Abstract
Background
Nickel exposure causes hepato-renal toxicity via oxidative stress. Medicinal plants with antioxidants properties are being explored as treatment options. In this study, the effect of ethanol extract of Nigella sativa (ENS) on nickel chloride (NiCl2)-induced hepato-renal damage was evaluated by monitoring biochemical and oxidative stress markers. Additionally, the antioxidant capacity and phytochemical constituents of ENS were quantified using HPLC and GC-MS.
Result
NiCl2 significantly increased (p < 0.05) aspartate aminotransferase, creatinine, sodium ion, chloride ion and malondialdehyde levels, while antioxidant enzymes were decreased in the organs except for kidney glutathione-S-transferase when compared to the control. However, ENS exerted inhibitory effect against NiCl2 toxicity in both organs by reversing the biomarkers towards control levels. ENS has a high antioxidant capacity and is rich in antioxidants including gallic acid, quercetin, eucalyptol and levomenthol that may have accounted for the improvement of hepato-renal health in co-exposed rats.
Conclusion
Our result suggests that amelioration of nickel chloride-induced hepato-renal pathology by ethanol extract of Nigella sativa was related to its antioxidant properties. Therefore, Nigella sativa could be valuable in the management of nickel-induced toxicity.
Similar content being viewed by others
Background
Nickel has several industrial applications including the manufacturing of stainless steel, batteries, utensils, cosmetics and electronic products [1]. It is also used as a catalyst and pigments in the food industries. Fossil fuel burning and other industrial activities result in the release of a large amount of nickel into the atmosphere every year [1, 2]. Occupational exposure to nickel at the workplace is common, while non-occupational exposure to the metal occurs in people who live in the vicinity of nickel related industries. This is particularly common in developing countries where regulations concerning effluent treatment are not strictly enforced [1,2,3]. Moreover, the general public is also chronically exposed to nickel leached from nickel-containing metal pipes, kitchen utensil and food processing equipment as well as cosmetics, tobacco and jewellery [4,5,6].
Several deleterious health hazards have been linked with exposure to this metal including allergic contact dermatitis, eczema, respiratory infections, asthma, bronchitis, dizziness, nausea, headache, diarrhoea, reproductive damage, neurological defects, diabetes, fever, heart attack, insomnia, itching and haemorrhages [7,8,9]. Moreover, epidemiological studies have found an increased occurrence of cancers of the breast, respiratory and gastrointestinal tracts in workers within nickel related industries [1].
The pathophysiological mechanisms of nickel toxicity are complex. However, one of the main mechanisms of nickel toxicity lies in its ability to alter bio-metal homeostasis and induce oxidative stress. Nickel induced reactive oxygen species (ROS) enhances lipid peroxidation and modulates antioxidant enzyme activities [10]. The ROS produced by nickel ion also oxidizes DNA resulting in elevation of 8-hydroxy-2′-deoxyguanosine in nickel-related workers [11]. Also, Ni-induced ROS production has been linked with apoptosis and inflammatory signalling including JNK, Nrf2/HO-1 TLR4/p38/CREB pathways [12,13,14].
Like other heavy metals, nickel toxicity is treated with chelating agents and synthetic antioxidants. Chelation treatment is however limited by redistribution of lethal metals, loss of essential metals, headache, nausea, hypertension, pressure, hepatotoxicity and nephrotoxicity [15]. Similarly, synthetic antioxidants are limited by negative health effects and restrictions [16]. Due to these limitations, medicinal plants with antioxidant properties are now being screened for natural antioxidants and other phytochemicals that could be valuable in the remediation or management of nickel toxicities [16]. The usefulness of Nigella sativa (N. sativa) was therefore explored in the present study.
The N. sativa is an annual herb in the Ranunculacae family that is commonly grown in the Middle East, Middle Asia and North Africa [17]. The seed is a common food additive in Middle Eastern and South Western cuisines and has been used since antiquity in traditional medicine for treating illness and improving physical performance [18,19,20]. It has been reported in traditional pharmacopoeia against wide range of ailments including, bronchitis, skin diseases, rheumatism, diarrhoea, headache, fever, diabetes, hypertension, obesity, amenorrhea, dysmenorrhea, asthma and fatigue [21,22,23,24]. The seed and oil are also used for the treatment of disorders of the nervous, cardiovascular, respiratory, digestive, and excretory and immune system [25]. Other pharmacological activities that have been reported in the seed include analgesic, immunomodulatory, anti-histaminic and anti-leukotrienes effects [17, 26,27,28].
Furthermore, N. sativa possesses a wide range of biological and pharmacological activities against bacteria, fungi, viruses and parasitic microbes [29,30,31]. It is also used in the management of psoriasis and cancers [32]. Recently, N. sativa was reported to exert protection against several food toxicants and ease the side effects of chemotherapeutic drugs including bromobenzene, tartrazine, cisplatin, thioacetamide and tramadol [33,34,35,36,37,38]. Potent antioxidants and anticancer agents in the plants including thymoquinone are well-documented [39]. Healing power and therapeutic benefits of N. sativa have been attributed to the powerful antioxidants present in it [34, 39].
Based on the reported antioxidant properties of the plant, we investigated the possible ameliorative effect of ethanol extract of Nigella sativa (ENS) in protecting against nickel chloride-induced hepato-renal damage in Wistar rat model. Additionally, we evaluated the extract for the presence of phenolics and other phytochemicals using high-performance liquid chromatography and gas chromatography-mass spectrometry.
Methods title
Chemicals
Nickel chloride, 2-thiobarbituric acid, reduced glutathione, and 5′5’dithiobis (2-nitrobenzoic acid) were purchased from Sigma Chemical Co., St. Louis, MO., while ethanol and hydrogen peroxide were obtained from Merck KGaA, Darmstadt Germany. 1-chloro-2, 4-dinitrobenzene was obtained from J.T. Baker Inc. Philipsburg NJ, USA. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein, total bilirubin, creatinine, urea, sodium ion (Na+), potassium ion (K+) and chloride ion (Cl−) Cobas diagnostic kits were obtained from Roche Diagnostic GnBH, Mannheim, Germany. All other chemicals used were of analytical grade.
Preparation of ethanol extract of Nigella sativa seeds
N.sativa seed was obtained from Al Khatim, Medina, Saudi Arabia. The seeds were milled and successively extracted for two days each with n-hexane, ethyl acetate and 70% ethanol. Briefly, 200 g of the milled Nigella sativa seeds were macerated in 1000 ml of n-hexane with constant shaking for forty-eight hours. The n-hexane extract was separated and the residue was subsequently soaked in 1000 ml ethyl acetate extract for another forty-eight-hours. Finally, the residue remaining after ethyl acetate extraction was soaked in 1000 ml of 70% ethanol for 48 h and the filtrate from the ethanol extract was concentrated in a rotary evaporator at 40 °C and then dried to constant weight in an oven at 40 °C.
Experimental animals
Sixteen female Wistar rats with average age and body weight of 7 weeks and 85 g respectively were obtained from Rawlab Farm Nigeria Enterprise, Ibadan and housed four per cage with wood shaven bedding in polypropylene cages under standard conditions at Bells University of Technology animal husbandry facility in conformity with a guide for the care and use of laboratory animals [40]. The rats were housed at room temperature and exposed to natural daily light-dark cycles. They were provided with water and standard rat chow ad libitum and were allowed to acclimate for two weeks before the commencement of treatment.
Experimental design
Animals were assigned in four different groups of four rats each and treated with deionized water, 2 mg/kg NiCl2, 50 mg/kg body weight ENS and 2 mg/kg NiCl2 + 50 mg/kg body weight ENS. Animals were assigned to groups based on their body weight to ensure even distribution and eliminate variation in initial mean body weights among the groups. The dose of nickel chloride was selected based on its environmental relevance and toxicity in a previous study [41], while the dose of ENS was selected based on our preliminary study and its reported ameliorative effect [42].
The NiCl2 was injected intraperitoneally once every Friday, while ENS was intubated by gavage daily throughout the 35-day study period. Animals were treated between 5.00 and 6.00 pm during the experiment. After the treatment schedule, the final weights of the animals were obtained before blood samples were collected from the retro-orbital plexus of each rat after a 12 h fast. The livers and kidneys were subsequently removed from test and control animals after they were euthanized by cervical dislocation. Before euthanasia, the rats were exposed to diethyl ether to anaesthetize them. The tissues were rinsed in ice-cold 1.15% KCl, blotted on a filter paper and weighed. The organ-body weight ratio was subsequently calculated for each animal.
Serum chemistry and oxidative stress assessment
Blood samples collected into heparinized tubes were allowed to stand for two hours before centrifugation at 3000 g for 15 min. Total protein, ALT, AST, total bilirubin, creatinine, urea, Na+, K+ and Cl− were determined in the resulting plasma using Cobas diagnostic kits and Cobas C311 chemistry autoanalyzer (Roche Diagnostic GnBH, Mannheim, Germany).
One part of the liver and left kidney of each rat was homogenized with cold 50 mM Tris-HCl buffer in a Potter- Elvehjem homogeniser and centrifuged at 10000 g for 20 min at 4 °C. The supernatants obtained were used for the determination of oxidative stress parameters. Lipid peroxidation was evaluated by TBARS formation as measured by malondialdehyde (MDA) following the method of Esterbauer and Cheeseman [43]. Catalase (CAT) activity was measured by monitoring the decomposition of H2O2 as described by Aebi et al [44]. Glutathione- S- transferase (GST) activity was determined according to the method of Habig et al. [45] based on the conjugation of 1-chloro-2,4-dinitrobenzene to reduced glutathione at 340 nm.
Histopathology
The other part of the liver and the right kidneys of each animal were fixed in 4% phosphate-buffered formalin, dehydrated in graded ethanol and embedded in paraffin. Tissues were cut into 5 μm sections, mounted on a clean slide and stained with haematoxylin-eosin dye. Pathological changes in both organs were examined by a trained pathologist who was blinded to the treatments.
Phytochemical analysis
Phytochemical screening of ENS was determined following standard methods described by Sofowora et al, [46], Trease and Evans [47] and Harborne [48].
HPLC analysis
The concentrations of caffeic acid, gallic acid, p-coumaric acid, apigenin, quercetin and amentoflavone were determined in ENS using the high-performance liquid chromatography. Briefly, 1.0 mg of ENS was dissolved in 1.0 ml of absolute methanol. The resulting solution was filtered through 0.45 m Millipore membrane filter. HPLC analysis of ENS was done using Agilent technologies HPLC 1100 series. The extract was separated on Zorbax eclipse XDB RP C8 (150 × 4.6 mm, 5 μm) column with the temperature set at 40 °C and a flow rate of 0.5 mL/min. Elution of the mobile phase was performed with acetonitrile and 0.2% acetic acid. The injection volume was 10 μL and the detection of the compounds was monitored at 257 nm. Sample identification was achieved by comparing the retention time and spectra of the compounds in the sample with that of reference standards.
Gas chromatography-mass spectrometry (GC-MS) analysis
Volatile compounds in ENS were detected by dissolving 1.0 mg of the sample in 1.0 ml of methanol, filtered with a micro-syringe and 1 μl aliquot was injected into a QP2010SE GC-MS Shimadzu gas chromatography-mass spectrometer (Shimadzu Co., Kyoto, Japan) equipped with a mass selective detector operating at 70 eV electron impact mode and capillary column (30 m × 0.25 mm, film thickness 0.25 μm). The column oven temperature was programmed from 60 to 290 °C at the rate of 4 °C/min. Initial and final temperatures were maintained for 3 and 10 min, respectively. The mass scanning range was 45–700 m/z, while ion source and interface temperatures were set at 230 and 250οC, respectively. The carrier gas was helium with a flow rate of 3.22 mL/min and pressure of 144.4 kPa. Compounds in ENS were identified by matching their mass spectra with those in the database of NIST computer data bank and by comparison of the fragmentation pattern with those reported in the literature.
Diphenyl-1-picrylhydrazyl (DPPH) scavenging activity
The DPPH scavenging activity of ENS was evaluated based on the reduction of DPPH to diphenylpicrylhydrazine by antioxidants as previously described by Dildar et al. [49]. Briefly, 3 mg of ENS was dissolved in 3 ml methanol and 100 μL of the different concentrations of ENS (10–500 μgml− 1) were mixed with 3 ml of DPPH solution (12 mg DPPH in 50 mL methanol). The absorbance was obtained at 517 nm after 30 min using a SM7504UV spectrophotometer (Uniscope, UK`). The DPPH scavenging activity was determined as:
where \( {A}_b \) = absorbance of DPPH∙ in solution without the extract
\( {A}_e \)= absorbance of DPPH∙ in the presence of the (extract)
The concentration of ENS that scavenge 50% of DPPH radicals (IC50) was calculated from the plot of percentage DPPH scavenging activity against sample concentration and expressed as 휇g/mL of the extract.
Total antioxidant capacity
Total antioxidant was determined based on the reduction of Mo (VI) to Mo (V) at acidic pH as described by Prieto et al, [50] with slight modification. Briefly, 1.0 ml of 1.0 mg/ml ENS was mixed with 1.0 ml of reagent solution containing 4.0 mM (NH4)2MoO4, 28.0 mM Na3PO4 and 0.6 M H2SO4. The mixture was incubated for 90 min at 95 °C in shaking water bath. The mixture was thereafter allowed cool at room and the absorbance was taken at 695 nm against a blank containing ethanol instead of the extract. The total antioxidant was calculated from an ascorbic standard curve and expressed as μg equivalent of ascorbic acid per ml.
Statistical analysis
Data obtained from all the test and control rats were analyzed using the 17th version of SPSS (SPSS Inc. Chicago, IL) and presented as mean ± SEM. One-way analysis of variance (ANOVA) and Duncan Multiple Range Test were used to determined intergroup differences and mean separation respectively. The level of significance was set at p < 0.05.
Results
The organ weight and relative organ weight ratio for both the liver and kidney of test and control animals are shown in Table 1. The result showed that there was no significant difference in liver and kidney weight as well as the relative liver weight in all the treatment groups. However, relative kidney weight was significantly (p < 0.05) reduced by 13.4% in NiCl2-treated group when compared with the control group. Administration of the ENS either alone or in combination with NiCl2 resulted in similar relative kidney weights with control.
The results obtained for the liver biomarkers following concurrent exposure to NiCl2 and ENS are presented in Table 2. The AST activity was significantly elevated (p < 0.05) in animals exposed to NiCl2 by 111.3% when compared to the control. In contrast, ENS reduced AST activity to 73.6%. The AST in the group that was given ENS alone was not significantly different from the control. Alanine aminotransferase (ALT) was increased by 44.4% in the NiCl2-treated rats when compared to the control. However, ALT activity was reduced to 20% in the group exposed simultaneously to ENS and NiCl2. The ALT activities were similar in the groups exposed to ENS alone and the control. Total protein (TP) and total bilirubin (TB) were not significantly different across the groups, although both parameters were elevated in NiCl2-treated group.
The effect of ENS on NiCl2-induced alteration of kidney health markers is presented in Table 3. Urea concentration was increased by 22.5% in the NiCl2-treated animals when compared to control, while creatinine was markedly (p < 0.05) increased by 74.1% also as compared to control value. Both markers were, however, reversed towards control value in the groups treated with ENS and NiCl2. The reversal was more pronounced (p < 0.05) for creatinine levels. The Na+ and Cl− were significantly (p < 0.05) elevated in rats administered NiCl2 by 15.4% and 23.2% respectively. In contrast, simultaneous administration of NiCl2 and ENS reduced Na+ and Cl− concentration to 10% and 2% respectively. The reduction was only significant (p < 0.05) for Cl− concentration. Concentrations of creatinine, urea, Na+ and Cl− in the group that was given ENS alone were not significantly different from the control. Similarly, plasma K+ was not significantly different across all the groups, although a decrease in K+ level was observed in the NiCl2-treated group.
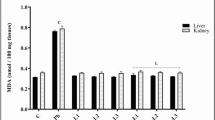
The results obtained for lipid peroxidation and antioxidant enzymes in test and control animals are presented in Table 4. Malondialdehyde (MDA) level was increased by 41.5% in the liver of NiCl2−treated rats when compared with the control, while kidney MDA of treated rats was significantly (p < 0.05) increased by 42.3% also as compared with the control. Simultaneous administration of ENS and NiCl2 reduced liver MDA and kidney MDA to 34% and 3.3% respectively. The MDA reduction was only significant (p < 0.05) in the kidney. The MDA level in both organs was not significantly different from the control in the group treated with ENS alone. Catalase activity was significantly (p < 0.05) decreased by 69.3% in the liver of rats administered NiCl2 when compared to the control, while a 40.1% decrease in kidney catalase activity was observed in the same set of animals as compared to control value. In contrast, liver catalase activity was markedly (p < 0.05) reduced to 40.1% in the co-treatment group that was given ENS and NiCl2. Kidney catalase activity was also reduced to 8.71% in the co-exposure group. Catalase activities in both organs of rats treated with ENS alone were not significantly different from the control. Liver GST was significantly (p < 0.05) decreased by 23.1% in the animals treated with NiCl2 when compared to the control. However, co-treatment with ENS improved liver GST activity to 15.9%. Contrary to the results obtained for liver GST, NiCl2 significantly (p < 0.05) increased kidney GST activity by 113.7% when compared to control value. However, ENS markedly (p < 0.05) reduced NiCl2- induced hike in GST activity to 14.7%. Liver and kidney GST activity were similar in the control group and those exposed to ENS alone.
The results of the histopathological evaluation of test and control animals are presented in Figs. 1 and 2. Control rats and those fed with ENS alone had normal and intact hepatic histological architecture (A and B), while NiCl2-induced alteration of hepatic architecture was evident by hepatocellular necrosis and atrophy of hepatic cords with Kupffer cell hyperplasia (C). However, simultaneous exposure to ENS and NiCl2 resulted in mild atrophy of hepatic cords (D). The renal histopathology result presented in Fig. 2 revealed normal architecture in the groups fed water and ENS (A and B), while treatment with NiCl2 led to tubular epithelial degeneration and necrosis (C) as well as nephrosis with casts (D) and congestion of glomerular capillaries (E). ENS prevented the lesion as evident by normal renal architecture observed in the co-treated group.
Ameliorative effect of ENS on NiCl2-induced alteration of hepatic histo-architecture in rats. Normal liver histology in the group treated with water and 50 mg/kg ENS alone a and b. Hepatocellular necrosis (black arrow) and atrophy of cord (arrow head) with Kupffer cell hyperplasia (blue arrow) in the group that received NiCl2 c. Mild atrophy of hepatic cords (arrow head) observed in the group that received a combination of ENS and NiCl2 d
Protective effect of ENS on NiCl2-induced alteration of renal histo-architecture in rats. Normal renal histology in the group treated with water a and 50 mg/kg ENS alone b. Tubular epithelial degeneration and necrosis c, Nephrosis with casts d and congestion of glomerular capillaries e observed in the group injected (i.p.) with NiCl2. Normal renal histology in the observed group that received a combination of ENS and NiCl2.f
The result of the phytochemical screening of ENS presented in Table 5 revealed that it is very rich in phenolic compounds, alkaloids, flavonoids, saponins and reducing sugar. Deoxy sugars, tannins, terpenoids and tannins were also detected in ENS. However, anthraquinone was not detected in ENS in the current study. The results of the DPPH scavenging and total antioxidant capacity presented in Table 6 showed that ENS has a DPPH scavenging activity of 145 ± 0.40 휇g/mL, while the total antioxidant was 186.90 ± 0.40 μgml−1AAE.
The HPLC chromatograms and quantities of some phenolic compound present in ENS are presented in Fig. 3 and Table 7 respectively. The concentration of the compounds is in the order quercetin > amentoflavone> apigenin> gallic acid> caffeic acid> p-coumaric acids. The GCMS analysis of ENS presented in Table 8 revealed the presence of 33 bioactive compounds. Major phytochemicals detected under our experimental conditions including 9Z,12Z-octadecadienoic acid methyl ester (27.8%) cyclohexanol,5-methyl-2-(1-methylethyl)-,(1.alpha.,2.beta.,5.alpha.)-(.+/−.)-(22%), 9E-octadecenoic acid methyl ester, (15.60%), eucalyptol (10%), hexadecanoic acid methyl ester (10%). Other beneficial phytochemicals detected in ENS are levomenthol (2.19%), cis-4-methoxy thujane (1.70%), 9Z-hexadecenoic acid, methyl ester, (1.46%), butylated hydroxytoluene (0.5%) and p-cymene (0.2%).
Discussion
The liver is a target of Ni toxicity. Clinical and experimental data have indicated that Ni toxicity is characterized by alteration of liver function profile [13, 51]. As evident in this study, the elevated ALT and AST observed in Ni- treated animal could be due to induction of hepatocellular injury. Both enzymes are localized in hepatocytes but are rapidly spilt into the bloodstream during hepatic membrane and hepatocellular damage. Changes in histo-architecture of liver including hepatocellular necrosis and atrophy of cord and kupffer cell hyperplasia observed in Ni-treated rats confirmed the toxicity of NiCl2 to liver cells. Similar lesions had been observed in the mice and fish models of nickel toxicity [7, 52]. In contrast, ENS acted antagonistically with NiCl2 to decrease plasma transaminase level as well as ameliorate Ni-induced hepatic lesion as evident by mild atrophy observed in the animals co-exposed to NiCl2 and ENS.
Creatinine and urea are by-products of cellular metabolism that are mainly excreted through the kidneys. Impairment in kidney function renders the kidney inefficient to excrete both compounds, resulting in their elevation in the plasma. Therefore, the elevation of both products in the group treated with NiCl2 could be due to the nephrotoxic effect of NiCl2 to renal cells. Earlier works have shown that NiCl2 is a nephrotoxin and similar elevation of creatinine and urea were observed in mice [53]. Renal impairment often results in perturbation of electrolytes balance. NiCl2-induced perturbation of electrolyte balance in the present study was characterized by increased plasma Na+ and Cl− with a concomitant decrease in K+. The cellular mechanism by which nickel affects electrolyte balance is not completely understood, but it may be secondary to its effects on mitochondria including depletion of ATP that may impair active transport of ions [54, 55]. Also, NiCl2 may alter tubular permeability or interfere with transporters including Na/K ATPase. For instance, Liapi et al [56] and Maiti et al [57] reported that NiCl2 inhibits Na/K ATPase in erythrocyte and brains of rats and fish. Inhibition of Na/K ATPase would decrease sodium reabsorption and could, in turn, elevate Cl−, since Cl− is co-transported with Na+. The evidence for NiCl2- induced histological damage to renal cells was confirmed by the presence of tubular epithelial degeneration and necrosis as well as nephrosis with casts and congestion of glomerular in treated rats. However, ENS protected the kidney against NiCl2- induced renal toxicity as evident by reversal of kidney function parameters and electrolyte imbalance towards the control as well as the apparently normal renal histo-architecture in the animals co-exposed to NiCl2 and ENS. Therefore, suggesting that ENS exerted protective role against NiCl2 treatment in the kidney.
Although the mechanism by which Ni ion cause tissue damage is not fully elucidated, accumulating evidence suggest that ROS generation play a significant role in nickel toxicity [58]. The result of the present study adds to the body of evidence in favour of an association between nickel exposure and oxidative stress. Increased MDA in the NiCl2- treated animals is indicative of lipid peroxidation. Ni ions initiate free radical generation through redox cycling that occurs when Ni2+ bind to protein [58]. In addition, nickel may facilitate bioaccumulation of iron that may partake in Fenton reaction and enhanced production of radicals including hydroxyl radicals that promotes membrane lipid peroxidation and oxidative stress [2]. Lipid peroxidation may lead to perturbation of membrane function, loss of enzyme activity and alteration of the antioxidant defence system. This may be responsible for the alteration of the endogenous antioxidants observed in this study, which was characterized by inhibition of liver and kidney catalase as well as liver GST. However renal GST activity was enhanced. A similar result was obtained by Misra et al [59]. Catalase plays an important role in maintaining physiological levels of hydrogen peroxide and eliminating peroxides generated during lipid peroxidation. Inhibition of catalase in the present study could be due to the utilization of the enzyme in eliminating H2O2 or deactivation of the enzymes by free radicals. This could enhance ROS-induced oxidative damage in both organs. The differential response of GST in the liver and kidney suggest that the effect NiCl2 on GST activity seems to be complex and tissue-specific. GST is a multifunctional family of enzymes that protect cells against oxidative damage and is involved in the detoxification of several electrophilic xenobiotics including environmental toxicants and products of oxidative membrane lipid peroxidation [60, 61]. Therefore, decreased liver GST activity observed in the present study could be due to inhibition of the enzyme by ROS. This may reflect inefficient detoxification and protection against Ni-induced oxidative stress. The kidney is more sensitive to nickel toxicity because Ni ions accumulate and are retained more in the kidney [2]. Therefore, the increase in kidney GST could be an adaptive response against enhanced oxidative stress induced by Ni2+. Alternatively, it could be suggestive of the onset of kidney diseases. Certain isoforms of GST are elevated at the early stage and during the progression of kidney diseases [62, 63]. The increase in renal GST observed in this study may also be due to NiCl2- induced damage to renal tubules. Increased renal GST-α and GST-π activities are associated with proximal and distal tubular damage in experimental drug and heavy metal models [64, 65].
Interestingly, ENS relieved the liver and kidney of NiCl2-induced oxidative stress as manifested by the attenuation of lipid peroxidation and reversal of both catalase and GST activities in the liver and kidney of the animals co-exposed to ENS and NiCl2. The high antioxidant capacity and free radical scavenging activity of ENS may be responsible for the fortification of antioxidant defences against nickel chloride-induced oxidative stress. This may be responsible for the observed reduction of lipid peroxidation as well as improvement in catalase and GST activities in both organs. Also, phytochemical screening of ENS showed that it contains beneficial phytochemicals including polyphenols and flavonoids. Polyphenols and flavonoids such as gallic acid, quercetin, amentoflavone, apigenin, caffeic acid and p-coumaric acid detected in ENS could account in part for the strong antioxidant capacity and inhibition of nickel induced oxidative stress as well as hepato-renal damage in this study. Some of these compounds have been demonstrated to modify oxidative stress-related pathways altered by environmental toxicants including nickel [66, 67]. Also, quercetin forms complex with nickel and accelerate its removal from the body [68].
Some of the volatile compounds detected in ENS by GCMS have been reported to have valuable biological effects and may have contributed to the amelioration of Ni-induced oxidative hepato-renal injury observed in the current study. For instance, the oxygenated terpenes, eucalyptol exerted hepatoprotection against TCDD and dexamethasone by inhibiting inflammation and oxidative stress [69, 70]. Similarly, levomenthol exhibited gastroprotective action against ethanol-induced ulcer through its anti-inflammatory, anti-apoptotic and antioxidant effects [71]. The fatty acid methyl esters that are abundant in ENS, 9, 12-Octadecadienoic acid (Z, Z)- methyl ester, 9-Octadecenoic acid methyl ester, (E)- and hexadecanoic acid methyl ester are potent free radical scavengers, antioxidants and anticancer agents [72,73,74]. The antioxidant properties of other compounds including p-cymene have also been documented [75]. Therefore, synergism between the different antioxidants present in ENS could be responsible for the high antioxidant capacity and amelioration of nickel chloride-induced hepato-renal damage in this study.
Conclusion
Collectively, our data support the involvement of oxidative stress in nickel chloride-induced hepato-renal damage. Ethanol extract of Nigella sativa ameliorated the oxidative damage in the liver and protected the kidney by evoking antioxidant enzyme response and attenuating lipid peroxidation. Therefore, suggesting that the ameliorative effect of ethanol extract of Nigella sativa may be related to its antioxidant properties.
Availability of data and materials
All data obtained during this study are included in this published article.
Abbreviations
- ENS:
-
Ethanol extract of Nigella sativa
- HPLC:
-
High-performance liquid chromatography
- GC-MS:
-
Gas chromatography-mass spectroscopy
- TCDD:
-
2, 3, 7, 8-tetrachlorodibenzo-p-dioxin
- DPPH:
-
Diphenyl-1-picrylhydrazyl
- AAE:
-
Ascorbic acid equivalent
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- TP:
-
Total protein
- TB:
-
Total bilirubin
- MDA:
-
Malondialdehyde
- GST:
-
Glutathione-S-transferase
- NIST:
-
National Institute of Standards and Technology
- LWT:
-
Liver weight
- RLWT:
-
Relative kidney weight
- KWT:
-
Kidney weight
- RKWT:
-
Relative kidney weight
- SPSS:
-
Statistical package for the social sciences
References
IARC. A review of human carcinogens, part C: arsenic, metals, fibres and dust. In: IARC monographs on the evaluation of carcinogenic risks to humans, vol. 100C. Lyon: World Health Organization; 2012. p. 169–211.
Cempel M, Janicka K. Distribution of nickel, zinc, and copper in rat organs after oral administration of nickel (II) chloride. Biol Trace Elem Res. 2002;90(1-3):215–60.
Agency for Toxic Substances and Disease Registry (1997). Toxicological profile for nickel (update). PublicHealth service, U.S. Department of Health and Human Services, Atlanta, GA.
Kamerud KL, Hobbie KA, Anderson KA. Stainless steel leaches nickel and chromium into foods during cooking. J Agric Food Chem. 2013;61(39):9495–501.
Talio MC, Marta OL, Fernánde P. Determination of nickel in cigarettes smoke by molecular fluorescence. Microchem J. 2011;99(2):486–91.
Torres F, Graças M, Melo M, Tosti A. Management of contact dermatitis due to nickel allergy: an update. Clin Cosmet Investig Dermatol. 2009;2:39–48.
Liu G, Sun L, Pan A, Zhu M, Zi L, Wang Z, Liu X, Ye XW, et al. Nickel exposure is associated with the prevalence of type 2 diabetes in Chinese adults. Int J Epidemiol. 2015;44:240–8.
Forgacs Z, Massányi P, Lukac N, Somosy Z. Reproductive toxicology of nickel – review, journal of environmental science and health Part A. Toxic Hazard Subs Environ Eng. 2012;47(9):1249–60.
Das KK, Das SN, Dhundas SA. Nickel, its adverse health effects and oxidative stress. Indian J Med Res. 2008;128:412–25.
Dahmen-Ben MI, Bellassoued K, Athmouni K, Naifar M, Chtourou H, Ayadi H, Makni-Ayadi F, Sayadi S, El Feki A, Dhouib A. Protective effect of Dunaliella sp., lipid extract rich in polyunsaturated fatty acids, on the hepatic and renal toxicity induced by nickel in rats. Toxicol Mech Methods. 2016;26(3):221–30.
Cheng Z, Cheng N, Shi D, Ren X, Gan T, Bai Y, et al. The relationship between Nkx2.1 and DNA oxidative damage repair in nickel smelting workers: Jinchang cohort study. Int J Environ Res Public Health. 2019. https://doi.org/10.3390/ijerph16010120.
Liu CM, Ma JQ, Liu SS, Feng ZJ, Wang AM. Puerarin protects mouse liver against nickel-induced oxidative stress and inflammation associated with the TLR4/p38/CREB pathway. Chem Biol Interact. 2016;243:29–34.
Liu CM, Zheng GH, Ming QL, Chao C, Sun JM. Sesamin protect mouse liver against nickel-induced oxidative DNA damage by the PI3K-Akt pathway. J Agric Food Chem. 2013;61(5):1146–54.
Zheng GH, Liu CM, Sun JM, Cheng C. Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquatic Toxicol. 2014. https://doi.org/10.1016/j.aquatox.2013.12.015.
Flora SJ, Pachauri V. Chelation in metal intoxication Int. J. Environ. Res. Public Health. 2010;7:2745–88.
Elangovan P, Pari L. Ameliorating effects of troxerutin on nickel-induced oxidative stress in rats. Redox Rep. 2013;18(6):224–32.
Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA. A review on the therapeutic potential of Nigella sativa: a miracle herb. Asian Pacific J Tropical Biomedicine. 2013;3(5):337–521.
Nakasugi T, Murakawa T, Shibuya K, Morimoto M. Deodorizing substance in black cumin (Nigella sativa L.) seed oil. J Oleo Sci. 2017;66(8):877–82.
Majdalawieh AF, Fayyad MW. Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. J Ayurveda Integ Med. 2016;7:173–18.
Zohary D, Hopf M. Domestication of plants in the old world (3rdedn). New York: Oxford University Press; 2000. p. 206.
Forouzanfar F, Bazzaz BSF, Hosseinzadeh H. Black cumin (Nigella sativa) and it's constituent (thymoquinone): a review on antimicrobial effects. Iran J Basic Med Sci. 2014;17(12):929–38.
Yarnell E, Abascal K (2001) Nigella sativa: holy herb of the Middle East. Alternative and Complementary Therapies17:99–105.
Namazi N, Larijani B, Ayati MH, Abdollahi M. The effects of Nigella sativa L. on obesity: a systematic review and meta-analysis. J Ethnopharmacol. 2018;219(12):173–81.
Koshak A, Koshak E, Heinrich M. Medicinal benefits of Nigella sativa in bronchial asthma: a literature review. Saudi Pharmaceutical Journal. 2017;25(8):1130–6.
Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H. Review on clinical trials of black seed (Nigella sativa ) and its active constituent, thymoquinone. J Pharmacopuncture. 2017;20(3):179–93.
Seghatoleslam M, Alipour F, Shafieian R, Hassanzadeh Z, Edalatmanesh MA, Sadeghnia HR, et al. The effects of Nigella sativa on neural damage after pentylenetetrazole induced seizures in rats. J Tradit Complement Med. 2016;6:262–8.
Perveen T, Haider S, Zuberi NA, Saleem S, Sadaf S, Batool Z. Increased 5-HT levels following repeated administration of Nigella sativa L. (black seed) oil produce antidepressant effects in rats. Sci Pharm. 2014;82(1):161–70.
Kanter M, Coskun O, Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch fürToxikol. 2006;80(4):217–40.
Oyero OG, Toyama M, Mitsuhiro N, Onifade AA, Hidaka A, Okamoto M. Selective inhibition of hepatitis C virus replication by alpha-zam, a Nigella sativa seed formulation. Afr J Tradit Complement Altern Med. 2016;13(6):144–8.
Shokri H. A review on the inhibitory potential of Nigella sativa against pathogenic and toxigenic fungi. Avicenna J Phytomed. 2016;6(1):21–33.
Ramadan MF. Nutritional value and applications of Nigella sativa essential oil: a mini-review. J Essent Oil Res. 2015;27:271–5.
Dwarampudi LP, Palaniswamy D, Nithyanantham M, Raghu PS. Antipsoriatic activity and cytotoxicity of ethanolic extract of Nigella sativa seeds. Pharmacogn Mag. 2012;8(32):268–72.
Al-Seeni MN, El RabeyH A, Al-Hamed AM, Zamazami MA. Nigella sativa oil protects against tartrazine toxicity in male rats. Toxicol Rep. 2018;5:146–55.
Farooqui Z, Ahmed F, Rizwan S, Shahid F, Khan AA, Khan F. Protective effect of Nigella sativa oil on cisplatin-induced nephrotoxicity and oxidative damage in rat kidney. Biomed Pharmacother. 2017;85:7–15.
Ahmad A, Al-Abbasi FA, Sadath S, Ali SS, Abuzinadah MF, Alhadrami HA, et al. Ameliorative effect of camel's milk and Nigella Sativa oil against thioacetamide-induced hepatorenal damage in rats. Phcog Mag. 2018;14:27–35.
Hamed MA, El-Rigal NS, Ali SA. Effects of black seed oil on the resolution of hepato-renal toxicity induced by bromobenzene in rats. Eur Rev Med Pharmacol Sci. 2018;17(5):569–81.
Abdel-Zaher AO, Abdel-Rahman MS, Elwasei FM. Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: role of nitric oxide and oxidative stress. Neurotoxicology. 2011;32(6):725–33.
Hassan AS, Ahmed JH, Sawsan S, Al-Haroon. A study of the effect of Nigella sativa (black seeds) in isoniazid (INH)-induced hepatotoxicity in rabbits. Indian J Pharmacol. 2012;44(6):678–82.
Bourgou S, Pichette A, Marzouk B, Legault J. Bioactivities of black cumin essential oil and its main terpenes from Tunisia. South Afr J Bot. 2010;76:210–6.
National Institute of Health (NIH). Guide for the care and use of laboratory animals. NIH Publication. 1985:85–23.
Gathwan KH, Al-Karkhi IHT, EA JAL-M. Hepatic toxicity of nickel chloride in mice. Res Chem Intermed. 2013;39:2537–42.
El-Sayed WM. Upregulation of chemoprotective enzymes glutathione by Nigella sativa (Black Seed) and thymoquinone in CCl4-intoxicated rats. Int J Toxicol. 2011;30(6):707–14.
Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Meth Enzymol. 1990;186:407–21.
Aebi HE (2011) Catalase. In: Bergmeyer HU (ed). Methods of enzymatic analysis. Verlag Chemie, Weinheim, Florida, p273.
Habig WH, Pabast MJ, Jakoby WB. Glutathione S- Transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9.
Sofowara A. Medicinal plants and traditional medicine in Africa. New York: Wiley; 1993. p. 97–145.
Trease GE, Evans WC. Pharmacognosy (11th edn). London: Bailliar Tindall; 1989. p. 60–75.
Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. London: Chapman and Hall Ltd; 1973. p. 279.
Dildar A, Muhammad MK, Ramsha S. Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antioxidants. 2015;4:394–409.
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41.
El-Shafei HM. Assessment of liver function among nickel-plating workers in Egypt. East Mediterr Health J. 2011;6:490–4.
Latif A, Ali M, Iqbal F. Histopathological responses of liver and kidney of a freshwater cyprinid, Labeo rohitato nickel sulphate. Pak J Zool. 2014;46(1):37–44.
Kadi IE, Dah Douh F. Vitamin C pretreatment protects from nickel-induced acute nephrotoxicity in mice. Arh Hig Rada Toksikol. 2016;67:210–5.
He M, Lu Y, Xu S, Mao ZL, Duan W, et al. MiRNA-210 modulates a nickel-induced cellular energy metabolism shift by repressing the iron-sulfur cluster assembly proteins ISCU1/2 in Neuro-2a cells. Cell Death Dis. 2014. https://doi.org/10.1038/cddis.2014.60.
Sousa CA, Soares HM, Soares EV. Nickel oxide nanoparticles trigger caspase- and mitochondria-dependent apoptosis in the yeast Saccharomyces cerevisiae. Chem Res Toxicol. 2019;32:245.
Liapi C, Zarros A, Theocharis S, Voumvourakis K, Anifantaki F, Gkrouzman E, Mellios Z, Skandali N, Al-Humadi H, Tsakiris S. Short-term exposure to nickel alters the adult rat brain antioxidant status and the activities of crucial membrane-bound enzymes: neuroprotection by L-cysteine. Biol Trace Elem Res. 2011;143(3):1673–81.
Maiti AK, Saha NC, Paul G, Dhara K. Mitochondrial respiratory chain inhibition and Na+K+ATPase dysfunction are determinant factors modulating the toxicity of nickel in the brain of Indian catfish Clarias batrachus L. Interdiscip Toxicol. 2018;1(2):306–15.
Das KK, Reddy RC, Bagoji IB, Das S, Bagali S, Mullur L, Khodnapur JP, Biradar MS. The primary concept of nickel toxicity – an overview. J Basic Clin Physiol Pharmacol. 2019;30(2):141–52.
Misra M, Rodriguez RE, Kasprzak KS. Nickel induced lipid peroxidation in the rat: correlation with nickel effect on antioxidant defence systems. Toxicol. 1990;64(1):1–17.
Allocati N, Masulli M, Ilio C, DiandFederici L. Glutathione transferases: substrates, inhibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018. https://doi.org/10.1038/s41389-017-0025-3.
Chang YC, Liu FP, Ma X, Li M-M, Li R, Li C-W, et al. Glutathione S-transferase A1 – a sensitive marker of alcoholic injury on primary hepatocytes. Hum Exp Toxicol. 2017;36(4):386–94.
Bieniaś B, Sikora P. Potential novel biomarkers of obstructive nephropathy in children with hydronephrosis. Dis Markers. https://doi.org/10.1155/2018/1015726.
Tesauro M, Nisticò S, Noce A, Tarantino A, Marrone G, Costa A, et al (2015). The possible role of glutathione-S-transferase activity in diabetic nephropathy. Int J Immunopathol Pharmacol 2015; 28(1): 129–133.
McMahon GM, Waikar SS. Biomarkers in nephrology. Am J Kidney Dis. 2013;62(1):165–78.
Wright LS, Kornguth SE, Oberley TD, Siegel FL. Effects of lead on glutathione-S-transferase expression in rat kidney: a dose-response study. Toxicol Sci. 2015;46:254–9.
An X, Zhou A, Yang Y, Yue W, Xin R, Tian C, Wu Y. Protective effects of gallic acid against NiSO4 induced toxicity through down-regulation of the Ras/ERK signalling pathway in BEAS-2B cells. Med Sci Monit. 2016;22:3446–54.
Liu Y, Guo M. Studies on transition metal-quercetin complexes using electrospray ionization tandem mass spectrometry. Molecules. 2015;20(5):8583–94.
de Castilho TS, Matias TB, Nicolini KP, Nicolini J. Study of interaction between metal ions and quercetin. Food Sci Human Wellness. 2018;7:215–9.
Ciftci O, Ozdemir I, Tanyildizi S, Yildiz S, Oguzturk H. Antioxidative effects of curcumin, β-myrcene and 1, 8-cineole against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol Ind Health. 2011;27(5):447–530.
Santos FA. 1, 8-cineole protects against liver failure in an in-vivo murine model of endotoxemic shock. J Pharm Pharmacol. 2001;53(4):505–11.
Rozza AL, Meira FF, Souza AR, Pellizzon CH. The gastroprotective effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS One doi. 2001. https://doi.org/10.1371/journal.pone.0086686.
Pinto MEA, Araújo SG, Morais MI, Nívea PSÁ, Caroline ML, Rosa CA, et al. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An Acad Bras Cienc. 2017;89(3):1671–81.
Dailey OD, Wang X, Chen F, Guohui H. Anticancer activity of branched-chain derivatives of oleic acid. Anticancer Res. 2011;31:3165–70.
Yu FR, Lian XZ, Guo HY, McGuire PM, Li RD, Wang R, et al. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J Pharm Pharm Sci. 2005;8:528–35.
de Oliveira TM, Fonseca de Carvalho RB, Fernandes da Costa IH, Lopes de Oliveira GA, de Souza AA, de Lima SG, et al. Evaluation of p-cymene, a natural antioxidant. Pharm Biol. 2015;53:423–4.
Acknowledgements
Not applicable.
Voucher specimen
Voucher specimen of this material has been identified by and deposited in the University of Ibadan herbarium, but a code has not been allocated to it, therefore the voucher code is not applicable.
Funding
This research did not receive a grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KA: Conception and design of the work, laboratory analysis, data acquisition, statistical analysis and manuscript writing. JA: Conception and design of the work, laboratory analysis, data acquisition and manuscript writing. OO: laboratory analysis and data acquisition. UY: laboratory analysis and data acquisition. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments were carried out according to recommendations of the ethical conditions approved by the Department of Chemical and Food Sciences, Bells University of Technology, Ota, Nigeria in conformity with standard ethics for handling and care of experimental animals [40].
Consent for publication
Not applicable.
Competing interests
Authors state no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akinwumi, K.A., Jubril, A.J., Olaniyan, O.O. et al. Ethanol extract of Nigella sativa has antioxidant and ameliorative effect against nickel chloride-induced hepato-renal injury in rats. Clin Phytosci 6, 64 (2020). https://doi.org/10.1186/s40816-020-00205-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-020-00205-9