Abstract
Peritonitis is an important complication of peritoneal dialysis. Several animal peritonitis models have been described, including bacterial and fungal models that are useful for studying inflammation in peritonitis. However, these models have limitations for investigating peritoneal fibrosis induced by acute inflammation and present difficulties in handling the infected animals. Animal models of peritonitis which induced peritoneal fibrosis are important for establishing new therapies to improve peritoneal damage induced by peritonitis. Here, we present an overview of representative animal models of peritoneal dialysis-associated infectious and non-infectious peritonitis, including our novel animal models (scraping and zymosan models) that mimic peritoneal injury associated with fibrosis and neoangiogenesis caused by bacterial or fungal peritonitis.
Similar content being viewed by others
Background
There are several reasons why peritonitis is important in peritoneal dialysis (PD) treatment. First, peritonitis remains an important cause of death in PD patients. The mortality rate for peritonitis is approximately 3% [1, 2], and peritonitis is a contributing cause of death in more than 10% of PD patients [3]. Second, peritonitis remains an important factor in withdrawal from PD. In the PD registry of the Nagoya group from both 2005 to 2007 [4] and 2010 to 2012 [5], the most common reasons for withdrawal from PD have been PD-related peritonitis, followed by dialysis failure/ultrafiltration failure and social problems such as lack of family support. PD peritonitis is primarily caused by gram-positive organisms that typically result from touch contamination. The mean incidence of peritonitis as reported twice from a study over a 3-year period was one episode every 42.8 [4] and 47.3 [5] patient-months. Third, peritonitis presents a risk for the development of encapsulating peritoneal sclerosis (EPS) [6]. The duration of peritonitis is independently associated with EPS [7]. In particular, fungal and Pseudomonas infections put patients at a higher risk for the development of EPS [8]. Fourth, peritonitis is one of the risks for a decrease in residual renal function. The number of peritonitis episodes has been reported to be an independent predictor of the development of anuria [9]. Fifth, peritonitis is an important cause of peritoneal membrane injury, which leads to peritoneal fibrosis, neoangiogenesis, and peritoneal dysfunction [10].
The characteristic features of chronic peritoneal damage in PD treatment are the loss of ultrafiltration capacity associated with morphological submesothelial fibrosis with extracellular matrix accumulation, and neoangiogenesis. The pathogenesis of peritoneal fibrosis is attributed to a combination of bioincompatible factors in PD fluid (PDF) and peritonitis, especially repeated episodes of peritonitis [11]. We have reported that uremia is associated with inflammation of the peritoneal membrane [12]. Histologically, acute peritonitis can cause morphological damage to the peritoneum [10, 13]. Detachment and disintegration of mesothelial cells is observed, along with the appearance of fibrin exudation and numerous infiltrating cells, ultimately resulting in internal structures becoming unrecognizable [6]. Therefore, peritonitis plays a crucial role in the development of peritoneal damage leading to peritoneal membrane failure.
Animal models of peritonitis-induced peritoneal fibrosis are important for establishing new therapies to improve peritoneal damage induced by peritonitis.
Peritonitis models induced by bacteria or fungus
There are several reports of animal models of peritonitis induced by bacteria or fungi (Table 1). The pathogenic microorganisms used to induce peritonitis include Staphylococcus aureus [14,15,16,17,18], Staphylococcus epidermidis [19, 20], Pseudomonas aeruginosa [21], and Candida albicans [22]. These models of infectious peritonitis have been mainly used to elucidate the mechanism of inflammation in the membrane and the mechanism of acute peritoneal membrane failure. However, the acute peritonitis model is not typically used to study peritoneal fibrosis.
A catheter-induced model of gram-positive bacterial peritonitis has been developed, which is an acute bacterial peritonitis model with bacteria originating from skin flora due to lack of aseptic precautions [23,24,25]. In subsequent studies, these researchers used a model of lipopolysaccharide (LPS)-induced peritonitis instead of the gram-positive bacteria-induced peritonitis model [26, 27]. They investigated the role of nitric oxide (NO) released by endothelial NO synthase (eNOS) in the gram-positive bacterial peritonitis model [23] and the LPS-induced peritonitis model [27] and suggested that the selective inhibition of eNOS might ameliorate the poor peritoneal function caused by acute peritonitis. They reported that mice injected with LPS developed a cloudy dialysate with increased white blood cell counts and NO metabolite levels and inflammatory cell infiltration in the peritoneum. These observations are similar to those of the gram-positive peritonitis model.
The mechanisms of inflammation were studied in the bacterial and fungal peritonitis models; however, these models were not used to investigate the long-term complications such as fibrosis and neoangiogenesis.
Non-infectious peritonitis models
Currently, the number of reports in which investigators use the non-infectious peritonitis model is increasing. The non-infectious model is convenient and useful for handling animals and performing experiments. We suggest that a model of peritoneal fibrosis induced in a peritonitis model will help identify new strategies for preventing peritoneal fibrosis. Many studies have used the LPS-induced peritoneal injury model [26,27,28,29,30,31,32,33,34,35,36]. LPS derived from Escherichia coli (Sigma, St. Louis, MO) is frequently used [26,27,28,29,30, 33, 35]. A method involving a single LPS dose was used to study peritoneal inflammation and dysfunction [26,27,28,29]. Rat peritoneal inflammation and significant changes in neoangiogenesis were caused by daily administration of PDF over 3 weeks following an initial exposure to LPS [29,30,31,32,33,34,35].
Another non-infectious peritonitis model induced by administration of lyophilized cell-free supernatants from Staphylococcus epidermidis has been used to study the regulation of inflammation and leukocyte trafficking [11, 37]. Hurst et al. showed that interleukin-6 (IL-6)/soluble IL-6 receptor trans-signaling, which involves signal transducer and activator of transcription 3 (STAT3) activation, regulates chemokine secretion and polymorphonuclear neutrophil apoptosis in the peritoneal cavity. These mechanisms of inflammation and leukocyte trafficking have been clearly shown in the non-infectious model.
Here, we introduce a model of peritoneal fibrosis that we generated in rats and mice that is induced by acute inflammation with mechanical scraping, the so-called “scraping model.”
Scraping model
We first reported the scraping model as a non-infectious, peritonitis-induced peritoneal fibrosis model [38]. After opening the rat abdomen under anesthesia, the right parietal peritoneum received hand-driven scratching for 1 min using the edge of a 15-ml centrifuge tube (Fig. 1). Rats freely consumed food with or without NaCl loading after surgery [38,39,40]. Similarly, in mice, the right parietal peritoneum was scraped for 90 s with the cap of an injection needle.
Procedures to generate scraping model. Used by permission from Methods Mol Biol [40]
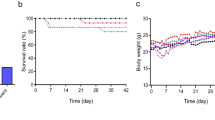
In this model (Fig. 2), neutrophil infiltration with fibrin exudation from the scraped peritoneum was demonstrated in 6 to 24 h after surgery. The predominant infiltrating cells switched to a mononuclear population on day 3, and inflammation gradually decreased thereafter. From days 7 to 14, the peritoneum became markedly thickened with the accumulation of alpha-smooth muscle actin (α-SMA)-positive fibroblasts and type III collagen. Mesothelial cells were not detected in 6 to 24 h after scraping, while approximately 30 and 70% of the total peritoneal length was covered with mesothelial cells on days 3 and 14, respectively. Increased CD31-positive blood vessel density was observed, which peaked at day 14. Transforming growth factor-β (TGF-β) and plasminogen activator inhibitor-1 (PAI-1), mainly expressed in the submesothelial compact zone, increased rapidly starting on day 3 and peaked at day 14. TGF-β and PAI-1 messenger RNA (mRNA) expression was upregulated from day 3 and peaked at day 7. In contrast, monocyte chemotactic protein-1 (MCP-1) mRNA rapidly increased and peaked at day 3. The pathology of this model in the early stage is characterized by strong infiltration of neutrophils and macrophages. The latter stage of this model is characterized by fibrosis and neoangiogenesis. In addition, peritoneal membrane permeability increased in rats that underwent bilateral scraping [38]. The pathological features and time course of this model are summarized in Figs. 2 and 3a.
Pathological findings of rat scraping model. Used by permission from Am J Physiology [38]
Using this model, we investigated the effects of mineralocorticoid receptor (MR) blockade with salt loading [38]. The local renin-angiotensin-aldosterone system (RAAS) is thought to play a role in peritoneal injury in PD patients [41]. Peritoneal mesothelial cells have been observed to express angiotensinogen, angiotensin-converting enzyme (ACE), and angiotensin II type 1 receptors (AT1R) [42, 43]. We found that MRs were expressed by rat fibroblasts and scraped peritoneum. Treatment with spironolactone suppressed macrophage infiltration, neoangiogenesis, and fibrosis, which is associated with the suppression of TGF-β and PAI-1 expression, thereby resulting in improvement of peritoneal dysfunction, including ultrafiltration, glucose transport, and albumin leakage [38]. The effects of spironolactone have also been shown in the LPS-induced peritoneal injury model [36].
In addition, we demonstrated the effects of atrial natriuretic peptide (ANP) in this model [39]. ANP has been used as a diuretic and vasodilator in clinical settings. ANP has been shown to play an important function in the inhibition of RAAS [44, 45]. ANP and brain natriuretic peptide (BNP) have been reported to prevent cardiac fibrosis [44, 46] and renal fibrosis [47,48,49], and to reduce infarct size in acute myocardial infarction [50]. We demonstrated that AT1R, ACE, and atrial natriuretic peptide receptor (NPR-A) mRNA expression were increased and peaked at days 14, 7, and 7, respectively. ANP administration resulted in a significant reduction in macrophage infiltration, fibrosis, and neoangiogenesis [39]. In this model, the salt loading progression of peritoneal fibrosis is likely to be involved in local RAAS activation. Administration of an MR blocker or ANP with antibiotics may prevent peritoneal membrane dysfunction associated with fibrosis and neoangiogenesis in human bacterial peritonitis. In a small study, 25 mg/day of spironolactone for 6 months was shown to reduce CD20 and collagen IV levels in the human peritoneal membrane [51]. Recently, the scraping model was used to study the effectiveness of cell therapy using the mesothelial cells to prevent peritoneal damage in PD patients [52].
Zymosan-induced fungal peritonitis model
By modifying the scraping model, we established the zymosan-induced fungal peritonitis model. Although fungal peritonitis is not common, yeast infection with the most common Candida species results in a poor outcome with high mortality [53,54,55]. The 2016 International Society for Peritoneal Dialysis guidelines recommends removal of the PD catheter in fungal peritonitis [56]. Several clinical observations have suggested that EPS could be induced by a single occurrence of fungal peritonitis [56,57,58,59,60]. The cell walls of many types of yeast activate various signaling reactions, including the complement system [61]. Complement maintains host homeostasis by eliminating microorganisms and irregular cells and also regulates cellular immunity. The complement system in the peritoneum is continuously active at low levels, and complement regulators (CRegs) regulate complement activation. Irregular activation of complement leads to tissue damage in many diseases [62, 63].
We demonstrated the expression of CRegs, Crry, CD55, and CD59 in rat peritoneum, especially along the mesothelial cell layer [64]. In rat peritoneum, combined blockade of Crry and CD59 induced severe focal inflammation with edema [65]. We examined the state of complement activation in the aforementioned rat scraping model, and C3 and C3b were transiently present in the inflammatory stage at day 3 [64]. Zymosan is abundant in the cell walls of fungi and activates the complement system through the alternative pathway [66].
We demonstrated that administration of zymosan after scraping promoted severe peritoneal injury that is pathologically similar to human fungal peritonitis. Zymosan (5 mg/rat/day, 2 mg/mouse/day) mixed with PDF was intraperitoneally injected into the rat or mouse abdominal cavity for up to 5 days after scraping the rat or mouse peritoneum [40, 64, 67]. Macroscopic findings in the zymosan rats showed the presence of a few white plaques at day 3, and yellow-white plaques at day 5, while no plaques were found in the control scraping model. Plaque fusion resulted in the formation of a yellow-white sheet covering the peritoneum with numerous small vessels running into the plaques, which suggests the occurrence of peritoneal neovascularization in the zymosan model at day 5. Peritoneal thickening associated with severe infiltration of inflammatory cells continued and remained present in the zymosan model at day 36, while the peritoneum was of normal appearance in the control scraping rats.
In recent experiments, we found that disease severity was affected by the lots of the zymosan (Sigma-Aldrich, St. Louis, MO). Expression of CRegs, Crry, CD55, and CD59 transiently decreased in the control scraping model at day 5. In contrast, CRegs expression was further decreased in the zymosan model at day 5 and continued decreasing up to day 18. Complement activation products, C3b and membrane attack complex (MAC), were clearly found in the zymosan model from days 1 to 5, and small amounts of these products remained at days 18 and 36. The time course of this model is summarized in Fig. 3b.
Systemic complement depletion by cobra venom factor or local suppression of complement activation by Crry-immunoglobulin or soluble complement receptor 1 dramatically reduced complement activation, peritoneal thickening, and inflammation. These findings clearly indicated that the zymosan model is a complement-dependent model of severe proliferative peritonitis [64]. Fungal peritonitis is known to be one of the causes of EPS. Subsequently, we successfully demonstrated that further enhancement of complement activation by inhibiting CRegs and enhancing systemic activation with cobra venom factor in the zymosan model induced fibrin exudation, which is the initial event of EPS [68].
Other models of non-infectious peritoneal injury associated with inflammation and fibrosis
Administration of PDF into the abdominal cavity of rats and mice by repeated intraperitoneal injection or implanting a catheter is a method used to study the pathophysiological changes of the peritoneum associated with PD [69,70,71,72,73,74,75,76,77,78,79,80,81,82,83], but a non-peritonitis model. Daily intraperitoneal injection of 4.25% glucose dialysate into the rat abdominal cavity for 1 week induced an increased peritoneal membrane transport rate and the absence of the peritoneal surface layer, as observed by electron microscopy [69, 70]. Daily injection of PDF (100 ml/kg, once or twice daily) was performed for up to 8 or 12 weeks to obtain morphological changes in the rat peritoneum [71,72,73]. Implantation of a silicon catheter into the rat abdomen was reported to amplify peritoneal inflammation from PDF through a foreign body reaction [74]. However, the peritoneum of rats that received only a puncture without infusion of any solution showed no functional or pathological changes [73, 75].
A model of renal insufficiency, such as 5/6 nephrectomy, was used in combination with PDF infusion to closely model the clinical situation of peritoneal dialysis patients and to understand the influence of PD on residual renal function [76, 77]. Daily intraperitoneal exposure of 1.5–3.0 ml of 4.25% glucose PDF for 4 or 5 weeks, with or without implanting a catheter, produced peritoneal dysfunction and morphological changes, such as fibrosis and neoangiogenesis, in mice [78,79,80,81,82,83].
Chronic intraperitoneal exposure to chemical irritation (chlorhexidine gluconate (CG)) is used as an experimental model of peritoneal fibrosis with inflammation and EPS. Suga et al. developed a CG-induced peritoneal fibrosis model in rats [84]. Daily injection of 0.1% CG in 15% ethanol, dissolved in 2–3 ml saline per 200 g body weight, was administered in the rat peritoneal cavity [85,86,87]. At day 7, the peritoneal tissue was partially thickened with edema and showed initial accumulation of connective tissues and modest cell migration. At day 14, significant alterations were found, including peritoneal thickening with edema, cell infiltration, and neoangiogenesis. At days 21 to 28, the peritoneal tissue was markedly thickened and showed remarkable proliferation of collagen fibers. The number of macrophages gradually increased in the thickened areas and reached a maximum at day 21. At day 28, neoangiogenesis had decreased, whereas collagen fibrils had accumulated. At day 35, fibrillary elements with cell infiltration occupied the submesothelial zone. Peritoneal resting for 3 weeks after 3 weeks of CG exposure ameliorated some functional parameters in the peritoneum; however, elevated peritoneal thickness and fibrosis continued during the resting period [88,89,90,91]. Placing an infusion pump in the rat abdominal cavity was reported as an alternative administration route for CG [92,93,94].
A lower dosage of CG is an option for producing mild peritoneal injury [95, 96]. Mice were given daily intraperitoneal administration of 0.3 ml or 10 ml saline/kg body weight containing 0.1% CG in 15% ethanol [97, 98]. Peritoneal fibrosis and increased infiltration of mononuclear cells were observed over time. Peritoneal fibrosis reached the chronic inflammatory stage, and macroscopic evidence of EPS was observed by 8 weeks. Lower doses of CG or shorter time courses produced milder and more infrequent development of peritoneal fibrosis [99, 100]. Recent studies showed that a standard peritoneal fibrosis model could be produced in mice following treatment with 0.1% CG every other day or three times a week for 1–3 weeks [101,102,103,104,105,106,107,108].
Glucose degradation products contained in PDF contribute to the biocompatibility of conventional PDF and are risk factors for EPS. Methylglyoxal (MGO) is an extremely toxic glucose degradation product, and administration of PDF containing MGO can be used as an animal peritoneal fibrosis model. Rats were given intraperitoneal injections of 100 ml/kg of 2.5% glucose PDF (pH 5.0) containing 20 mM MGO every day for 3 weeks [109,110,111]. Peritoneal function decreased significantly, and fibrous peritoneal thickening with proliferation of mesenchymal-like mesothelial cells and abdominal cocoon was induced. The combination of low doses of MGO and adenine-induced renal failure accelerated the progression of fibrous peritoneal thickening, whereas both MGO and renal failure alone did not [112]. Intraperitoneal injection of PDF (100 ml/kg) containing 20 or 40 mM MGO for five consecutive days per week for 3 weeks induced peritoneal injury in mice [113, 114]. We clearly showed the presence of severe lymphangiogenesis in the diaphragm of both CG and MGO models [96, 114]. TGF-β is a central mediator of peritoneal fibrosis. Overexpression of TGF-β1 driven by intraperitoneal adenovirus administration induced peritoneal fibrosis through epithelial mesenchymal transition, neoangiogenesis, and poor peritoneal function in mice [115,116,117,118] and rats [119, 120]. Other chemical irritants, such as deoxycholate [121], household bleach [122] and acidic solutions [123], were also reported to produce peritoneal inflammation, fibrosis, and abdominal cocoon in rats.
Conclusions
Non-infectious peritonitis models are convenient and useful for animal handling and performing experiments. The peritoneum in the scraping model showed signs of peritonitis initially and fibrosis at a later stage. These pathological changes, along with alterations in solute transport, mimic those observed in bacterial peritonitis. This model is useful for exploring strategies for the treatment and prevention of peritoneal fibrosis and membrane failure. The zymosan model is useful for studying the mechanisms of fungal peritonitis and the drugs used to reduce peritoneal damage induced by fungal peritonitis. Anti-complement therapy might be useful as a therapeutic in human fungal peritonitis and related peritoneal damage. Other non-infectious models, such as CG and MGO models, are also useful for investigating the pathophysiology of fibrosis with inflammation, angiogenesis, and lymphangiogenesis.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ANP:
-
Atrial natriuretic peptide
- AT1R:
-
Angiotensin II type 1 receptors
- BNP:
-
Brain natriuretic peptide
- CG:
-
Chlorhexidine gluconate
- CRegs:
-
Complement regulators
- eNOS:
-
Endothelial NO synthase
- IL-6:
-
Interleukin-6
- LPS:
-
Lipopolysaccharide
- MAC:
-
Membrane attack complex
- MCP-1:
-
Monocyte chemotactic protein-1
- MGO:
-
Methylglyoxal
- MR:
-
Mineralocorticoid receptor
- NO:
-
Nitric oxide
- NPR-A:
-
Natriuretic peptide receptor
- PD:
-
Peritoneal dialysis
- PDF:
-
PD fluid
- RAAS:
-
Renin-angiotensin-aldosterone system
- STAT3:
-
Signal transducer and activator of transcription 3
- TGF-β:
-
Transforming growth factor-β
- α-SMA:
-
Alpha-smooth muscle actin
References
Brown MC, Simpson K, Kerssens JJ, Mactier RA, Scottish Renal Registry. Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post-millennium (2000–2007). Perit Dial Int. 2011;31:639–50.
Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002–2003. Perit Dial Int. 2009;29:297–302.
Pajek J, Hutchison AJ, Bhutani S, Brenchley PE, Hurst H, Perme MP, et al. Outcomes of peritoneal dialysis patients and switching to hemodialysis: a competing risks analysis. Perit Dial Int. 2014;34:289–98.
Mizuno M, Ito Y, Tanaka A, Suzuki Y, Hiramatsu H, Watanabe M, et al. Peritonitis is still an important factor for withdrawal from peritoneal dialysis therapy in the Tokai area of Japan. Clin Exp Nephrol. 2011;15:727–37.
Mizuno M, Ito Y, Suzuki Y, Sakata F, Saka Y, Hiramatsu T, et al. Recent analysis of status and outcomes of peritoneal dialysis in the Tokai area of Japan: the second report of the Tokai peritoneal dialysis registry. Clin Exp Nephrol. 2016;20:960–97.
Tawada M, Ito Y, Hamada C, Honda K, Mizuno M, Suzuki Y, et al. Vascular endothelial cell injury is an important factor in the development of encapsulating peritoneal sclerosis in long-term peritoneal dialysis patients. PLoS One. 2016;11:e0154644.
Nakao M, Yokoyama K, Yamamoto I, Matsuo N, Tanno Y, Ohkido I, et al. Risk factors for encapsulating peritoneal sclerosis in long-term peritoneal dialysis: a retrospective observational study. Ther Apher Dial. 2014;18:68–73.
Kawanishi H, Moriishi M. Epidemiology of encapsulating peritoneal sclerosis in Japan. Perit Dial Int. 2005;25(Suppl 4):S14–8.
Szeto CC, Kwan BC, Chow KM, Chung S, Yu V, Cheng PM, et al. Predictors of residual renal function decline in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 2015;35:180–8.
Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–9.
Devuyst O, Margetts PJ, Topley N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol. 2010;21:1077–85.
Sawai A, Ito Y, Mizuno M, Suzuki Y, Toda S, Ito I, et al. Peritoneal macrophage infiltration is correlated with baseline peritoneal solute transport rate in peritoneal dialysis patients. Nephrol Dial Transplant. 2011;26:2322–32.
Verger C, Luger A, Moore HL, Nolph KD. Acute changes in peritoneal morphology and transport properties with infectious peritonitis and mechanical injury. Kidney Int. 1983;23:823–31.
Catalan MP, Esteban J, Subirá D, Egido J, Ortiz A, Grupo de Estudios Peritoneales de Madrid-FRIAT/IRSIN. Inhibition of caspases improves bacterial clearance in experimental peritonitis. Perit Dial Int. 2003;23:123–6.
Calame W, Afram C, Blijleven N, Hendrickx RJ, Namavar F, Beelen RH. Establishing an experimental infection model for peritoneal dialysis: effect of inoculum and volume. Perit Dial Int. 1993;13(Suppl 2):S79–80.
Welten AG, Zareie M, van den Born J, ter Wee PM, Schalkwijk CG, Driesprong BA, et al. In vitro and in vivo models for peritonitis demonstrate unchanged neutrophil migration after exposure to dialysis fluids. Nephrol Dial Transplant. 2004;19:831–9.
van Westrhenen R, Westra WM, van den Born J, Krediet RT, Keuning ED, Hiralall J, et al. Alpha-2-macroglobulin and albumin are useful serum proteins to detect subclinical peritonitis in the rat. Perit Dial Int. 2006;26:101–7.
Akman S, Koyun M, Gelen T, Coskun M. Comparison of intraperitoneal antithrombin III and heparin in experimental peritonitis. Pediatr Nephrol. 2008;23:1327–30.
Mactier RA, Moore H, Khanna R, Shah J. Effect of peritonitis on insulin and glucose absorption during peritoneal dialysis in diabetic rats. Nephron. 1990;54:240–4.
Gallimore B, Gagnon RF, Richards GK. Response of chronic renal failure mice to peritoneal Staphylococcus epidermidis challenge: impact of repeated peritoneal instillation of dialysis solution. Am J Kidney Dis. 1989;14:184–95.
Finelli A, Burrows LL, DiCosmo FA, DiTizio V, Sinnadurai S, Oreopoulos DG, et al. Colonization-resistant antimicrobial-coated peritoneal dialysis catheters: evaluation in a newly developed rat model of persistent Pseudomonas aeruginosa peritonitis. Perit Dial Int. 2002;22:27–31.
Kretschmar M, Hube B, Bertsch T, Sanglard D, Merker R, Schröder M, et al. Germ tubes and proteinase activity contribute to virulence of Candida albicans in murine peritonitis. Infect Immun. 1999;67:6637–42.
Ni J, Moulin P, Gianello P, Feron O, Balligand JL, Devuyst O. Mice that lack endothelial nitric oxide synthase are protected against functional and structural modifications induced by acute peritonitis. J Am Soc Nephrol. 2003;14:3205–16.
Combet S, Van Landschoot M, Moulin P, Piech A, Verbavatz JM, Goffin E, et al. Regulation of aquaporin-1 and nitric oxide synthase isoforms in a rat model of acute peritonitis. J Am Soc Nephrol. 1999;10:2185–96.
Ferrier ML, Combet S, van Landschoot M, Stoenoiu MS, Cnops Y, Lameire N, et al. Inhibition of nitric oxide synthase reverses changes in peritoneal permeability in a rat model of acute peritonitis. Kidney Int. 2001;60:2343–50.
Ni J, Cnops Y, McLoughlin RM, Topley N, Devuyst O. Inhibition of nitric oxide synthase reverses permeability changes in a mouse model of acute peritonitis. Perit Dial Int. 2005;25(Suppl 3):S11–4.
Ni J, McLoughlin RM, Brodovitch A, Moulin P, Brouckaert P, Casadei B, et al. Nitric oxide synthase isoforms play distinct roles during acute peritonitis. Nephrol Dial Transplant. 2010;25:86–96.
Breborowicz A, Połubinska A, Wu G, Tam P, Oreopoulos DG. N-acetylglucosamine reduces inflammatory response during acute peritonitis in uremic rats. Blood Purif. 2006;24:274–81.
Korybalska K, Wieczorowska-Tobis K, Polubinska A, Wisniewska J, Moberly J, Martis L, et al. L-2-oxothiazolidine-4-carboxylate: an agent that modulates lipopolysaccharide-induced peritonitis in rats. Perit Dial Int. 2002;22:293–300.
Kim YL, Kim SH, Kim JH, Kim SJ, Kim CD, Cho DK, et al. Effects of peritoneal rest on peritoneal transport and peritoneal membrane thickening in continuous ambulatory peritoneal dialysis rats. Perit Dial Int. 1999;19(Suppl 2):S384–7.
Margetts PJ, Kolb M, Yu L, Hoff CM, Gauldie J. A chronic inflammatory infusion model of peritoneal dialysis in rats. Perit Dial Int. 2001;21(Suppl 3):S368–72.
Margetts PJ, Gyorffy S, Kolb M, Yu L, Hoff CM, Holmes CJ, et al. Antiangiogenic and antifibrotic gene therapy in a chronic infusion model of peritoneal dialysis in rats. J Am Soc Nephrol. 2002;13:721–8.
Park SH, Lee EG, Kim IS, Kim YJ, Cho DK, Kim YL. Effect of glucose degradation products on the peritoneal membrane in a chronic inflammatory infusion model of peritoneal dialysis in the rat. Perit Dial Int. 2004;24:115–22.
Nie J, Hao W, Dou X, Wang X, Luo N, Lan HY, et al. Effects of Smad7 overexpression on peritoneal inflammation in a rat peritoneal dialysis model. Perit Dial Int. 2007;27:580–8.
Song SH, Kwak IS, Yang BY, Lee DW, Lee SB, Lee MY. Role of rosiglitazone in lipopolysaccharide-induced peritonitis: a rat peritoneal dialysis model. Nephrology (Carlton). 2009;14:155–63.
Zhang L, Hao JB, Ren LS, Ding JL, Hao LR. The aldosterone receptor antagonist spironolactone prevents peritoneal inflammation and fibrosis. Lab Invest. 2014;94:839–50.
Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–14.
Nishimura H, Ito Y, Mizuno M, Tanaka A, Morita Y, Maruyama S, et al. Mineralocorticoid receptor blockade ameliorates peritoneal fibrosis in new rat peritonitis model. Am J Physiol Renal Physiol. 2008;294:F1084–93.
Kato H, Mizuno T, Mizuno M, Sawai A, Suzuki Y, Kinashi H, et al. Atrial natriuretic peptide ameliorates peritoneal fibrosis in rat peritonitis model. Nephrol Dial Transplant. 2012;27:526–36.
Mizuno M, Ito Y. Rat models of acute and/or chronic peritoneal injuries including peritoneal fibrosis and peritoneal dialysis complications. Methods Mol Biol. 2016;1397:35–43.
Nessim SJ, Perl J, Bargman JM. The renin-angiotensin-aldosterone system in peritoneal dialysis: is what is good for the kidney also good for the peritoneum? Kidney Int. 2010;78:23–8.
Noh H, Ha H, Yu MR, Kim YO, Kim JH, Lee HB. Angiotensin II mediates high glucose-induced TGF-beta1 and fibronectin upregulation in HPMC through reactive oxygen species. Perit Dial Int. 2005;25:38–47.
Kiribayashi K, Masaki T, Naito T, Ogawa T, Ito T, Yorioka N, et al. Angiotensin II induces fibronectin expression in human peritoneal mesothelial cells via ERK1/2 and p38 MAPK. Kidney Int. 2005;67:1126–35.
Tsuneyoshi H, Nishina T, Nomoto T, Kanemitsu H, Kawakami R, Unimonh O, et al. Atrial natriuretic peptide helps prevent late remodeling after left ventricular aneurysm repair. Circulation. 2004;110(11 Suppl 1):II174–9.
Kasama S, Furuya M, Toyama T, Ichikawa S, Kurabayashi M. Effect of atrial natriuretic peptide on left ventricular remodelling in patients with acute myocardial infarction. Eur Heart J. 2008;29:1485–94.
Ito T, Yoshimura M, Nakamura S, Nakayama M, Shimasaki Y, Harada E, et al. Inhibitory effect of natriuretic peptides on aldosterone synthase gene expression in cultured neonatal rat cardiocytes. Circulation. 2003;107:807–10.
Kasahara M, Mukoyama M, Sugawara A, Makino H, Suganami T, Ogawa Y, et al. Ameliorated glomerular injury in mice overexpressing brain natriuretic peptide with renal ablation. J Am Soc Nephrol. 2000;11:1691–701.
Suganami T, Mukoyama M, Sugawara A, Mori K, Nagae T, Kasahara M, et al. Overexpression of brain natriuretic peptide in mice ameliorates immune-mediated renal injury. J Am Soc Nephrol. 2001;12:2652–63.
Makino H, Mukoyama M, Mori K, Suganami T, Kasahara M, Yahata K, et al. Transgenic overexpression of brain natriuretic peptide prevents the progression of diabetic nephropathy in mice. Diabetologia. 2006;49:2514–24.
Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–93.
Vazquez-Rangel A, Soto V, Escalona M, Toledo RG, Castillo EA, Polanco Flores NA, et al. Spironolactone to prevent peritoneal fibrosis in peritoneal dialysis patients: a randomized controlled trial. Am J Kidney Dis. 2014;63:1072–4.
Kitamura S, Horimoto N, Tsuji K, Inoue A, Takiue K, Sugiyama H, et al. The selection of peritoneal mesothelial cells is important for cell therapy to prevent peritoneal fibrosis. Tissue Eng Part A. 2014;20:529–39.
Nagappan R, Collins JF, Lee WT. Fungal peritonitis in continuous ambulatory peritoneal dialysis—the Auckland experience. Am J Kidney Dis. 1992;20:492–6.
Wang AY, Yu AW, Li PK, Lam PK, Leung CB, Lai KN, et al. Factors predicting outcome of fungal peritonitis in peritoneal dialysis: analysis of a 9-year experience of fungal peritonitis in a single center. Am J Kidney Dis. 2000;36:1183–92.
Felgueiras J, del Peso G, Bajo A, Hevia C, Romero S, Celadilla O, et al. Risk of technique failure and death in fungal peritonitis is determined mainly by duration on peritoneal dialysis: single-center experience of 24 years. Adv Perit Dial. 2006;22:77–81.
Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36:481–508.
Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant. 1998;13:154–9.
Lee HY, Kim BS, Choi HY, Park HC, Kang SW, Choi KH, et al. Sclerosing encapsulating peritonitis as a complication of long-term continuous ambulatory peritoneal dialysis in Korea. Nephrology (Carlton). 2003;8(Suppl 1):S33–9.
Gupta S, Woodrow G. Successful treatment of fulminant encapsulating peritoneal sclerosis following fungal peritonitis with tamoxifen. Clin Nephrol. 2007;68:125–9.
Trigka K, Dousdampanis P, Chu M, Khan S, Ahmad M, Bargman JM, et al. Encapsulating peritoneal sclerosis: a single-center experience and review of the literature. Int Urol Nephrol. 2011;43:519–26.
Sorenson WG, Shahan TA, Simpson J. Cell wall preparations from environmental yeasts: effect on alveolar macrophage function in vitro. Ann Agric Environ Med. 1998;5:65–71.
Mizuno M, Morgan BP. The possibilities and pitfalls for anti-complement therapies in inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2004;3:87–96.
Mizuno M. A review of current knowledge of the complement system and the therapeutic opportunities in inflammatory arthritis. Curr Med Chem. 2006;13:1707–17.
Mizuno M, Ito Y, Hepburn N, Mizuno T, Noda Y, Yuzawa Y, et al. Zymosan, but not lipopolysaccharide, triggers severe and progressive peritoneal injury accompanied by complement activation in a rat peritonitis model. J Immunol. 2009;183:1403–12.
Mizuno T, Mizuno M, Morgan BP, Noda Y, Yamada K, Okada N, et al. Specific collaboration between rat membrane complement regulators Crry and CD59 protects peritoneum from damage by autologous complement activation. Nephrol Dial Transplant. 2011;26:1821–30.
Rawal N, Pangburn MK. C5 convertase of the alternative pathway of complement. Kinetic analysis of the free and surface-bound forms of the enzyme. J Biol Chem. 1998;273:16828–35.
Kim H, Mizuno M, Furuhashi K, Katsuno T, Ozaki T, Yasuda K, et al. Rat adipose tissue-derived stem cells attenuate peritoneal injuries in rat zymosan-induced peritonitis accompanied by complement activation. Cytotherapy. 2014;16:357–68.
Mizuno M, Ito Y, Mizuno T, Harris CL, Suzuki Y, Okada N, et al. Membrane complement regulators protect against fibrin exudation increases in a severe peritoneal inflammation model in rats. Am J Physiol Renal Physiol. 2012;302:F1245–51.
Guo QY, Peng WX, Cheng HH, Ye RG, Lindholm B, Wang T. Hyaluronan preserves peritoneal membrane transport properties. Perit Dial Int. 2001;21:136–42.
Wang T, Cheng HH, Liu SM, Wang Y, Wu JL, Peng WX, et al. Increased peritoneal membrane permeability is associated with abnormal peritoneal surface layer. Perit Dial Int. 2001;21(Suppl 3):S345–8.
Chunming J, Miao Z, Cheng S, Nana T, Wei Z, Dongwei C, et al. Tanshinone IIA attenuates peritoneal fibrosis through inhibition of fibrogenic growth factors expression in peritoneum in a peritoneal dialysis rat model. Ren Fail. 2011;33:355–62.
Lee EA, Oh JH, Lee HA, Kim SI, Park EW, Park KB, et al. Structural and functional alterations of the peritoneum after prolonged exposure to dialysis solutions: role of aminoguanidine. Perit Dial Int. 2001;21:245–53.
Musi B, Braide M, Carlsson O, Wieslander A, Albrektsson A, Ketteler M, et al. Biocompatibility of peritoneal dialysis fluids: long-term exposure of nonuremic rats. Perit Dial Int. 2004;24:37–47.
Flessner MF, Credit K, Richardson K, Potter R, Li X, He Z, et al. Peritoneal inflammation after twenty-week exposure to dialysis solution: effect of solution versus catheter-foreign body reaction. Perit Dial Int. 2010;30:284–93.
Zeltzer E, Klein O, Rashid G, Katz D, Korzets Z, Bernheim J. Intraperitoneal infusion of glucose-based dialysate in the rat—an animal model for the study of peritoneal advanced glycation end-products formation and effect on peritoneal transport. Perit Dial Int. 2000;20:656–61.
Nakao A, Nakao K, Takatori Y, Kojo S, Inoue J, Akagi S, et al. Effects of icodextrin peritoneal dialysis solution on the peritoneal membrane in the STZ-induced diabetic rat model with partial nephrectomy. Nephrol Dial Transplant. 2010;25:1479–88.
Kihm LP, Müller-Krebs S, Klein J, Ehrlich G, Mertes L, Gross ML, et al. Benfotiamine protects against peritoneal and kidney damage in peritoneal dialysis. J Am Soc Nephrol. 2011;22:914–26.
Wang J, Jiang ZP, Su N, Fan JJ, Ruan YP, Peng WX, et al. The role of peritoneal alternatively activated macrophages in the process of peritoneal fibrosis related to peritoneal dialysis. Int J Mol Sci. 2013;14:10369–82.
Duan WJ, Yu X, Huang XR, Yu JW, Lan HY. Opposing roles for Smad2 and Smad3 in peritoneal fibrosis in vivo and in vitro. Am J Pathol. 2014;184:2275–84.
Yu JW, Duan WJ, Huang XR, Meng XM, Yu XQ, Lan HY. MicroRNA-29b inhibits peritoneal fibrosis in a mouse model of peritoneal dialysis. Lab Invest. 2014;94:978–90.
Aroeira LS, Lara-Pezzi E, Loureiro J, Aguilera A, Ramírez-Huesca M, González-Mateo G, et al. Cyclooxygenase-2 mediates dialysate-induced alterations of the peritoneal membrane. J Am Soc Nephrol. 2009;20:582–92.
Loureiro J, Aguilera A, Selgas R, Sandoval P, Albar-Vizcaíno P, Pérez-Lozano ML, et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol. 2011;22:1682–95.
Loureiro J, Sandoval P, del Peso G, Gónzalez-Mateo G, Fernández-Millara V, Santamaria B, et al. Tamoxifen ameliorates peritoneal membrane damage by blocking mesothelial to mesenchymal transition in peritoneal dialysis. PLoS One. 2013;8:e61165.
Suga H, Teraoka S, Ota K, Komemushi S, Furutani S, Yamauchi S, et al. Preventive effect of pirfenidone against experimental sclerosing peritonitis in rats. Exp Toxicol Pathol. 1995;47:287–91.
Mishima Y, Miyazaki M, Abe K, Ozono Y, Shioshita K, Xia Z, et al. Enhanced expression of heat shock protein 47 in rat model of peritoneal fibrosis. Perit Dial Int. 2003;23:14–22.
Nishino T, Miyazaki M, Abe K, Furusu A, Mishima Y, Harada T, et al. Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress peritoneal fibrosis in rats. Kidney Int. 2003;64:887–96.
Io H, Hamada C, Ro Y, Ito Y, Hirahara I, Tomino Y. Morphologic changes of peritoneum and expression of VEGF in encapsulated peritoneal sclerosis rat models. Kidney Int. 2004;65:1927–36.
Bozkurt D, Hur E, Ulkuden B, Sezak M, Nar H, Purclutepe O, et al. Can N-acetylcysteine preserve peritoneal function and morphology in encapsulating peritoneal sclerosis? Perit Dial Int. 2009;29(Suppl 2):S202–5.
Bozkurt D, Sipahi S, Cetin P, Hur E, Ozdemir O, Ertilav M, et al. Does immunosuppressive treatment ameliorate morphology changes in encapsulating peritoneal sclerosis? Perit Dial Int. 2009;29(Suppl 2):S206–10.
Ertilav M, Hur E, Bozkurt D, Sipahi S, Timur O, Sarsik B, et al. Octreotide lessens peritoneal injury in experimental encapsulated peritoneal sclerosis model. Nephrology (Carlton). 2011;16:552–7.
Huddam B, Başaran M, Koçak G, Azak A, Yalçın F, Reyhan NH, et al. The use of mycophenolate mofetil in experimental encapsulating peritoneal sclerosis. Int Urol Nephrol. 2015;47:1423–8.
Komatsu H, Uchiyama K, Tsuchida M, Isoyama N, Matsumura M, Hara T, et al. Development of a peritoneal sclerosis rat model using a continuous-infusion pump. Perit Dial Int. 2008;28:641–7.
Kanda R, Hamada C, Kaneko K, Nakano T, Wakabayashi K, Hara K, et al. Paracrine effects of transplanted mesothelial cells isolated from temperature-sensitive SV40 large T-antigen gene transgenic rats during peritoneal repair. Nephrol Dial Transplant. 2014;29:289–300.
Wakabayashi K, Hamada C, Kanda R, Nakano T, Io H, Horikoshi S, et al. Adipose-derived mesenchymal stem cells transplantation facilitate experimental peritoneal fibrosis repair by suppressing epithelial-mesenchymal transition. J Nephrol. 2014;27:507–14.
Saito H, Kitamoto M, Kato K, Liu N, Kitamura H, Uemura K, et al. Tissue factor and factor v involvement in rat peritoneal fibrosis. Perit Dial Int. 2009;29:340–51.
Kinashi H, Ito Y, Mizuno M, Suzuki Y, Terabayashi T, Nagura F, et al. TGF-β1 promotes lymphangiogenesis during peritoneal fibrosis. J Am Soc Nephrol. 2013;24:1627–42.
Ishii Y, Sawada T, Shimizu A, Tojimbara T, Nakajima I, Fuchinoue S, et al. An experimental sclerosing encapsulating peritonitis model in mice. Nephrol Dial Transplant. 2001;16:1262–6.
Sawada T, Ishii Y, Tojimbara T, Nakajima I, Fuchinoue S, Teraoka S. The ACE inhibitor, quinapril, ameliorates peritoneal fibrosis in an encapsulating peritoneal sclerosis model in mice. Pharmacol Res. 2002;46:505–10.
Tanabe K, Maeshima Y, Ichinose K, Kitayama H, Takazawa Y, Hirokoshi K, et al. Endostatin peptide, an inhibitor of angiogenesis, prevents the progression of peritoneal sclerosis in a mouse experimental model. Kidney Int. 2007;71:227–38.
Fukuoka N, Sugiyama H, Inoue T, Kikumoto Y, Takiue K, Morinaga H, et al. Increased susceptibility to oxidant-mediated tissue injury and peritoneal fibrosis in acatalasemic mice. Am J Nephrol. 2008;28:661–8.
Yoshio Y, Miyazaki M, Abe K, Nishino T, Furusu A, Mizuta Y, et al. TNP-470, an angiogenesis inhibitor, suppresses the progression of peritoneal fibrosis in mouse experimental model. Kidney Int. 2004;66:1677–85.
Nakav S, Kachko L, Vorobiov M, Rogachev B, Chaimovitz C, Zlotnik M, et al. Blocking adenosine A2A receptor reduces peritoneal fibrosis in two independent experimental models. Nephrol Dial Transplant. 2009;24:2392–9.
Kokubo S, Sakai N, Furuichi K, Toyama T, Kitajima S, Okumura T, et al. Activation of p38 mitogen-activated protein kinase promotes peritoneal fibrosis by regulating fibrocytes. Perit Dial Int. 2012;32:10–9.
Yokoi H, Kasahara M, Mori K, Ogawa Y, Kuwabara T, Imamaki H, et al. Pleiotrophin triggers inflammation and increased peritoneal permeability leading to peritoneal fibrosis. Kidney Int. 2012;81:160–9.
Nishino T, Ashida R, Obata Y, Furusu A, Abe K, Miyazaki M, et al. Involvement of lymphocyte infiltration in the progression of mouse peritoneal fibrosis model. Ren Fail. 2012;34:760–6.
Sekiguchi Y, Hamada C, Ro Y, Nakamoto H, Inaba M, Shimaoka T, et al. Differentiation of bone marrow-derived cells into regenerated mesothelial cells in peritoneal remodeling using a peritoneal fibrosis mouse model. J Artif Organs. 2012;15:272–82.
Hirose M, Nishino T, Obata Y, Nakazawa M, Nakazawa Y, Furusu A, et al. 22-Oxacalcitriol prevents progression of peritoneal fibrosis in a mouse model. Perit Dial Int. 2013;33:132–42.
Yokoi H, Kasahara M, Mori K, Kuwabara T, Toda N, Yamada R, et al. Peritoneal fibrosis and high transport are induced in mildly pre-injured peritoneum by 3,4-dideoxyglucosone-3-ene in mice. Perit Dial Int. 2013;33:143–54.
Hirahara I, Kusano E, Yanagiba S, Miyata Y, Ando Y, Muto S, et al. Peritoneal injury by methylglyoxal in peritoneal dialysis. Perit Dial Int. 2006;26:380–92.
Hirahara I, Ishibashi Y, Kaname S, Kusano E, Fujita T. Methylglyoxal induces peritoneal thickening by mesenchymal-like mesothelial cells in rats. Nephrol Dial Transplant. 2009;24:437–47.
Hirahara I, Sato H, Imai T, Onishi A, Morishita Y, Muto S, et al. Methylglyoxal induced basophilic spindle cells with podoplanin at the surface of peritoneum in rat peritoneal dialysis model. Biomed Res Int. 2015;2015:289751.
Onishi A, Akimoto T, Morishita Y, Hirahara I, Inoue M, Kusano E, et al. Peritoneal fibrosis induced by intraperitoneal methylglyoxal injection: the role of concurrent renal dysfunction. Am J Nephrol. 2014;40:381–90.
Kitamura M, Nishino T, Obata Y, Furusu A, Hishikawa Y, Koji T, et al. Epigallocatechin gallate suppresses peritoneal fibrosis in mice. Chem Biol Interact. 2012;195:95–104.
Terabayashi T, Ito Y, Mizuno M, Suzuki Y, Kinashi H, Sakata F, et al. Vascular endothelial growth factor receptor-3 is a novel target to improve net ultrafiltration in methylglyoxal-induced peritoneal injury. Lab Invest. 2015;95:1029–43.
Liu L, Shi CX, Ghayur A, Zhang C, Su JY, Hoff CM, et al. Prolonged peritoneal gene expression using a helper-dependent adenovirus. Perit Dial Int. 2009;29:508–16.
Patel P, Sekiguchi Y, Oh KH, Patterson SE, Kolb MR, Margetts PJ. Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int. 2010;77:319–28.
Margetts PJ, Hoff C, Liu L, Korstanje R, Walkin L, Summers A, et al. Transforming growth factor β-induced peritoneal fibrosis is mouse strain dependent. Nephrol Dial Transplant. 2013;28:2015–27.
Padwal M, Siddique I, Wu L, Tang K, Boivin F, Liu L, et al. Matrix metalloproteinase 9 is associated with peritoneal membrane solute transport and induces angiogenesis through β-catenin signaling. Nephrol Dial Transplant. 2016. doi:10.1093/ndt/qfw076.
Margetts PJ, Kolb M, Galt T, Hoff CM, Shockley TR, Gauldie J. Gene transfer of transforming growth factor-beta1 to the rat peritoneum: effects on membrane function. J Am Soc Nephrol. 2001;12:2029–39.
Margetts PJ, Bonniaud P, Liu L, Hoff CM, Holmes CJ, West-Mays JA, et al. Transient overexpression of TGF-β1 induces epithelial mesenchymal transition in the rodent peritoneum. J Am Soc Nephrol. 2005;16:425–36.
Gotloib L, Wajsbrot V, Cuperman Y, Shostak A. Acute oxidative stress induces peritoneal hyperpermeability, mesothelial loss, and fibrosis. J Lab Clin Med. 2004;143:31–40.
Levine S, Saltzman A. Abdominal cocoon: an animal model for a complication of peritoneal dialysis. Perit Dial Int. 1996;16:613–6.
Nakamoto H, Imai H, Ishida Y, Yamanouchi Y, Inoue T, Okada H, et al. New animal models for encapsulating peritoneal sclerosis—role of acidic solution. Perit Dial Int. 2001;21(Suppl 3):S349–53.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (YI, # 20590972).
Funding
There is no funding. The authors declare that no financial conflict of interest exists.
Availability of data and materials
Figures 1 and 2 were used under permission from Methods Mol Biol [40] and Am J Physiology [38].
Authors’ contributions
YI and HK planned the study, searched and collected the literatures, and wrote the manuscript. All the authors wrote the manuscript partly. TK, YS, and MM discussed the contents of the manuscript with YI and HK. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ito, Y., Kinashi, H., Katsuno, T. et al. Peritonitis-induced peritoneal injury models for research in peritoneal dialysis review of infectious and non-infectious models. Ren Replace Ther 3, 16 (2017). https://doi.org/10.1186/s41100-017-0100-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-017-0100-4