Abstract
Background
Available data in the literature comparing different induction chemotherapy (IC) regimens on locoregionally advanced nasopharyngeal carcinoma (NPC) are scarce. The purpose of the present study was to evaluate the outcomes of locoregionally advanced NPC patients who were treated with taxane, cisplatin and 5-fluorouracil (TPF) or cisplatin and 5-fluorouracil (PF) as IC followed by concurrent chemoradiotherapy (CCRT).
Methods
In total, 1879 patients with locoregionally advanced NPC treated with IC and CCRT from a prospectively maintained database were included in the present observational study. We compared overall survival (OS), disease-specific survival (DSS), distant metastasis-free survival (DMFS), and locoregional relapse-free survival, using the propensity score method.
Results
In total, 1256 patients received TPF or PF as IC backbone. The TPF group showed significantly better OS (hazard ratio [HR], 0.660; 95% confidence interval [CI] 0.442–0.986; P = 0.042), DSS (HR, 0.624; 95% CI 0.411–0.947; P = 0.027) and DMFS (HR, 0.589; 95% CI 0.406–0.855; P = 0.005) compared with the PF group in multivariable analyses. Propensity score matching identified 294 patients in each cohort and confirmed that TPF was associated with significantly improved 5-year OS (88.1% vs. 80.7%; P = 0.042), DSS (88.5% vs. 80.7%; P = 0.021) and DMFS (87.9% vs. 78.6%; P = 0.012) rates compared with the PF group. There were no significant differences in locoregional relapse-free survival before or after matching.
Conclusions
In our study, IC with the TPF regimen combined with CCRT showed improved long-term survival for the patients with locoregionally advanced NPC compared with the PF regimen. However, a prospective randomized clinical trial to validate these findings is necessary.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is a distinct form of head and neck cancer in terms of its etiology, epidemiology, pathology, clinical presentation, and treatment responses [1, 2]. Because of its unique anatomical location and mild, non-specific symptoms, 60–70% of patients present with locoregionally advanced disease at diagnosis [3]. Due to its radiosensitive properties and deep-seated location, radiotherapy is the cornerstone of initial treatment [2, 4, 5]. Moreover, concurrent chemoradiotherapy (CCRT) has been shown to improve survival and is considered the standard-of-care after the landmark intergroup 0099 trial [6]. However, distant metastasis remains a key problem; more than 30–40% of locoregionally advanced NPC patients will develop distant metastasis after standard therapy [7]. Thus, a more efficacious treatment regimen is needed.
Induction chemotherapy (IC) has advantages over adjuvant chemotherapy, including improved tolerability and the early eradication of micrometastases [8, 9]. Recently, several randomized studies and meta-analyses have demonstrated that IC significantly improved disease-free survival (DFS), overall survival (OS) and distant metastasis-free survival (DMFS) [10,11,12,13,14]. Based on these encouraging results, sequential IC followed by CCRT has been included as an option in both the National Comprehensive Cancer Network (category III evidence) [15] and the EHNS–ESMO–ESTRO clinical practice guidelines (category IIB evidence) [16]. Nevertheless, the best IC regimen for NPC has not been defined.
Several randomized trials have demonstrated that OS and progression free survival (PFS) in head and neck cancer were significantly increased by an IC regimen consisting of taxane, cisplatin, and fluorouracil (TPF) compared with cisplatin and fluorouracil (PF) [17, 18]. These trials confirmed TPF as the optimal IC treatment regimen for head and neck cancer. In locoregionally advanced NPC, Sun et al. [11] found that compared with CCRT alone, IC based on TPF plus CCRT significantly improved OS; the 3-year OS rate of the IC group was 92%, which was higher than the group with IC based on PF in previous randomized trials [14, 19]. It remains unknown whether TPF significantly prolongs survival compared to PF as an IC regimen in locoregionally advanced NPC. Thus, we conducted a retrospective, propensity score-matched (PSM) analysis of locoregionally advanced NPC patients who received IC, which either did or did not contain taxane in combination with cisplatin and fluorouracil (TPF or PF, respectively).
Patients and methods
Patient selection
We identified patients with newly diagnosed, biopsy proven, stage III–IVb NPC according to the American Joint Committee on Cancer classification system who were treated at Sun Yat-sen University Cancer Center between January 1, 2000 and June 1, 2013. Patients who received IC + CCRT as primary treatment (with either TPF or PF as the IC backbone) were included. Patients who had received other anticancer agents in addition to these initial treatments, had missing medical data, or died during radiotherapy were excluded. Additional information, including demographics, pathological diagnosis, date of diagnosis, imaging results, family history, smoking history, Karnofsky Performance Status (KPS), chemotherapy pattern and drugs, radiation technology and dosage, and follow-up were collected from the hospital information system and paper medical records. T- and N-stages were re-categorized based on the original magnetic resonance imaging (MRI)/computed tomography (CT) imaging, and all patients were re-staged according to the 7th edition of the American Joint Committee on Cancer classification system.
This study was performed in accordance with the Institutional Review Boards of our institution. Written informed consent was obtained from each patient, including signed consent for tissue analysis and consent to be recorded for potential medical research at the time of sample acquisition.
IC
TPF-treated patients received docetaxel (60 mg/m2) or paclitaxel (150 mg/m2) and cisplatin (60 mg/m2) as a 4-h intravenous infusion on day 1, followed by fluorouracil (600 mg/m2) as a 24-h continuous infusion on days 1–5. Patients in the PF arm received intravenous cisplatin (100 mg/m2), followed by fluorouracil (1000 mg/m2) per day as a continuous 24-h infusion for 5 days. The cycles were repeated every 3 weeks.
CCRT
RT was given to the nasopharynx and neck using intensity-modulated radiotherapy (IMRT) or two-dimensional radiotherapy (2D-CRT) 5 days/week. The IMRT dose-volume histograms of the treatment targets and critical normal structures were evaluated. The prescribed dose was 70 Gy to the primary tumor, and 60–66 Gy to any involved cervical lymph nodes in 30–32 fractions. 2D-CRT-accumulated radiation doses were 68–76 Gy, with 2 Gy per fraction applied to the primary tumor, and 60–66 Gy applied to involved cervical lymph nodes. Our policy was to accept a plus or minus 5% variation across the target. All patients received a concurrent chemotherapy regimen of cisplatin weekly or every 3 weeks during radiotherapy.
Follow-up
The date of last follow-up was defined as the last image study and/or clinic visit and/or telephone follow-up. The final follow-up data were updated on October 8, 2015. Patients received follow-up every 3 months for the first 3 years after IC + CCRT, every 6 months for the next 2 years, and then annually, including physical examinations, chest X-ray, abdominal ultrasonography, MRI of the head and neck and/or bone scan, until the end of the study. All survival data were calculated from the date of diagnosis to the date of each event or the last follow-up.
Statistical analysis
The primary outcomes were OS, DSS, DMFS and locoregional relapse-free survival (LRFS). OS, DSS, DMFS and LRFS were defined as the time from diagnosis to death from any cause, death resulting from NPC or treatment complications, the first distant metastasis, or to the first locoregional relapse. All data, including diagnoses of metastasis and/or local–regional relapse, were audited by the first three co-authors and the last author. Hematological and gastrointestinal reactions were evaluated for acute IC-associated toxicity and were classified based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
We analyzed the clinical characteristics and toxicities of the two treatment groups using the Chi squared test. Survival curves for the original unmatched and PSM cohorts were analyzed using the Kaplan–Meier method and log-rank tests. A multivariable Cox regression analysis was used to adjust for IC regimens, sex, age, smoking, the cisplatin dose of concurrent chemotherapy (CDDP dose), time to RT, number of IC cycles, T-stage, N-stage, clinical stage and radiation techniques with a forward logistic regression method and analysis, including covariates that were statistically significant in univariable analysis of the PSM cohort. The results of this analysis are presented as hazard ratios (HRs) with 95% confidence intervals (CIs).
A propensity score analysis was undertaken to adjust for potential biases associated with factors related to receiving specific treatments [20]. Propensity scores were computed by logistic regression for each patient based on the presumed covariates, which included sex, age (≤ 45/> 45), smoking (yes/no), BMI (< 19/19–24/> 24), KPS (< 90/≥ 90), T-stage, N-stage, clinical stage and radiation techniques. The PSM, was generated using all reported covariates with a one-to-one nearest neighbor matching algorithm at a caliper of 0.2. We used SPSS version 22.0 (IBM, Armonk, NY, USA) for statistical analyses, and PSM analyses were performed using R (version 3.2.3). Statistical significance was set at 0.05, and all tests were two-tailed.
Results
Study cohort characteristics
Between January 2000 and June 2013, 1879 locoregionally advanced NPC patients were treated with IC + CCRT, among which, 1256 received PF or TPF as the IC backbone (Fig. 1). Among these 1256 patients, 315 (25.1%) were treated with TPF + CCRT and 941 (74.9%) received PF + CCRT. The male:female ratio of the entire cohort was approximately 3:1. Before matching, patients who received TPF as IC were more likely to receive IMRT (P < 0.001). After matching, the distribution of radiation techniques was well-balanced between the two groups. Also, all reported parameters were balanced among the two groups, and no statistical differences were detected. Baseline characteristics of the study cohort are shown in Table 1.
Survival outcomes
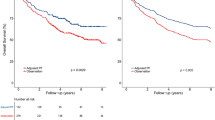
With a median follow-up of 65 (range: 3–174) months, the 5-year OS, DSS, DMFS and LRFS rates for the entire cohort were 80.1, 80.6, 82.9, and 90.7%, respectively. The 5-year OS, DSS, DMFS and LRFS rates for the TPF vs. the PF group were 87.8% vs. 78% (P < 0.001, Fig. 2a), 88.1% vs. 78.7% (P < 0.001, Fig. 2b), 88.6% vs. 80.6% (P = 0.008, Fig. 2c), and 89.2% vs. 91.0% (P = 0.582, Fig. 2d), respectively.
Kaplan–Meier survival curves based on the IC regimens cisplatin and fluorouracil (PF) versus taxane, cisplatin and fluorouracil (TPF) for the entire cohort. a Overall survival, b disease-free survival, c distant metastasis-free survival, and d locoregional relapse-free survival. IC induction chemotherapy
A multivariate analysis was performed using a Cox proportional hazards model to adjust for the various prognostic factors (Table 2). Consistent with the univariate results, multivariate analysis revealed that the TPF-based IC regimen was associated with significantly improved 5-year OS (HR, 0.660; 95% CI 0.442–0.986; P = 0.042), DSS (HR, 0.624; 95% CI 0.411–0.947; P = 0.027) and DMFS (HR, 0.589; 95% CI 0.406–0.855; P = 0.005). There were no significant differences in LRFS (HR, 1.213; 95% CI 0.719–2.047; P = 0.469).
Propensity score
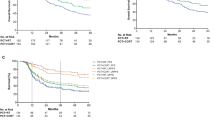
Propensity score-matched identified 294 patients in each cohort: the 5-year OS rates for patients treated with TPF and PF were 88.1 and 80.7%, respectively (P = 0.042, Fig. 3a), the 5-year DSS rates were 88.5 and 80.7% (P = 0.021, Fig. 3b), the 5-year DMFS rates were 87.9 and 78.6% (P = 0.012, Fig. 3c), and the 5-year LRFS rates were 89.5 and 91.0% (P = 0.517, Fig. 3d). Multivariate analysis was also performed in the PSM cohort to adjust for various prognostic factors (Table 3). This multivariate analysis confirmed that an IC regimen of TPF significantly improved OS (HR, 0.581; 95% CI 0.371–0.910; P = 0.018), DSS (HR, 0.543; 95% CI 0.343–0.859; P = 0.009) and DMFS (HR, 0.551; 95% CI 0.357–0.850; P = 0.007) in the PSM cohort. There were no significant differences in LRFS (HR, 1.179; 95% CI 0.667–2.084; P = 0.572).
Kaplan–Meier survival curves based on the IC regimens cisplatin and fluorouracil (PF) versus taxane, cisplatin and fluorouracil (TPF) for the propensity-matched cohort. a Overall survival, b disease-free survival, c distant metastasis-free survival, and d locoregional relapse-free survival. IC induction chemotherapy
Toxicity
Patients toxicities in the PSM cohort were retrospectively evaluated. Regarding hematologic toxicities, the incidences of grade 3/4 anemia (6.5% vs. 2.7%, P = 0.047) and grade 3/4 thrombocytopenia (8.8% vs. 3.4%, P = 0.009) were higher in the PF group. The incidence of grade 3/4 neutropenia was higher in the TPF group (40.1% vs. 29.6%, P = 0.009). With respect to non-hematologic toxicities, grade 3/4 nausea/vomiting occurred more frequently in the PF group (61.2% vs. 48.6%, P = 0.003). Neither group experienced serious liver damage or acute renal toxicity. Acute toxicities according to the National Cancer Institute Common Toxicity Criteria, version 4.0 are listed in Table 4.
Discussion
In a large, single institution-based cohort of locoregionally advanced NPC patients who received IC + CCRT, we found that a TPF-based IC regimen significantly improved OS, DSS and DMFS compared with a PF regimen. The TPF regimen also showed acceptable toxicities. To control for potential confounders, a PSM analysis was performed, which also confirmed the consistency of these results.
Updated meta-analyses and systematic reviews of clinical trials have demonstrated a survival advantage to the addition of concomitant chemotherapy to radiotherapy in patients with locoregionally advanced NPC [12]. However, distant metastasis remains a critical issue, as more than 30% of patients with locoregionally advanced NPC develop distant metastases after CCRT, ultimately succumbing to the disease [7, 21]. Benefits have been seen regarding the eradication of distant micrometastases and reduced locoregional failure with IC; the use of chemotherapy as IC before radiation is an attractive model. In a randomized phase II comparison of chemoradiotherapy (cisplatin) with or without IC, Hui et al. [10] reported that the 3-year PFS and OS rates were 88 and 94% in the IC group, respectively, and 60 and 68% in the control group without IC, respectively. Moreover, Sun et al. [11] conducted a randomized phase III study to compare three cycles of induction docetaxel, cisplatin and continuous intravenous fluorouracil followed by CCRT with CCRT alone. IC significantly increased the 3-year failure-free survival, OS and DMFS rates of their patient population. Most recently, a phase III multicenter randomized controlled trial reported that IC improved 3-year DFS (P = 0.028) and DMFS rates (P = 0.056) compared with CCRT alone in locoregionally advanced NPC [14]. Despite the demonstrated merit of adding IC to CCRT for locoregionally advanced NPC treatment from intense investigations of this approach, studies comparing different IC regimens are scarce.
The PF regimen is the most commonly used IC treatment strategy. In the 1990s, Hareyama et al. [22] conducted a randomized study to compare two cycles of PF-based IC followed by radiotherapy with radiotherapy alone. The 5-year DMFS for patients in the IC + radiotherapy arm was 74% compared with 56% for patients in the radiotherapy-only arm. Moreover, a randomized study of 408 patients designed to compare two cycles of induction floxuridine plus carboplatin followed by radiotherapy with or without concurrent carboplatin (IC + CCRT vs. IC + radiotherapy) for patients with locoregionally advanced NPC was performed, and 5-year OS rates of 70.3 and 71.7% (P = 0.734) were found in the IC + CCRT and IC + radiotherapy groups, respectively [19].
Taxanes are microtubule-stabilizing drugs that have been extensively used as effective chemotherapeutic agents for solid tumors treatment [23, 24]. The TAX 323 study was the first to demonstrate the benefits of adding docetaxel to cisplatin and 5-fluorouracil as an IC for locoregionally advanced head and neck cancer. Patients in the TPF group experienced a significant 27% reduction in mortality and an improved median OS of 4.3 months [17]. Later, the TAX 324 study [25] and the GORTEC laryngeal study showed that TPF was significantly better than PF at improving survival, local control, and organ preservation and was associated with manageable toxicity [18, 26].
Because the epidemiology, histology, clinical behavior, and treatment responses of NPC differ from other head and neck cancers, the efficacy of adding a taxane to PF as an IC regimen for locoregionally advanced NPC patients is unclear. In a phase II trial designed to compare TPF with PF as IC regimens in locoregionally advanced NPC patients under the age of 21, Casanova et al. [27] found no differences between the two groups in terms of efficacy or toxicity. This negative result may be attributable to the small sample size and/or the specific adolescent patient population. Recently, a prospective, randomized non-inferiority study evaluated the benefits and side effects of two cycles of TPF as IC compared with two cycles of PF for locoregionally advanced NPC patients. After a short follow-up of approximately 36 months, Jin et al. [28] found no significant differences in PFS between the two groups. However, the small sample size and short-term follow-up period limit these results.
We conducted this retrospective study to compare the outcomes of locoregionally advanced NPC patients who were treated with TPF or PF as IC followed by CCRT in a large cohort with long-term follow-up. We found that TPF-based IC significantly improved long-term OS, DSS and DMFS compared with PF. Interestingly, the 3-year OS and DSS rates for patients treated with TPF and PF were not significantly different. Rather, significant difference presented gradually after 3 years, especially at 5 years, which may partially explain the negative results of the study by Jin et al. In their study, the median follow-up was only 36 months, which may not have been long enough to observe differences between the two groups. Locoregional relapse and/or distant metastasis are the major reasons for treatment failure and death in locoregionally advanced NPC patients [29]. Previous studies have reported that death from locoregional relapse and/or distant metastasis primarily occurs during the first 3 years after diagnosis [11, 14]. In our study, the median OS of patients who developed locoregional relapse or distant metastasis was 38.5 months, and more than 60% of the patients were alive after 3 years of diagnosis. Moreover, in our cohort, more patients died of disease failure after 3 years. Therefore, we believe that greater than 3-year follow-up is needed for NPC patients to determine therapeutic efficacy.
Induction chemotherapy may shrink the primary tumor, providing a wider margin for radiotherapy and reduced organ toxicities. Previous clinical trials have reported that these two IC regimens can achieve satisfactory locoregional control rates [11, 14]. In our study, the 5-year LRFS rates for the whole cohort were both satisfactory and showed no difference between the TPF and PF groups (91.0 and 89.5%, respectively). It is notable that a significantly larger proportion of patients in the PF group underwent 2D-RT techniques. This means, that to some extended, the addition of IC to CCRT was helpful for the local–regional control of NPC regardless of radiation technique. In contrast, the survival benefits of the TPF regimen could also be attributed to a decrease in distant metastasis. Distant metastasis is the most important and lethal outcome for NPC patients; thus, this result suggested that the TPF regimen may provide a better long-term survival and control of distant metastasis in patients who are at a high risk of disease dissemination.
With regard to toxicity, the two regimens were well tolerated, and nearly all patients completed more than two cycles of IC. Acute toxicities during IC were mainly hematologic (neutropenia and anemia), and these incidences were uncomplicated and manageable. Notably, neutropenia was more common in the TPF arm (40.1%) than in the PF arm (29.6%), whereas anemia was more common in the PF arm than the TPF arm. Moreover, the incidence of grade 3/4 nausea/vomiting was higher in the PF group than in the TPF group. The reduced fluorouracil and cisplatin doses administered in the TPF regimen likely minimized gastrointestinal reactions, nut may attributable to the higher anemia rates. Additionally, the additional of a taxane may be responsible for bone marrow suppression.
There were several limitations to this analysis that must be considered. First, although our cohort is likely to be representative of the majority of patients diagnosed with NPC in South China, this was a single-center study. Second, although we used PSM, a method designed to minimize the impact of observed confounders, to account for potential confounders, this was a retrospective analysis, and the results may have been subject to residual confounding variables. For example, we had incomplete data of acute toxicity, such as mucositis, odynophagia, or infectious fever and plasma Epstein–Barr virus DNA, which is one of the major limitations of this study.
Conclusions
In summary, TPF as an induction regimen before CCRT may improve disease control for locoregionally advanced NPC compared with the PF regimen. A prospective randomized clinical trial to validate these results is necessary.
References
Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30(2):114–9.
Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–24. https://doi.org/10.1016/s0140-6736(15)00055-0.
Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73(5):1326–34. https://doi.org/10.1016/j.ijrobp.2008.07.062.
Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22(3):233–44. https://doi.org/10.1016/j.semradonc.2012.03.008.
Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li WF, et al. Prognostic scoring system for locoregional control among the patients with nasopharyngeal carcinoma treated by intensity-modulated radiotherapy. Chin J Cancer. 2013;32(9):494–501. https://doi.org/10.5732/cjc.013.10121.
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–7.
Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53(1):12–22.
Li WF, Chen L, Sun Y, Ma J. Induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer. 2016;35(1):94. https://doi.org/10.1186/s40880-016-0157-4.
Qiu WZ, Huang PY, Shi JL, Xia HQ, Zhao C, Cao KJ. Neoadjuvant chemotherapy plus intensity-modulated radiotherapy versus concurrent chemoradiotherapy plus adjuvant chemotherapy for the treatment of locoregionally advanced nasopharyngeal carcinoma: a retrospective controlled study. Chin J Cancer. 2016;35:2. https://doi.org/10.1186/s40880-015-0076-9.
Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242–9. https://doi.org/10.1200/JCO.2008.18.1545.
Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–20. https://doi.org/10.1016/S1470-2045(16)30410-7.
Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–55. https://doi.org/10.1016/s1470-2045(15)70126-9.
Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol. 2005;23(6):1118–24. https://doi.org/10.1200/JCO.2005.12.081.
Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14–23. https://doi.org/10.1016/j.ejca.2016.12.039.
National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology for Head and Neck Cancer, Version 1, 2016. http://www.nccn.org/index.asp. Accessed 6 May 2016.
Chan AT, Gregoire V, Lefebvre JL, Licitra L, Hui EP, Leung SF, et al. Nasopharyngeal cancer: EHNS–ESMO–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii83–5. https://doi.org/10.1093/annonc/mds266.
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–15.
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–704.
Huang PY, Cao KJ, Guo X, Mo HY, Guo L, Xiang YQ, et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;48(10):1038–44. https://doi.org/10.1016/j.oraloncology.2012.04.006.
Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837–49. https://doi.org/10.1002/sim.5705.
Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. https://doi.org/10.1016/j.radonc.2013.10.020.
Hareyama M, Sakata K, Shirato H, Nishioka T, Nishio M, Suzuki K, et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer. 2002;94(8):2217–23. https://doi.org/10.1002/cncr.10473.
Colevas AD, Posner MR. Docetaxel in head and neck cancer: a review. Am J Clin Oncol. 1998;21(5):482–6.
Dreyfuss AI, Clark JR, Norris CM, Rossi RM, Lucarini JW, Busse PM, et al. Docetaxel: an active drug for squamous cell carcinoma of the head and neck. J Clin Oncol. 1996;14(5):1672–8.
Janoray G, Pointreau Y, Garaud P, Chapet S, Alfonsi M, Sire C, et al. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, ±docetaxel for larynx preservation. J Natl Cancer Inst. 2016;108(4). https://doi.org/10.1093/jnci/djv368.
Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011;12(2):153–9. https://doi.org/10.1016/s1470-2045(10)70279-5.
Casanova M, Ozyar E, Patte C, Orbach D, Ferrari A, Veyrat-Follet C, et al. International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5-fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother Pharmacol. 2016;77(2):289–98. https://doi.org/10.1007/s00280-015-2933-2.
Jin T, Qin WF, Jiang F, Jin QF, Wei QC, Tang XW, et al. Interim analysis of a prospective randomized non-inferiority trial of cisplatin and fluorouracil induction chemotherapy with or without docetaxel in nasopharyngeal carcinoma. Oncotarget. 2016. https://doi.org/10.18632/oncotarget.10903.
Guan Y, Liu S, Wang HY, Guo Y, Xiao WW, Chen CY, et al. Long-term outcomes of a phase II randomized controlled trial comparing intensity-modulated radiotherapy with or without weekly cisplatin for the treatment of locally recurrent nasopharyngeal carcinoma. Chin J Cancer. 2016;35:20. https://doi.org/10.1186/s40880-016-0081-7.
Authors’ contributions
Conception and design: WXX, YQX, XG; provision of study material or patients: YQX; data collection and analysis: GYL, WXX, XL, LRK, YFY, YHY, HL, JY, WZQ, XJH, WZL; data analysis and interpretation: HL; contribution of financial support: YQX, XG, XL; manuscript writing: GYL, XL, YSW. All authors read and approved the final manuscript.
Acknowledgements
This study was partly funded by the National Natural Science Foundation of China (NSFC) (program Grants 81472525, 81672680 and 81572665) and the Science and Technology Planning Project of Guangdong Province, China (program Grant 2014A050503033 and 2016A050502011). We thank American Journal Experts for their professional language editing service.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated and/or analyzed during the current study are publicly available in the SYSUCC. The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the Approval Number of RDDA2017000289.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was performed in accordance with the Institutional Review Boards of SYSUCC. Written informed consent was issued by each patient, including signed consent for tissue analysis and consent to be recorded for potential medical research at the time of sample acquisition.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, GY., Lv, X., Wu, YS. et al. Effect of induction chemotherapy with cisplatin, fluorouracil, with or without taxane on locoregionally advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis. Cancer Commun 38, 21 (2018). https://doi.org/10.1186/s40880-018-0283-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-018-0283-2