Abstract
Background
Maternal metabolic health during the prenatal period is an established determinant of cardiometabolic disease risk. Many studies have focused on poor offspring outcomes after exposure to poor maternal health, while few have systematically appraised the evidence surrounding the role of maternal exercise in decreasing this risk.
The aim of this study is to characterize and quantify the specific impact of prenatal exercise on children’s cardiometabolic health markers, at birth and in childhood.
Methods
A systematic review of Scopus, MEDLINE, EMBASE, CENTRAL, CINAHL, and SPORTDiscus up to December 2017 was conducted.
Randomized controlled trials (RCTs) and prospective cohort studies of prenatal aerobic exercise and/or resistance training reporting eligible offspring outcomes were included.
Four reviewers independently identified eligible citations and extracted study-level data. The primary outcome was birth weight; secondary outcomes, specified a priori, included large-for-gestational age status, fat and lean mass, dyslipidemia, dysglycemia, and blood pressure. We included 73 of the 9804 citations initially identified. Data from RCTs was pooled using random effects models. Statistical heterogeneity was quantified using the I2 test. Analyses were done between June and December 2017 and the search was updated in December 2017.
Results
Fifteen observational studies (n = 290,951 children) and 39 RCTs (n = 6875 children) were included. Observational studies were highly heterogeneous and had discrepant conclusions, but globally showed no clinically relevant effect of exercise on offspring outcomes. Meta-analyzed RCTs indicated that prenatal exercise did not significantly impact birth weight (mean difference [MD] − 22.1 g, 95% confidence interval [CI] − 51.5 to 7.3 g, n = 6766) or large-for-gestational age status (risk ratio 0.85, 95% CI 0.51 to 1.44, n = 937) compared to no exercise. Sub-group analyses showed that prenatal exercise reduced birth weight according to timing (starting after 20 weeks of gestation, MD − 84.3 g, 95% CI − 142.2, − 26.4 g, n = 1124), type of exercise (aerobic only, MD − 58.7 g, 95% CI − 109.7, − 7.8 g; n = 2058), pre-pregnancy activity status (previously inactive, MD − 34.8 g, 95% CI − 69.0, − 0.5 g; n = 2829), and exercise intensity (light to moderate intensity only, MD − 45.5 g, 95% CI − 82.4, − 8.6 g; n = 2651). Fat mass percentage at birth was not altered by prenatal exercise (0.19%, 95% CI − 0.27, 0.65%; n = 130); however, only two studies reported this outcome. Other outcomes were too scarcely reported to be meta-analyzed.
Conclusions
Prenatal exercise does not causally impact birth weight, fat mass, or large-for-gestational-age status in a clinically relevant way. Longer follow up of offspring exposed to prenatal exercise is needed along with measures of relevant metabolic variables (e.g., fat and lean mass).
Protocol Registration
Protocol registration number: CRD42015029163.
Similar content being viewed by others
Key Points
-
In general, birth weight is not impacted in a clinically significant manner by prenatal exercise programs.
-
Our understanding of the impact of prenatal exercise on the child’s metabolic health and future risk of cardiometabolic disease is limited as very few trials and cohort studies examined other health indicators aside from birth weight—an imperfect marker of health and future outcomes.
-
Few trials and cohort studies followed up the children to see potential lasting effects of maternal prenatal exercise on their metabolic health.
-
Clinicians looking to counsel their clients might highlight that while prenatal exercise is perfectly safe for the baby, the best evidence currently available indicate exercise is not sufficient by itself to protect the child against cardiometabolic diseases.
Background
Obesity and metabolic syndrome are two of the most common chronic diseases among children [1,2,3]. Recent evidence suggests these conditions have their roots in utero as maternal obesity, dyslipidemia, and hyperglycemia are associated with child cardiometabolic health [4,5,6,7,8,9]. Animal- and population-based studies suggest that prenatal exposures may influence offspring development and cardiometabolic risk in childhood [10, 11]. Moderate-intensity exercise at least three times per week can maintain or improve maternal physical fitness [12] and cardiovascular health during pregnancy through a decrease in blood pressure [13], plasma triglycerides [13, 14], and insulin resistance [15,16,17]. Therefore, prenatal exercise could create a beneficial fetal milieu and reduce the risk of obesity and metabolic syndrome for the offspring by regulating weight and cardiometabolic factors at birth and later in childhood. However, comprehensive syntheses of high-quality evidence on this topic are scarce.
Previous systematic reviews examining the role of prenatal exercise on offspring outcomes have been conducted with heterogeneous results and the interpretation of their findings are limited for several reasons: (1) the primary outcome of interest for previous systematic reviews varied (birth weight or large-for-gestational age (LGA) status); (2) inclusion criteria were variable (types of exercise targeted; inclusion of diet as an intervention; type and number of databases queried; low quality study designs); (3) there were flaws in methodological rigor (type of analysis, pooling heterogeneous studies together); and (4) few evaluated health outcomes in offspring beyond birth weight. The reviews that found a reduction in birth weight (from − 440 g, 95% confidence interval [CI] − 610 to − 270 g [18], to − 31 g, 95%CI − 57 to − 24 g [19], compared to sedentary controls) or LGA status after exposure to prenatal exercise pooled randomized trials and observational studies [18], included interventions that combined exercise and dietary changes [20], or used fixed effect models to analyze the data [19]. Fixed effect models assume that one true effect size is shared by all the included studies regardless of the population or type of exercise studied. Thus, utilizing a fixed effects model considers less variability in the primary studies, and is more likely to reach statistical significance with a large enough sample size [21]. Random effect models should, thus, be preferred when dealing with complex physiological conditions like the effect of different prenatal exercises undergone by different populations on offspring parameters. Other reviews [12, 22, 23] did not find any significant impact of prenatal exercise on birth weight, but were also limited by their specific scope [12, 23] or restricted search strategy [22].
To overcome these limitations and update past reviews [12, 18,19,20, 22, 23], we synthesized evidence from randomized controlled trials and prospective cohort studies separately to assess the impact of prenatal exercise on offspring cardiometabolic risk factors including weight, adiposity, and blood pressure at birth and in childhood while investigating the effects of maternal body mass index (BMI) and training variables. The clinical question guiding this review was: “Does maternal exercise training elicit short- and/or long-term cardiometabolic health benefits in offspring, compared to no exercise training?”
Methods
Study Design
Using a protocol designed a priori (PROSPERO #CRD42015029163), we conducted our systematic review using methodological approaches outlined in the Cochrane Handbook for Systematic Reviews [24] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [25]. The search was targeted to identify studies to address the specific research question: Do regular aerobic and/or resistance exercises during pregnancy, compared to no exercise, reduce the risk of cardiometabolic disease in offspring? We defined regular exercise as voluntary movements done to improve or maintain fitness on a weekly basis for at least a month. The review team, composed of researchers in the fields of physiology, exercise, and developmental origins of health and disease, formulated the review question, reviewed the search strategies and review methods, and provided input throughout the review process.
Literature Search Strategy
We searched Medline (Ovid), EMBASE (Ovid), CENTRAL (the Cochrane Library—Wiley), from inception to May 2016 for studies on prenatal pregnancy and cardiometabolic outcomes in the offspring. We added Scopus (Elsevier), CINAHL (EBSCO), and SPORTDiscus (EBSCO) to our target databases following comments from reviewers and reran the search through all six databases in May 2017. The Cochrane Highly Sensitive Search Strategy [26] was used as a model for searching; we designed search strategies specific to each database (see example on PROSPERO registration page). In order to identify ongoing or planned trials, we searched the World Health Organization’s International Clinical Trials Registry Platform (ClinicalTrials.gov). In addition to electronic searching, we hand-searched the bibliographies of relevant narrative and systematic reviews as well as those of included studies for additional citations. The search was rerun in December 2017 to include additional citations. Reference management was performed in EndNote™ (ver. 16, Thompson Reuters).
Study Selection
We used a two-step process for study selection. First, all titles and abstracts of search results were screened independently in duplicate (by LG, NH, JLH, and CO) to determine if a study met the general inclusion criteria. The same reviewers independently examined the full texts of relevant citations. Disagreements were resolved by discussion between the reviewers or by third-party adjudication (LG or DSK), as needed. We included only randomized controlled trials or prospective cohort studies examining the impact of exercise undergone during pregnancy (from conception to delivery) on offspring cardiometabolic outcomes (see Table 1 for detailed inclusion and exclusion criteria). We hoped to reduce bias by selecting studies with the best experimental and observational designs; observational cohorts were included with the expectation that follow up data may be provided to inform long term offspring outcomes. No other restrictions, including language or publication status, were considered. The primary outcome measure was birth weight. Secondary outcomes included offspring fat and lean mass at birth, LGA status and weight, fat and lean mass, blood pressure, dyslipidemia, and dysglycemia at any time in childhood.
Data Extraction and Quality Assessment
Three reviewers (LG, NH, and JLH) extracted data from included full texts using a standardized and piloted data extraction form. Extracted data included funding sources, demographics of the enrolled mothers and children (country, gestational age at randomization, maternal age at randomization, pre-pregnancy BMI, maternal smoking status at randomization; child age at latest follow-up), details of the prenatal exercise (type of exercise: aerobic or resistance training, frequency, intensity, timing during pregnancy; exercise measure: self-report or supervised), and predetermined offspring outcomes as described above and in Tables 1 and 2. When a trial reported results for more than one time period, results at birth and at the longest follow up were extracted separately. Intent-to-treat analysis was preferred when the data was presented accordingly. Data management was performed using Microsoft Word 2007 (Microsoft Corp).
We evaluated the internal validity of included studies with the Cochrane Collaboration’s Risk of Bias tools [24, 27], in duplicate (LG and CO). Study authors were contacted if data as published was incomplete for the needs of the review.
Data Synthesis and Analysis
We quantitatively analyzed data from the included studies using Review Manager (RevMan version 5.3.5, the Cochrane Collaboration). Pooled dichotomous data, calculated based on the generic inverse variance method, are presented as a risk ratio (RR) and pooled continuous data are expressed as a mean difference (MD), with 95% confidence intervals (CI). Only random effects models were used as populations and interventions varied. Statistical heterogeneity was explored and quantified using the I2 test. After validation against the associated protocols and/or trial registration information available, evidence of selective reporting found for any of the included trials was marked in the risk of bias assessment. A sensitivity analysis grouping only studies with low or unclear risk of bias was done for the primary outcome. Our pre-defined sub-group analyses, which were planned only for our primary outcome (birth weight) and only with data from randomized controlled trials (RCTs), were maternal pre-pregnancy activity level (inactive vs. active), maternal pre-pregnancy BMI, type of intervention (resistance training only vs. aerobic training only vs. combined), timing of exercise (starting in the first half of pregnancy, i.e., < 20 gestational weeks, vs. starting after the first half, i.e., ≥ 20 gestational weeks), country of origin, and internal validity (high vs. moderate/low quality). These sub-groups were intended to help pinpoint which components, if any, were responsible for the effect observed. Relevant components could then be highlighted in research and clinical interactions to promote better effectiveness. All tests of statistical inference reflect a two-sided α of 0.05.
Results
Search Results
Of 9804 citations identified through the literature search, 54 unique studies conducted between 1993 and 2017 were included, of which 15 were cohort studies [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] and 39 RCTs [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81] with 19 companion publications [82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] (see Fig. 1 for the flow chart and Tables 2 and 3 for studies characteristics). Nine additional studies not included in the previous meta-analyses were included [28, 51, 52, 55, 65, 70, 76, 77, 80]. Most studies (12) were conducted in the USA [31, 33, 36, 37, 41, 42, 47, 49, 61, 66, 73, 92]; others were from Australia and New Zealand [29, 62, 101], Brazil [43, 52, 53, 63, 68, 74, 81], Canada [28, 65], Colombia [72], Croatia [80], Denmark [30, 32, 34, 35, 40], Finland [54], Iran [56, 57], Kosovo [67], The Netherlands [69], Norway [55, 60, 102], Spain [44,45,46, 48, 50, 70, 79], Sweden [71], and the UK [38, 39, 76]. No study recruited previously active women, 16 recruited inactive women [43, 44, 46, 49, 52, 53, 55,56,57,58, 66,67,68, 70, 73, 92, 102], and the rest did not use previous exercise levels as a criterion. Most studies did not consider maternal BMI in their inclusion criteria, although eight specifically recruited women with overweight and/or obesity [51, 55, 64, 68, 69, 75, 77, 81], and three [49, 57, 65] women without overweight. The majority of experimental trials included healthy pregnant women, except five who targeted women with type 1 [61] or gestational diabetes [47, 52, 69, 74, 80], whereas observational cohorts were locally representative [31, 32, 41, 42], nationally representative [28, 30, 34, 35, 38,39,40], or convenience samples [29, 36, 37]. The funnel plot indicates that studies where exposure to prenatal exercise reduced birth weight were probably more likely to be published, at least for the smaller studies (Additional file 1: Fig. S1; especially seen for smaller studies). Concerning internal validity, most trials were adjudicated as of low or unclear risk of bias [43,44,45,46, 49, 52, 53, 57,58,59, 63, 67, 68, 74, 75, 83] (see Additional file 2: Fig. S2). The remaining trials were considered as high risk of bias due to their high dropout rates and unclear/inefficient blinding methods. All cohorts but one [29] were adjudicated at serious risk of bias, usually due to selection bias or missing data (Additional file 3: Fig. S3).
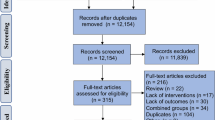
Flow diagram of literature search and study selection according to the 2009 Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) with modifications [25]. *Companion articles represent reports involving the same study population for the same intervention trial, including conference abstracts reporting findings from previous or subsequent full-length publications
Evidence from Observational Studies
Primary Outcome: Birth weight
Fifteen observational studies investigated the relationship between exercise in pregnancy and offspring cardiometabolic outcomes (see Table 2). They were too heterogenous in terms of exposure assessment and outcome reporting to be meta-analyzed. For the primary outcome, two cohorts found an increased average birth weight after exposure to exercise [31, 33] (from + 109 g, p < 0.05 to + 276 g, 95%CI 54, 497, n = 434), nine found no impact [28, 29, 32, 34, 36, 37, 39, 41, 42] (n = 143,432), and three lower average birth weight after prenatal exercise [30, 35, 40] (from − 23 g, 95% CI − 44 to − 1, to − 0.72 g, 95% CI − 1.3 to − 0.1 g, n = 142,420).
Secondary Outcomes
Two studies found investigated the risk of being born LGA after exposure to prenatal exercise. Although both found a similar reduction in risk, none were statistically significant (RR 0.83, 95% CI 0.67, 1.02, n = 1913 [28]; prevalence 0.73%, 95% CI 0.10, 5.18%; n = 20,458 [41]). Two studies reported long-term secondary outcomes after following up offspring at 7.1 [40] and 15.5 years old [38]. No significant relationships were found with exposure to prenatal exercise on BMI, blood pressure, blood lipids, or fasting glucose in these studies after adjustment for confounders.
Evidence from Randomized Controlled Trials
Primary Outcome
For the primary outcome, 38 trials involving 6766 pregnant women provided data for the meta-analysis of birth weight (see Table 3). Compared to control condition (no prenatal exercise), there was no difference in birth weight after exercise interventions delivered in pregnancy at any time period, frequency, or intensity of exercise (mean difference (MD): − 22.1 g, 95% confidence interval [CI] − 51.5 to 7.3 g; I2 22%; see Fig. 2). Restricting to the studies with healthy populations did not yield very different results (MD − 23.6 g, 95% CI − 54.7, 7.5; I2 23%, 31 trials, n = 5777). The moderate statistical heterogeneity led us to conduct our pre-defined sub-group analyses. Sub-grouping by maternal BMI indicated that prenatal exercise had no effect according to pre-pregnancy BMI categories (see Fig. 2), either < 25 kg/m2 (MD − 69.4, 95% CI − 210.3, 71.5; I2 54%; four trials, n = 831), > 25 kg/m2 (MD − 49.5, 95% CI − 112.1, 13.2; I2 0%; eight trials, n = 960), or > 30 kg/m2 (MD − 151.1, 95% CI − 528.9, 226.7; I2 60%; two trials; n = 106). Sub-grouping according to maternal pre-pregnancy activity level (inactive vs. active) indicated that prenatal exercise could reduce birth weight in previously inactive women (MD − 34.8 g, 95% CI − 69.0, − 0.5 g; I2 0%; 18 trials, n = 2829); however, as no study specifically included active women, we could not assess that sub-group (Fig. 3). Sub-grouping according to type of exercise showed that aerobic-only training similarly reduced birth weight (MD − 58.7 g, 95% CI − 109.7, − 7.8; I2 12%; 17 studies, n = 2058), but resistance training only (5 trials, n = 543), or combined regimens (16 trials, n = 4183) had non-significant effects (see Fig. 4). Prenatal exercise regimens starting after the 20th week of pregnancy marginally reduced birth weight (MD − 84.3 g, 95% CI − 142.2, − 26.4 g; I2 0%, n = 1124), whereas interventions starting before this time had no impact on birth weight (20 studies, n = 3853, see Fig. 5). Interventions that were light to moderate intensity reduced birth weight (MD − 45.5 g, 95% CI − 82.4, − 8.6 g; I2 3%; 9 trials, n = 2651) but not those that were moderate to vigorous intensity (25 trials, n = 2992; Fig. 6). Finally, frequency of exercise did not impact birth weight, whether interventions were less than three times a week (4 trials, n = 1131) or at least that frequent (31 trials, n = 5408; Fig. 7). Restricting to studies with low to moderate risk of bias did not yield different results (MD − 10.9, 95% CI − 42.1, 20.4; I2 0%; 17 trials, n = 3418; data not shown); however, studies from developing countries were more likely to find that prenatal exercise reduced birth weight (MD − 78.7 g, 95% CI − 135.4, − 22.0 g; I2 0%; 12 trials, n = 1120) compared to studies in developed countries (MD − 8.3 g, 95% CI − 43.8, 27.11 g; I2 32%; 26 trials, n = 5646; data not shown).
Secondary Outcomes
Only LGA status and fat mass at birth could be meta-analyzed. Data concerning the other outcomes were either not reported by more than one study or were not clinically homogenous enough to be pooled (e.g., collected at different ages). Prenatal exercise did not reduce the risk of LGA (RR 0.85, 95% CI 0.51, 1.44; I2 58%; seven studies; n = 937; Fig. 8) nor impact fat mass percentage (MD 0.19, 95% CI − 0.27, 0.65%; I2 10%; two studies; n = 130; Fig. 9). Qualitatively, the two studies that followed up offspring after birth did not indicate any significant impact of prenatal exercise on weight [62, 92] or fat mass [62, 92] at 17 days or 6 months old. No RCT reported on offspring blood pressure, blood glucose, or blood lipids.
Discussion
Exercise is an established cornerstone for optimizing women’s metabolic health, and prenatal exercise is safe for mothers and fetus [103]. As evidence is accumulating that in utero exposures have a major influence on the fetus’ future cardiometabolic health [10], the positive maternal impacts of exercise on women’s cardiometabolic health have recently been posited to extend to exposed fetuses [23]. Although recent meta-analyses stated prenatal exercise might prevent giving birth to larger babies [18, 19, 22], we found contrasting results from both high quality observational cohorts and RCTs which indicated that prenatal exercise does not impact average birth weight in a significant manner. None of the included cohort studies found a clinically relevant birth weight difference (i.e., ≥ 300 g [104]) after exposure to various kinds of prenatal exercise. Thus, even though the methodological differences made it difficult to compare the studies and explain their opposing results (prenatal exercise increasing vs. decreasing birth weight), none of the reviewed cohorts reported clinically relevant impacts of prenatal exercise on birth weight. As few cohorts followed children into childhood or measured other variables than weight, the long-term impact of maternal exercise on offspring cardiometabolic health remains unclear. Long-term follow-up of pregnancy cohorts is needed to discern the influence of exercise in pregnancy and child health.
Similar to results observed by prospective cohorts, we did not find a clinically relevant effect of prenatal aerobic and/or strength training interventions on child birth weight, LGA status, or birth fat mass. Although prenatal exercise led to statistically significant birth weight reduction in some sub-group analyses, the mean effect varied from − 0.5 to − 84 g, which are clinically negligible impacts [104]. We were limited in our ability to examine the impact of prenatal exercise on other important health outcomes (childhood blood pressure, glucose, lipids, and fat mass) because they were not measured or reported by the trials, therefore the long-term impact of exposure to prenatal exercise on cardiometabolic health of offspring could not be assessed.
Birth weight is a very common marker of infant health due to its ease of measurement and its historically frequent association with future health outcomes [105]. Nonetheless, recent work in the field of developmental origins of health and disease indicate that weight is only a crude marker of health. For example, some studies indicate that offspring born small for gestational age are leaner later in life [106, 107] while others found that these offspring were at increased risk of obesity [108,109,110]. Likewise, some studies found increased markers of cardiometabolic risk in LGA offspring [111, 112] while others did not find evidence of increased risk [113,114,115]. Thus, in order to adequately assess the potentially protective effects of prenatal exercise on offspring cardiometabolic health, it is imperative to measure other relevant markers (e.g., body composition, blood glucose, and lipids) at birth and later in childhood. Our unexpected null results provide cautionary evidence that exercise by itself is not sufficient to impact birth weight, as some have argued [28, 116]. On the other hand, they suggest that women can safely participate in the type of activity they prefer (aerobic or resistance) at the intensity and frequency that suits them, which might increase adherence.
Strengths and Limitations
Strengths of this systematic review include isolating the causal impact of prenatal exercise (vs. other interventions like dietary modifications); restricting to high-quality designs to reduce bias (prospective cohorts and RCTs); considering outcomes other than weight to assess the impact of prenatal exercise on offspring health; considering maternal and training variables not assessed by previous reviews (timing, intensity and frequency of intervention, maternal BMI, country of origin); using random effect models for all analyses; and using a protocol established a priori. Despite these strengths, the review has some limitations. First, only studies assessing aerobic and/or strength training were included, discarding studies where other forms of prenatal exercise (e.g., yoga [117]) were measured. This choice was made because current recommendations [118, 119] are focused on those two types of exercise. However, as > 80% of active pregnant women report engaging in some kind of aerobic training [120], we are confident our results are representative of real-life prenatal exercise habits and are therefore relevant for clinicians and researchers. Second, our predefined sub-groups addressed only one variable at a time (e.g., maternal BMI, timing of exercise). It is possible that evaluating the interaction by grouping according to many parameters (e.g., among women with a BMI > 25, those who starting exercising < 20 gestational weeks) through a meta-regression might be more informative. However, such analyses were not planned a priori so another study would be needed to answer this limitation. Third, there was marked heterogeneity in research designs, assessments of exercise dose (frequency, intensity, duration, adherence), and reports of offspring outcomes, making direct comparisons between studies difficult. Accordingly, we refrained from pooling results that we considered too heterogeneous and were careful in not over-interpreting the results.
Evidence Gaps
It is imperative that future trials report determinants of offspring cardiometabolic health other than birth weight, such as adiposity, plasma glucose and lipids, and blood pressure early in life and ideally at multiple times throughout childhood to define the long term impact of exposure to prenatal exercise on offspring cardiometabolic health. Indeed, there are indications that higher blood pressure [121], glycemia [5], and dyslipidemia [122] early in life are related to future metabolic syndrome, diabetes, and cardiovascular diseases, whereas birth weight is a crude marker [113, 123]. Follow up of data for these parameters in childhood would provide important tools to public health authorities to help determine if and how prenatal exercise improves offspring cardiovascular risk factors in both the short and long term. Indeed, assessing offspring fat and lean mass might be more informative than only weight. Additionally, offspring should be periodically reassessed as there is a dearth of longitudinal data concerning offspring exposed to exercise interventions in the literature. A sample size calculation based on the RCTs included indicate that matched groups of at least 268 participants (536 participants total) are needed to detect a birth weight difference between groups at 90% power and with a 0.05 double-sided α. However based on our analyses, future interventions should include components other than exercise (such as a dietary intervention) if the intent is to have an impact on birth weight. Finally, more diverse participants in terms of pre-pregnancy activity level and body composition are needed in future studies to understand how exercise interventions in pregnancy modulate the relationship between maternal physiology, offspring body composition, and cardiometabolic health. Clinicians looking to counsel their clients might want to highlight that while prenatal exercise is perfectly safe for the baby, the best evidence currently available indicates it is not sufficient by itself to protect the child against cardiometabolic diseases.
Conclusion
In summary, high-quality studies analyzed with conservative statistics show that the impact of prenatal exercise on birth weight is not clinically relevant. This impact might be more important in previously less active women and when the exercise program has light to moderate intensity and starts in the second half of pregnancy. Due to the scarcity of studies collecting parameters other than birth weight and/or following up offspring in childhood, there is limited evidence about the relationship of prenatal exercise and long-term offspring cardiometabolic health. Thus, there is a great need for the collection of data other than weight and for the long-term follow up of offspring exposed to exercise to better define the impact of prenatal exercise on offspring cardiometabolic risk throughout life. Researchers and clinicians intending to impact the health of the future generations should consider adding other components (such as dietary components) to their exercise interventions.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- LGA:
-
Large for gestational age
- MD:
-
Mean difference
- RCT:
-
Randomized controlled trials
- RR:
-
Risk ratio
References
Rodd C, Sharma AK. Recent trends in the prevalence of overweight and obesity among Canadian children. Can Med Assoc J. 2016; https://doi.org/10.1503/cmaj.150854.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14. https://doi.org/10.1001/jama.2014.732.
Kim J, Lee I, Lim S. Overweight or obesity in children aged 0 to 6 and the risk of adult metabolic syndrome: a systematic review and meta-analysis. J Clin Nurs. 2017;26(23–24):3869–80. https://doi.org/10.1111/jocn.13802.
Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60(1):5–20.
Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–6. https://doi.org/10.1542/peds.2004-1808.
Huang R-C, de Klerk NH, Smith A, Kendall GE, Landau LI, Mori TA, et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care. 2011;34(4):1019–25.
Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University diabetes in pregnancy center. Diabetes Care. 1998;21(Suppl 2):B142–9.
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–41.
Nicholas LM, Rattanatray L, Morrison JL, Kleemann DO, Walker SK, Zhang S, et al. Maternal obesity or weight loss around conception impacts hepatic fatty acid metabolism in the offspring. Obesity (Silver Spring). 2014;22(7):1685–93. https://doi.org/10.1002/oby.20752.
Winett L, Wallack L, Richardson D, Boone-Heinonen J, Messer L. A framework to address challenges in communicating the developmental origins of health and disease. Curr Environ Health Rep. 2016;3(3):169–77. https://doi.org/10.1007/s40572-016-0102-3.
Blaize AN, Pearson KJ, Newcomer SC. Impact of maternal exercise during pregnancy on offspring chronic disease susceptibility. Exercise & Sport Sciences Reviews. 2015;43(4):198–203.
Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane database of systematic reviews (Online). 2006;3:CD000180.
Loprinzi PD, Fitzgerald EM, Woekel E, Cardinal BJ. Association of physical activity and sedentary behavior with biological markers among U.S. pregnant women. J Women's Health (Larchmt). 2013;22(11):953–8. https://doi.org/10.1089/jwh.2013.4394.
Mudd LM, Evenson KR. Review of impacts of physical activity on maternal metabolic health during pregnancy. Curr Diab Rep. 2015;15(2):572. https://doi.org/10.1007/s11892-014-0572-3.
Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: a randomised controlled trial. Br J Sports Med. 2012;46(9):656–61. https://doi.org/10.1136/bjsports-2011-090009.
Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33(7):1457–9. https://doi.org/10.2337/dc09-2336.
Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab. 2009;35(5):418–21. https://doi.org/10.1016/j.diabet.2009.04.008.
Leet T, Flick L. Effect of exercise on birthweight. Clin Obstet Gynecol. 2003;46(2):423–31. https://doi.org/10.1097/00003081-200306000-00021.
Wiebe HW, Boule NG, Chari R, Davenport MH. The impact of prenatal exercise on fetal growth: a meta-analysis. Can J Diabetes. 2014;38:S64.
Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. https://doi.org/10.1136/bmj.e2088.
Mansourian M, Mohammadi R, Marateb HR, Yazdani A, Goodarzi-Khoigani M, Molavi S. Comprehensive maternal characteristics associated with birth weight: Bayesian modeling in a prospective cohort study from Iran. Journal of Research in Medical Sciences. 2017;22(9):107.
Perales M, Santos-Lozano A, Ruiz JR, Lucia A, Barakat R. Benefits of aerobic or resistance training during pregnancy on maternal health and perinatal outcomes: a systematic review. Early Hum Dev. 2016;94:43–8.
Moyer C, Reoyo OR, May L. The influence of prenatal exercise on offspring health: a review. Clinical Medicine Insights Women's health. 2016;9:37–42.
Higgins JPT, Green S, Cochrane C. Cochrane handbook for systematic reviews of interventions. Chichester; Hoboken: Wiley-Blackwell; 2008.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery (London, England). 2010;8(5):336–41. https://doi.org/10.1016/j.ijsu.2010.02.007.
Robinson K, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol. 2002;31(1):150. https://doi.org/10.1093/ije/31.1.150.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 https://doi.org/10.1136/bmj.i4919.
Bisson M, Croteau J, Guinhouya BC, Bujold E, Audibert F, Fraser WD, et al. Physical activity during pregnancy and infant's birth weight: results from the 3D birth cohort. BMJ Open Sport Exerc Med. 2017;3(1):e000242.
Duncombe D, Skouteris H, Wertheim EH, Kelly L, Fraser V, Paxton SJ. Vigorous exercise and birth outcomes in a sample of recreational exercisers: a prospective study across pregnancy. Aust N Z J Obstet Gynaecol. 2006;46(4):288–92.
Fleten C, Stigum H, Magnus P, Nystad W. Exercise during pregnancy, maternal prepregnancy body mass index, and birth weight. Obstet Gynecol. 2010;115(2 PART 1):331–7.
Hatch MC, Shu XO, McLean DE, Levin B, Begg M, Reuss L, et al. Maternal exercise during pregnancy, physical fitness, and fetal growth. Am J Epidemiol. 1993;137(10):1105–14.
Hegaard HK, Petersson K, Hedegaard M, Ottesen B, Dykes AK, Henriksen TB, et al. Sports and leisure-time physical activity in pregnancy and birth weight: a population-based study. Scand J Med Sci Sports. 2010;20(1):e96–102.
Johnson AA, Knight EM, Edwards CH, Oyemade UJ, Cole OJ, Westney OE, et al. Selected lifestyle practices in urban African American women—relationships to pregnancy outcome, dietary intakes and anthropometric measurements. J Nutr. 1994;124(6 SUPPL):963S–72S.
Juhl M, Kogevinas M, Andersen PK, Andersen AM, Olsen J. Is swimming during pregnancy a safe exercise? Epidemiology. 2010;21(2):253–8.
Juhl M, Olsen J, Andersen PK, Nohr EA, Andersen AM. Physical exercise during pregnancy and fetal growth measures: a study within the Danish National Birth Cohort. American Journal of Obstetrics & Gynecology. 2010;202(1):63.e1–8.
Jukic A, Evenson K, Daniels J, Herring AH, Wilcox A, Hartmann K. A prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birthweight. Am J Epidemiol. 2010;171:S33.
Magann EF, Evans SF, Weitz B, Newnham J. Antepartum, intrapartum, and neonatal significance of exercise on healthy low-risk pregnant working women. Obstet Gynecol. 2002;99(3):466–72.
Millard LAC, Lawlor DA, Fraser A, Howe LD. Physical activity during pregnancy and offspring cardiovascular risk factors: findings from a prospective cohort study. BMJ Open. 2013;3(9) (no pagination):e003574.
Nieuwenhuijsen MJ, Northstone K, Golding J. Swimming and birth weight. Epidemiology (Cambridge, Mass). 2002;13(6):725–8.
Schou Andersen C, Juhl M, Gamborg M, Sorensen TI, Nohr EA. Maternal recreational exercise during pregnancy in relation to Children's BMI at 7 years of age. International Journal of Pediatrics. 2012;2012:920583.
Snapp CA, Donaldson SK. Gestational diabetes mellitus: physical exercise and health outcomes. Biological Research for Nursing. 2008;10(2):145–55.
Sternfeld B, Quesenberry CP, Eskenazi B, Newman LA. Exercise during pregnancy and pregnancy outcome. / Pratique de l ' exercice physique pendant la grossesse et son incidence sur la grossesse. Medicine & Science in Sports & Exercise. 1995;27(5):634-40.
Baciuk EP, Pereira RI, Cecatti JG, Braga AF, Cavalcante SR. Water aerobics in pregnancy: cardiovascular response, labor and neonatal outcomes. Reprod Health. 2008; https://doi.org/10.1186/1742-4755-5-10.
Barakat R, Lucia A, Ruiz JR. Resistance exercise training during pregnancy and newborn's birth size: a randomised controlled trial. International journal of obesity. 2005) 2009; https://doi.org/10.1038/ijo.2009.150.
Barakat R, Pelaez M, Cordero Y, Perales M, Lopez C, Coteron J, et al. Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol. 2016; https://doi.org/10.1016/j.ajog.2015.11.039.
Barakat R, Pelaez M, Lopez C, Montejo R, Coteron J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: results of a randomized controlled trial. Journal of maternal-fetal & neonatal medicine. 2013; https://doi.org/10.3109/14767058.2012.696165.
Avery MD, Leon AS, Kopher RA. Effects of a partially home-based exercise program for women with gestational diabetes. Obstet Gynecol. 1997;89(1):10-5.
Barakat R, Perales M, Bacchi M, Coteron J, Refoyo I. A program of exercise throughout pregnancy. Is it safe to mother and newborn? American journal of health promotion: AJHP. 2014;29(1):2–8.
Clapp JF, Kim H, Burciu B, Lopez B. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol. 2000; https://doi.org/10.1067/mob.2000.107096.
Cordero FY, Mottola FM, Vargas FJ, Blanco FM, Barakat FR. Exercise is associated with a reduction in gestational diabetes mellitus. Med Sci Sports Exerc. 2015;47(7):1328–33. https://doi.org/10.1249/MSS.0000000000000547.
Daly N, Farren M, McKeating A, O'Higgins A, Mullaney L, Turner MJ. Effect of a randomized controlled trial of an intensive medically supervised exercise program designed to improve maternal glucose control on gestational weight gain. Am J Obstet Gynecol. 2017;216(1 Supplement 1):S24.
de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2010;203:556–6. e1–6
De Oliveria Melo AS, Silva JLP, Tavares JS, Barros VO, Leite DFB, Amorim MMR. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: a randomized controlled trial. Obstet Gynecol. 2012;120(2 PART 1):302–10.
Erkkola R, Mäkelä M. Heart volume and physical fitness of parturients. Ann Clin Res. 1976;8(1):15–21.
Garnaes KK, Nyrnes SA, Salvesen KA, Salvesen O, Morkved S, Moholdt T. Effect of supervised exercise training during pregnancy on neonatal and maternal outcomes among overweight and obese women. Secondary analyses of the ETIP trial: a randomised controlled trial. PLoS One. 2017;12(3) (no pagination):e0173937.
Garshasbi A, Faghih Zadeh S. The effect of exercise on the intensity of low back pain in pregnant women. Int J Gynecol Obstet. 2005;88(3):271–5.
Ghodsi Z, Asltoghiri M. Effects of aerobic exercise training on maternal and neonatal outcome: a randomized controlled trial on pregnant women in Iran. JPMA The Journal of the Pakistan Medical Association. 2014;64(9):1053–6.
Guelfi KJ, Ong MJ, Crisp NA, Fournier PA, Wallman KE, Grove JR, et al. Regular exercise to prevent the recurrence of gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2016;128(4):819–27. https://doi.org/10.1097/aog.0000000000001632.
Haakstad LA, Bo K. Exercise in pregnant women and birth weight: a randomized controlled trial. BMC Pregnancy & Childbirth. 2011;11:66.
Hellenes OM, Vik T, Løhaugen GC, Salvesen K, Stafne SN, Mørkved S, et al. Regular moderate exercise during pregnancy does not have an adverse effect on the neurodevelopment of the child. Acta paediatrica (Oslo, Norway : 1992). 2015; https://doi.org/10.1111/apa.12890.
Hollingsworth DR, Moore TR. Postprandial walking exercise in pregnant insulin-dependent (type I) diabetic women: reduction of plasma lipid levels but absence of a significant effect on glycemic control. American Journal of Obstetrics & Gynecology. 1987;157(6):1359–63.
Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab. 2010; https://doi.org/10.1210/jc.2009-2255.
Kasawara KT, Burgos CS, do Nascimento SL, Ferreira NO, Surita FG, Pinto ESJL. Maternal and perinatal outcomes of exercise in pregnant women with chronic hypertension and/or previous preeclampsia: a randomized controlled trial. ISRN obstetrics and gynecology. 2013;2013:857047. https://doi.org/10.1155/2013/857047.
Kong KL, Campbell CG, Foster RC, Peterson AD, Lanningham-Foster L. A pilot walking program promotes moderate-intensity physical activity during pregnancy. Med Sci Sports Exerc. 2014;46(3):462–71.
Labonte-Lemoyne E, Curnier D, Ellemberg D. Exercise during pregnancy enhances cerebral maturation in the newborn: a randomized controlled trial. Journal of Clinical & Experimental Neuropsychology: official. J Int Neuropsychol Soc. 2017;39(4):347–54.
Marquez-Sterling S, Perry AC, Kaplan TA, Halberstein RA, Signorile JF. Physical and psychological changes with vigorous exercise in sedentary primigravidae. Med Sci Sports Exerc. 2000;
Murtezani A, Paçarada M, Ibraimi Z, Nevzati A, Abazi N. The impact of exercise during pregnancy on neonatal outcomes: a randomized controlled trial. J Sports Med Phys Fitness. 2014;54(6):802–8.
Nascimento SL, Surita FG, Parpinelli M, Siani S, Pinto e Silva JL. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: a randomised clinical trial. BJOG: An International Journal of Obstetrics and Gynaecology. 2011;118(12):1455–63.
Oostdam N, Poppel MN, Wouters MG, Eekhoff EM, Bekedam DJ, Kuchenbecker WK, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG : an international journal of obstetrics and gynaecology. 2012; https://doi.org/10.1111/j.1471-0528.2012.03366.x.
Perales M, Calabria I, Lopez C, Franco E, Coteron J, Barakat R. Regular exercise throughout pregnancy is associated with a shorter first stage of labor. Am J Health Promot. 2017; https://doi.org/10.4278/ajhp.140221-QUAN-79.
Petrov Fieril K, Glantz A, Fagevik Olsen M. The efficacy of moderate-to-vigorous resistance exercise during pregnancy: a randomized controlled trial. Acta Obstet Gynecol Scand. 2015;94(1):35–42.
Pinzon DC, Zamora K, Martinez JH, Florez-Lopez ME, de Plata AC, Mosquera M, et al. Type of delivery and gestational age is not affected by pregnant Latin-American women engaging in vigorous exercise: a secondary analysis of data from a controlled randomized trial. Revista de salud publica (Bogota, Colombia). 2012;14(5):731–43.
Price BB, Amini SB, Kappeler K. Exercise in pregnancy: effect on fitness and obstetric outcomes—a randomized trial. Medicine and science in sports and exercise. 2012; https://doi.org/10.1249/MSS.0b013e318267ad67.
Ramos JG, Bgeginski R, Opperman ML, Martins-Costa S, Delevatti R, Schuch R, et al. Effect of aerobic training in pregnant women diagnosed with gestational diabetes: a preliminary report. Pregnancy Hypertension. 2015;5(1):105.
Seneviratne SN, Jiang Y, Derraik J, McCowan L, Parry GK, Biggs JB, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG : an international journal of obstetrics and gynaecology. 2016; https://doi.org/10.1111/1471-0528.13738.
Ussher M, Lewis S, Aveyard P, Manyonda I, West R, Lewis B, et al. Physical activity for smoking cessation in pregnancy: randomised controlled trial. BMJ (Clinical research ed). 2015;350:h2145.
Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Sun Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216(4):340–51.
Kihlstrand M, Stenman B, Nilsson S, Axelsson O. Water-gymnastics reduced the intensity of back/low back pain in pregnant women. Acta Obstet Gynecol Scand. 1999;78(3):180. https://doi.org/10.1080/j.1600-0412.1999.780302.x.
Ruiz JR, Perales M, Pelaez M, Lopez C, Lucia A, Barakat R. Supervised exercise-based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin Proc. 2013;88(12):1388–97. https://doi.org/10.1016/j.mayocp.2013.07.020.
Sklempe Kokic I, Ivanisevic M, Biolo G, Simunic B, Kokic T, Pisot R. Combination of a structured aerobic and resistance exercise improves glycaemic control in pregnant women diagnosed with gestational diabetes mellitus. A randomised controlled trial. Women and birth. 2017; https://doi.org/10.1016/j.wombi.2017.10.004.
Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Kroeff LR, et al. Aerobic exercise and submaximal functional capacity in overweight pregnant women: a randomized trial. Obstet Gynecol. 2005;106(2):243–9. https://doi.org/10.1097/01.aog.0000171113.36624.86.
Barakat R, Pelaez M, Montejo R, Refoyo I, Coteron J. Exercise throughout pregnancy does not cause preterm delivery: a randomized, controlled trial. J Phys Act Health. 2014; https://doi.org/10.1123/jpah.2012-0344.
Barakat R, Ruiz JR, Stirling JR, Zakynthinaki M, Lucia A. Type of delivery is not affected by light resistance and toning exercise training during pregnancy: a randomized controlled trial. Am J Obstet Gynecol. 2009; https://doi.org/10.1016/j.ajog.2009.06.004.
Cavalcante SR, Cecatti JG, Pereira RI, Baciuk EP, Bernardo AL, Silveira C. Water aerobics II: maternal body composition and perinatal outcomes after a program for low risk pregnant women. Reprod Health. 2009;6(1) https://doi.org/10.1186/1742-4755-6-1.
Chiavaroli V, Hopkins S, Biggs J, Derraik J, Rodrigues RO, Cutfield W et al. Regular, moderate intensity maternal exercise reduces birth weight but increases the risk of later childhood adiposity. International journal of pediatric endocrinology conference: 8th biennial scientific meeting of the asia pacific paediatric endocrine society, APPES 2014 Australia conference start: 20141029 conference end: 20141101 2017.
Guelfi KJ, Ong MJ, Fournier PA, Wallman KE, Grove JR, Doherty DA, et al. Does supervised home-based exercise during pregnancy reduce the recurrence of gestational diabetes? A randomised controlled trial. Journal of Paediatrics and Child Health. 2015;51:35.
Haakstad L, Bo K. Exercise during pregnancy—does it impact offspring birth weight parameters? J Sci Med Sport. 2012; https://doi.org/10.1016/j.jsams.2012.11.828.
Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Effects of exercise training on maternal hormonal changes in pregnancy. Clin Endocrinol. 2011; https://doi.org/10.1111/j.1365-2265.2010.03964.x.
Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Sex differences in fetal growth in response to exercise in pregnancy. Reprod Sci. 2013;1:211A–2A.
Kasawara KT, Burgos CS, Costa ML, JL ES. PP046. Adherence to exercise with bicycle during pregnancy in women with risk of preeclampsia. Pregnancy Hypertension. 2012;2(3):266–7.
Kasawara KT, Burgos CSG, Nascimento SL, Costa ML, Surita F, Pinto ESJL. Effects of exercise on maternal and neonatal outcomes in pregnant women with chronic hypertension and/or previous preecampsia: a randomized clinical trial. Pregnancy Hypertension. 2012;2(3):185–6.
Kong KL, Campbell C, Wagner K, Peterson A, Lanningham-Foster L. Impact of a walking intervention during pregnancy on post-partum weight retention and infant anthropometric outcomes. J Dev Orig Health Dis. 2014; https://doi.org/10.1017/S2040174414000117.
Phelan S, Hart CN, Phipps MG, Abrams B, Schaffner A, Adams A, et al. Maternal weight gain and behaviors during pregnancy predict offspring weight in the "fit for delivery" study. Obesity. 2011;19:S52.
Ramirez-Velez R. Effect of recommended physical activity dose on obstetrical, neonatal and maternal metabolic outcomes in pregnant Latina women. Ann Nutr Metab. 2013;63:984.
Ramirez-Velez R, Bustamante J, Czerniczyniec A, Aguilar de Plata AC, Lores-Arnaiz S. Effect of exercise training on eNOS expression, NO production and oxygen metabolism in human placenta. PLoS ONE [Electronic Resource]. 2013;8(11):e80225.
Seneviratne S, Parry G, Jiang Y, Mc Cowan L, Cutfield W, Rodrigues R et al. A randomized controlled trial on the effects of antenatal exercise on birth weight and neonatal body composition. International Journal of Pediatric Endocrinology Conference: 8th Biennial Scientific Meeting of the Asia Pacific Paediatric Endocrine Society, APPES. 2014(pagination).
Seneviratne SN, Jiang Y, McCowan LM, Parry G, Gusso S, Rodrigues R, et al. The improve randomised controlled trial: non-weight bearing antenatal exercise in overweight and obese women increases neonatal adiposity. Endocrine reviews conference: 97th annual meeting and expo of the endocrine society. In: ENDO 2015 United States conference start: 20150305 conference end: 20150308; 2017.
Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552–9.
Van Poppel M, Oostdam N, Wouters M, Eekhoff M, Van Mechelen W. FitFor2: effects of an exercise training program on the incidence of gestational diabetes. J Sci Med Sport. 2012;15:S342–S3.
Van Poppel MN, Oostdam N, Wouters MG, Eekhoff MM, Van Mechelen W. A training program for women at risk for gestational diabetes. Diabetes 2011;60:A636.
Seneviratne SN, Jiang Y, Derraik J, McCowan L, Parry GK, Biggs JB, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG. 2016;123(4):588–97. https://doi.org/10.1111/1471-0528.13738.
Haakstad LA, Bø K. Exercise in pregnant women and birth weight: a randomized controlled trial. BMC pregnancy and childbirth. 2011; https://doi.org/10.1186/1471-2393-11-66.
Committee A. ACOG Committee opinion no. 650: physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2015;126(6):e135–42. https://doi.org/10.1097/aog.0000000000001214.
Bell R, Palma S. Antenatal exercise and birthweight. Aust N Z J Obstet Gynaecol. 2000;40(1):70–3.
Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):647–61. https://doi.org/10.1093/ije/dyq267.
Kramer MS, Martin RM, Bogdanovich N, Vilchuk K, Dahhou M, Oken E. Is restricted fetal growth associated with later adiposity? Observational analysis of a randomized trial. Am J Clin Nutr. 2014;100(1):176–81. https://doi.org/10.3945/ajcn.113.079590.
Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29–36.
Biosca M, Rodriguez G, Ventura P, Samper MP, Labayen I, Collado MP, et al. Central adiposity in children born small and large for gestational age. Nutr Hosp. 2011;26(5):971–6. https://doi.org/10.1590/s0212-16112011000500008.
Crume TL, Scherzinger A, Stamm E, McDuffie R, Bischoff KJ, Hamman RF, et al. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring). 2014;22(2):608–15. https://doi.org/10.1002/oby.20565.
Dolan MS, Sorkin JD, Hoffman DJ. Birth weight is inversely associated with central adipose tissue in healthy children and adolescents. Obesity (Silver Spring). 2007;15(6):1600–8. https://doi.org/10.1038/oby.2007.189.
Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111(3):e221–6.
Kuhle S, Allen AC, Veugelers PJ. Perinatal and childhood risk factors for overweight in a provincial sample of Canadian grade 5 students. Int J Pediatr Obes. 2010;5(1):88–96. https://doi.org/10.3109/17477160903060028.
Kuhle S, Maguire B, Ata N, MacInnis N, Dodds L. Birth weight for gestational age, anthropometric measures, and cardiovascular disease markers in children. J Pediatr. 2017;182:99–106. https://doi.org/10.1016/j.jpeds.2016.11.067.
Nordman H, Voutilainen R, Laitinen T, Antikainen L, Huopio H, Heinonen S, et al. Growth and cardiovascular risk factors in Prepubertal children born large or small for gestational age. Horm Res Paediatr. 2016;85(1):11–7. https://doi.org/10.1159/000441652.
Perng W, Hajj H, Belfort MB, Rifas-Shiman SL, Kramer MS, Gillman MW, et al. Birth size, early life weight gain, and Midchildhood Cardiometabolic health. J Pediatr. 2016;173:122–30 e1 https://doi.org/10.1016/j.jpeds.2016.02.053.
Bell RJ, Palma SM, Lumley JM. The effect of vigorous exercise during pregnancy on birth-weight. Aust N Z J Obstet Gynaecol. 1995;35(1):46-51.
Narendran S, Nagarathna R, Gunasheela S, Nagendra HR. Efficacy of yoga in pregnant women with abnormal Doppler study of umbilical and uterine arteries. J Indian Med Assoc. 2005;103(1):12–4. 6-7
ACOG. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171.
Davies GA, Wolfe LA, Mottola MF, MacKinnon C, Arsenault MY, Bartellas E, et al. Exercise in pregnancy and the postpartum period. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2003;25(6):516–29.
Evenson KR, Savitz DA, Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatr Perinat Epidemiol. 2004;18(6):400.
Pivarnik JM, Mudd LM, White EE, Schlaff RA, Peyer KL. Physical activity during pregnancy and offspring characteristics at 8-10 years. J Sports Med Phys Fitness. 2014;54(5):672–9.
Tanvig M, Vinter CA, Jorgensen JS, Wehberg S, Ovesen PG, Beck-Nielsen H, et al. Effects of lifestyle intervention in pregnancy and anthropometrics at birth on offspring metabolic profile at 2.8 years: results from the Lifestyle in Pregnancy and Offspring (LiPO) study. J Clin Endocrinol Metab. 2015;100(1):175–83. https://doi.org/10.1210/jc.2014-2675.
Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–13. https://doi.org/10.3945/ajcn.2008.27416.
Funding
No funding was received specifically for this review. L.G. was supported by a Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarship—Doctoral (CGS-D) Award, a Fonds de recherche en santé—Québec doctoral training award, the University of Manitoba Graduate Enhancement of Tri-Council Stipends program, and by a Heart and Stroke Foundation (HSF) operating grant (to J.M.M.). D.S.K. was supported by a CIHR CGS-D Award, a Manitoba Heath Research Council Studentship, a CIHR Strategic Training Initiative in Health Research (STIHR) Knowledge Translation Canada Student Fellowship, a CIHR STIHR Population Intervention for Chronic Disease Prevention Fellowship, and a HSF Dr. Dexter Harvey Award. J.L.H. was supported by a Vanier Canada Scholarship. NCH was supported by a University of Manitoba Graduate Fellowship. JMM holds grants from the CIHR, the Canadian Diabetes Association, the Manitoba Institute of Child Health, the HSF, the Cosmopolitan Foundation, and the Lawson Foundation. TAD holds grants from the HSF and the CIHR. LG, JMM, and TAD were also supported by a Research Manitoba grant to the DEVOTION research cluster. None of the supporting agencies were involved in the design, conduct, or approval of this manuscript.
Availability of Data and Materials
The data supporting our findings can be found in the published manuscripts cited and included.
Author information
Authors and Affiliations
Contributions
LG, TAD, and JMM conceptualized the idea for the study. LG conducted the literature search, reviewed manuscripts according to the selection criteria, and drafted the manuscript. JLH and CO acted as second reviewers and screened citations and full texts for inclusion. JLH additionally contributed to elaborating the inclusion/exclusion criteria and made a table. DSK contributed to the structure of the manuscript. NCH contributed to wording of the manuscript. JLH, DSK, TAD, and JMM revised the manuscript for important intellectual content. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval
No ethics approval was required as only secondary (published) data was used.
Consent for Publication
Not applicable.
Competing Interests
Laetitia Guillemette, Jacqueline L. Hay, D. Scott Kehler, Naomi C. Hamm, Christopher Oldfield, Jonathan M. McGavock, and Todd A. Duhamel declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional Files
Additional file 1:
Figure S1. Funnel plot of included randomized controlled trials that contributed birth weight data, with each trial represented by a gray circle (n = 34). The horizontal axis represents the standardized mean difference. The vertical axis represents the standard error of the mean. Individual study results are represented by the open circles. The vertical line in the plot represents the pooled effect size. The poor symmetry specifically in smaller studies might indicate a publication bias favoring studies that found a reduction in birth weight following prenatal exercise. (EPS 77 kb)
Additional file 2:
Figure S2. Summary of risk of bias for individual studies following the Cochrane tool. Low risk of bias is indicated by the plus sign, high risk of bias by the minus sign and unclear risk of bias by the question mark. (EPS 980 kb)
Additional file 3:
Figure S3. Summary of risk of bias for individual studies following the Risk Of Bias In Non-randomized Studies of Interventions tool. The possible categories of risk of bias are: Low (green), Moderate (Mod; blue), Serious (red), Critical (gray), and No information (NI; yellow). (TIF 111 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Guillemette, L., Hay, J.L., Kehler, D.S. et al. Exercise in Pregnancy and Children’s Cardiometabolic Risk Factors: a Systematic Review and Meta-Analysis. Sports Med - Open 4, 35 (2018). https://doi.org/10.1186/s40798-018-0148-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40798-018-0148-x