study showed that the cumulative VA patency after VAIVT was significantly higher in patients treated with antiplatelet agents than in those who were not treated with antiplatelet agents. This outcome is biologically plausible because several recent reports have suggested that high levels of local oxidative stress or inflammatory mediators are involved in development of VAF [35,36,37,38,39]. Several recent studies showed that some mediators such as heme oxygenase-1 and heme oxygenase-2, monocyte chemoattractant protein-1, Kruppel-like factor-2, and TGF-b1 play an important role in AVF dysfunction in mouse model [40, 41]. Antiplatelet agents, such as aspirin, clopidogrel, and cilostazol, have been reported to reduce oxidative stress and inflammation [42, 43]. For example, these agents have a great effect on suppression of the onset or progression of vascular disease after invasive treatment for major CVD and PAD [44,45,46,47,48,49,50,51]. In addition, our results are consistent with those of a recent systematic review showing that antiplatelet agents reduced VAF incidence in AVFs [32].
In the present study, there were no serious bleeding events related to antiplatelet agent treatment; this finding is consistent with that of a recent meta-analysis that showed that antiplatelet agents are not associated with serious adverse events [32].
A recent study has shown that protein-bound uremic toxins, such as indoxyl sulfate, resulted from the metabolism of dietary tryptophan were associated with vascular access patency after VAIVT [52]. Uremic toxins were not measured in the present study, which may have influenced vascular access outcomes between the two groups. However, no participant had dysphagia and all participants were educated regarding the medical nutrition therapy for chronic kidney disease.
Our study has several limitations. First, this retrospective cohort study at a single institution included a small number of patients. Because the proportional assumption of age was violated, selection bias related to age may have influenced the analysis result. Second, cause-specific risk of VAF after VAIVT could not be analyzed. Third, the rate of patients with radiocephalic fistula was significantly higher in the non-antiplatelet group than in the antiplatelet group. Although there might be selection bias such as technical problem and selection bias involving surgeon skill, AVF anastomosis type was not significantly associated with VAF. Finally, because all study subjects were hospitalized, their overall condition was worse than the general dialysis population, which might have led to a selection bias.
In Japan, antiplatelet therapy for the treatment or prevention of VAF has not been covered by the Japanese health insurance system. Therefore, in the present state, it is not possible to administer antiplatelet agents against VAF to patients with VAF.
Conclusions
This retrospective study on a cohort of patients with VAF at a single institution revealed that administration of antiplatelet agents was associated with a reduced VAF rate after VAIVT and that there was no significant difference in serious adverse event rates between the two groups. Overall, these results suggest that antiplatelet agents are useful in reducing the development of VAF after VAIVT. We, interventional nephrologists, believe that some vascular access failure pathology may be implicated in platelet and inflammation, but there is little existing evidence. Therefore, progress of research in this field is expected in future.
Abbreviations
- AVF:
-
Arteriovenous fistula
- CHF:
-
Congestive heart failure
- CKD:
-
Chronic kidney disease
- CVD:
-
Cardiovascular disease
- DAPT:
-
Dual antiplatelet therapy
- DM:
-
Diabetes mellitus
- DN:
-
Diabetic nephropathy
- HD:
-
Hemodialysis
- IHD:
-
Ischemic heart disease
- JSDT:
-
Japanese Society for Dialysis Therapy
- PAD:
-
Peripheral artery disease
- RAAS:
-
Rennin–angiotensin–aldosterone system
- VA:
-
Vascular access
- VAF:
-
Vascular access failure
- VAIVT:
-
Vascular access intervention therapy
References
Kukita K, Ohira S, Amano I, Naito H, Azuma N, Ikeda K, et al. 2011 update Japanese Society for Dialysis Therapy Guidelines of vascular access construction and repair for chronic hemodialysis. Ther Apher Dial. 2015;19(Suppl 1):1–39.
Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7(4):523–35.
Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, et al. Establishment and maintenance of vascular access in incident hemodialysis patients: a prospective cost analysis. J Am Soc Nephrol. 2005;16(1):201–9.
Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360(21):2191–201.
Tanner NC, Da Silva A. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database Syst Rev. 2015;7:CD002786.
Group VAW. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176–247.
Forauer AR, Hoffer EK, Homa K. Dialysis access venous stenoses: treatment with balloon angioplasty--1- versus 3-minute inflation times. Radiology. 2008;249(1):375–81.
Safa AA, Valji K, Roberts AC, Ziegler TW, Hye RJ, Oglevie SB. Detection and treatment of dysfunctional hemodialysis access grafts: effect of a surveillance program on graft patency and the incidence of thrombosis. Radiology. 1996;199(3):653–7.
Leskovar B, Furlan T, Poznič S, Potisek M, Adamlje A, Ključevšek T. Ultrasound-guided percutaneous endovascular treatment of arteriovenous fistula/graft. Clin Nephrol. 2017;88(13):61–4.
Wakabayashi M, Hanada S, Nakano H, Wakabayashi T. Ultrasound-guided endovascular treatment for vascular access malfunction: results in 4896 cases. J Vasc Access. 2013;14(3):225–30.
Gray RJ. Percutaneous intervention for permanent hemodialysis access: a review. J Vasc Interv Radiol. 1997;8(3):313–27.
Kim SM, Ko HK, Noh M, Ko GY, Kim MJ, Kwon TW, et al. Factors affecting patency following successful percutaneous intervention for dysfunctional hemodialysis vascular access. Ann Vasc Surg. 2018;47:54–61.
Cho S, Lee YJ, Kim SR. Clinical experience with ultrasound guided angioplasty for vascular access. Kidney Res Clin Pract. 2017;36(1):79–85.
Tordoir JHM, Zonnebeld N, van Loon MM, Gallieni M, Hollenbeck M. Surgical and endovascular intervention for dialysis access maturation failure during and after arteriovenous fistula surgery: review of the evidence. Eur J Vasc Endovasc Surg. 2018;55(2):240–8.
Gray RJ, Sacks D, Martin LG, Trerotola SO. Committee SoIRTA. Reporting standards for percutaneous interventions in dialysis access. J Vasc Interv Radiol. 2003;14(9 Pt 2):S433–42.
Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN. A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg. 2007;45(2):420–6.
Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, et al. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int. 1999;56(1):275–80.
Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan - 2012 version. J Atheroscler Thromb. 2013;20(6):517–23.
Sadaghianloo N, Declemy S, Jean-Baptiste E, Haudebourg P, Robino C, Islam MS, et al. Radial artery deviation and reimplantation inhibits venous juxta-anastomotic stenosis and increases primary patency of radial-cephalic fistulas for hemodialysis. J Vasc Surg. 2016;64(3):698–706.e1.
Turmel-Rodrigues L, Mouton A, Birmelé B, Billaux L, Ammar N, Grézard O, et al. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant. 2001;16(12):2365–71.
Beathard GA, Arnold P, Jackson J, Litchfield T, Lifeline POFR. Aggressive treatment of early fistula failure. Kidney Int. 2003;64(4):1487–94.
Natário A, Turmel-Rodrigues L, Fodil-Cherif M, Brillet G, Girault-Lataste A, Dumont G, et al. Endovascular treatment of immature, dysfunctional and thrombosed forearm autogenous ulnar-basilic and radial-basilic fistulas for haemodialysis. Nephrol Dial Transplant. 2010;25(2):532–8.
Park JY, Yoo CH. On postoperative day balloon angioplasty for salvage of newly-placed, flow-limiting native arteriovenous fistula. Vasc Specialist Int. 2015;31(1):20–4.
Jeon EY, Cho YK, Cho SB, Yoon DY, Suh SO. Predicting factors for successful maturation of autogenous haemodialysis fistulas after salvage percutaneous transluminal angioplasty in diabetic nephropathy: a study on follow-up Doppler ultrasonography. Iran J Radiol. 2016;13(1):e32559.
Park HS, Lee YH, Kim HW, Baik JH, Won YS, Park CW, et al. Usefulness of assisted procedures for arteriovenous fistula maturation without compromising access patency. Hemodial Int. 2017;21(3):335–42.
Woods JD, Turenne MN, Strawderman RL, Young EW, Hirth RA, Port FK, et al. Vascular access survival among incident hemodialysis patients in the United States. Am J Kidney Dis. 1997;30(1):50–7.
Astor BC, Coresh J, Powe NR, Eustace JA, Klag MJ. Relation between gender and vascular access complications in hemodialysis patients. Am J Kidney Dis. 2000;36(6):1126–34.
Ravani P, Marcelli D, Malberti F. Vascular access surgery managed by renal physicians: the choice of native arteriovenous fistulas for hemodialysis. Am J Kidney Dis. 2002;40(6):1264–76.
Shin DH, Rhee SY, Jeon HJ, Park JY, Kang SW, Oh J. An increase in mean platelet volume/platelet count ratio is associated with vascular access failure in hemodialysis patients. PLoS One. 2017;12(1):e0170357.
Kaufman JS, O’Connor TZ, Zhang JH, Cronin RE, Fiore LD, Ganz MB, et al. Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol. 2003;14(9):2313–21.
Trimarchi H, Young P, Forrester M, Schropp J, Pereyra H, Freixas E. Clopidogrel diminishes hemodialysis access graft thrombosis. J Vasc Access. 2005;6(1):29–33.
Palmer SC, Di Micco L, Razavian M, Craig JC, Ravani P, Perkovic V, et al. Antiplatelet therapy to prevent hemodialysis vascular access failure: systematic review and meta-analysis. Am J Kidney Dis. 2013;61(1):112–22.
Hiremath S, Holden RM, Fergusson D, Zimmerman DL. Antiplatelet medications in hemodialysis patients: a systematic review of bleeding rates. Clin J Am Soc Nephrol. 2009;4(8):1347–55.
Hu H, Patel S, Hanisch JJ, Santana JM, Hashimoto T, Bai H, et al. Future research directions to improve fistula maturation and reduce access failure. Semin Vasc Surg. 2016;29(4):153–71.
Kang L, Grande JP, Farrugia G, Croatt AJ, Katusic ZS, Nath KA. Functioning of an arteriovenous fistula requires heme oxygenase-2. Am J Physiol Renal Physiol. 2013;305(4):F545–52.
Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, et al. MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol. 2011;22(1):43–8.
Kang L, Hillestad ML, Grande JP, Croatt AJ, Barry MA, Farrugia G, et al. Induction and functional significance of the heme oxygenase system in pathological shear stress in vivo. Am J Physiol Heart Circ Physiol. 2015;308(11):H1402–13.
Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, et al. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int. 2008;74(1):47–51.
Lee T, Misra S. New insights into dialysis vascular access: molecular targets in arteriovenous fistula and arteriovenous graft failure and their potential to improve vascular access outcomes. Clin J Am Soc Nephrol. 2016;11(8):1504–12.
Janardhanan R, Yang B, Vohra P, Roy B, Withers S, Bhattacharya S, et al. Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney Int. 2013;84(2):338–52.
Kang L, Grande JP, Hillestad ML, Croatt AJ, Barry MA, Katusic ZS, et al. A new model of an arteriovenous fistula in chronic kidney disease in the mouse: beneficial effects of upregulated heme oxygenase-1. Am J Physiol Renal Physiol. 2016;310(6):F466–76.
Kambayashi J, Liu Y, Sun B, Shakur Y, Yoshitake M, Czerwiec F. Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003;9(28):2289–302.
Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19(11):42.
Kakkos SK, Nicolaides A, Griffin M, Sabetai M, Dhanjil S, Thomas DJ, et al. Factors associated with mortality in patients with asymptomatic carotid stenosis: results from the ACSRS study. Int Angiol. 2005;24(3):221–30.
Hobson RW, Krupski WC, Weiss DG. Influence of aspirin in the management of asymptomatic carotid artery stenosis. VA Cooperative Study Group on Asymptomatic Carotid Stenosis. J Vasc Surg. 1993;17(2):257–63 discussion 63-5.
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236.
Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305–16.
Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61(3 Suppl):2S–41S.
Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299(18):2164–71.
Iida O, Yokoi H, Soga Y, Inoue N, Suzuki K, Yokoi Y, et al. Cilostazol reduces angiographic restenosis after endovascular therapy for femoropopliteal lesions in the Sufficient Treatment of Peripheral Intervention by Cilostazol study. Circulation. 2013;127(23):2307–15.
Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47(5):975–81.
Wu CC, Hsieh MY, Hung SC, Kuo KL, Tsai TH, Lai CL, et al. Serum indoxyl sulfate associates with postangioplasty thrombosis of dialysis grafts. J Am Soc Nephrol. 2016;27(4):1254–64.
Acknowledgements
The authors are grateful to all investigators and contributors of our study.
Funding
The study was supported by JSPS KAKENHI (Grant Number JP17K16071).
Availability of data and materials
Please contact the corresponding author for data requests.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
TM, MN, NS, HT, YH, and MN are members of the Division of Nephrology and Endocrinology at the University of Tokyo Hospital, and AM and HK are members of the Department of Urology at the University of Tokyo Hospital.
Ethics approval and consent to participate
This study was approved by the institutional review board of the University of Tokyo [IRB number 2879-(6)] and was conducted in accordance with the guidelines of the Declaration of Helsinki.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Box plot showing the age distribution of the patients in this study. People over 71 years old was defined as the older age. Box plot explanation: upper horizontal line of box, 75th percentile; lower horizontal line of box, 25th percentile; horizontal bar within box, median; upper horizontal bar outside box, maximum; lower horizontal bar outside box, minimum. (TIF 523 kb)
Additional file 2:
Table S1. Results of the proportional hazards assumption by a Schoenfeld residuals test. (DOCX 19 kb)
Additional file 3:
Figure S2. Scaled Schoenfeld residuals for each factor. The residual was estimated as the time-dependent coefficient beta (t) vs. transformed time. The proportional hazards assumption of age was violated (p < 0.001), but that of the other factors was fitted (p > 0.05) (Additional file 2: Table S1). a Age, b Older age, c Sex, d DN, e NS, f CGN, g IgAN, h Other or unknown, i Smoking history, j DM, k Dyslipidemia, l CVD, m IHD, n CHF, o Stroke, p PAD, q RASI, r Statin, s Warfarin, t Antiplatelet agents, u Radiocephalic, v Brachiocephalic (ZIP 1081 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mizuno, T., Nakamura, M., Satoh, N. et al. Patency with antiplatelet treatment after vascular access intervention therapy: a retrospective observational study. Ren Replace Ther 4, 43 (2018). https://doi.org/10.1186/s41100-018-0184-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-018-0184-5