Abstract
Background
The main mechanism involved in the pathogenesis of autoimmunity is an uncontrolled inflammatory response against self-antigens. Therefore, anti-inflammatory factors, such as the intake of bioactive compounds and a physically active lifestyle, may decrease or cease the development of autoimmune diseases. Type 1 diabetes (T1D) is an autoimmune disease characterized by pancreatic β cell destruction. The non-obese diabetic (NOD) mouse is a model of spontaneous T1D and is the model most similar to human disease.
Methods
To determine the effects of exercise training and curcumin supplementation on T1D progression, 48 NOD mice, 5 weeks old, were randomly divided into four groups: control, curcumin supplementation, trained, and trained plus curcumin. Every 2 weeks, blood glucose was measured using a glucometer. At the end of 20 weeks, a histopathological procedure was used to assess immune cells infiltration into pancreatic β cells (insulitis).
Results
Moderate intensity exercise training has the potential to protect pancreatic β cells against an immune response in vivo. However, curcumin supplementation failed to attenuate insulitis in NOD mice.
Conclusions
These data provide evidence that exercise training can mitigate T1D development in genetically susceptible mice.
Similar content being viewed by others
Key Points
-
Exercise training decreased immune cells infiltration in vivo in a spontaneous mouse model of type 1 diabetes.
-
Curcumin supplementation did not attenuate insulitis alone or improve exercise training effects.
-
The effect of exercise training is present regardless of IL-6 and TNF-alpha alteration.
Background
Intense inflammatory response is a main feature of autoimmune diseases. Type 1 diabetes (T1D), for instance, occurs due to a chronic inflammatory response with enough severity to destroy most of the pancreatic β cells [1]. The incidence of some autoimmune diseases, including T1D, has been increasing worldwide over the past decades [2–5], and environmental factors are the most accepted explanation for this phenomenon. For example, vitamin D status [6] and the hygiene hypothesis [7] are being investigated. Currently, physical inactivity and processed food intake during childhood appear to be linked to the development of chronic [8–10] and inflammatory disease [11] in early stages of life. Furthermore, the Overload Hypothesis suggests an association between a modern lifestyle and beta cell overload, which could make these cells more vulnerable to an autoimmune response [12].
Intervention using functional foods with anti-inflammatory properties could decrease or halt the autoimmune response, leading to a delay or even prevention of autoimmunity. Dietary patterns characterized by high bioactive compounds, such as a Mediterranean diet, promote an “anti-inflammatory environment” in humans [13]. Among several compounds, curcumin, a polyphenol found in the rhizome of Curcuma longa, is one of the most studied bioactive compounds due to its anti-inflammatory properties [14–16]. Additionally, studies have shown that physical exercise also promotes anti-inflammatory responses through several mechanisms, such as increasing antioxidant activity [17], releasing myokines and hormones [18], modulating immune cell metabolism [19], and decreasing inflammation signaling [20].
Currently, the non-obese diabetic (NOD) mouse, a model of spontaneous T1D, is the model that is most similar to human T1D [21]. Over the last 30 years, it has been used in research to improve the science of autoimmunity [22]. As in humans, T1D occurs in NOD mice due to immune cell infiltration into pancreatic islets (insulitis) and subsequent destruction of β cells. Additionally, it has been demonstrated by ample literature that TNF-α, a pro-inflammatory cytokine, has an important role in the pathogenesis of T1D in NOD mice [23–27].
Some studies have tested bioactive compounds in NOD mice. Supplementation with cocoa flavonoids or green tea catechin reduced diabetes incidence in this animal model [28, 29]. However, no studies have tested the potential of exercise training to prevent T1D in NOD mice. Therefore, this study aimed to investigate the effects of exercise training and curcumin supplementation on T1D progression in NOD mice.
Methods
Animals
Forty-eight female NOD mice, 5 weeks old, were randomly divided into four groups (n = 12): control (C), curcumin supplementation (CUR), trained (T), and trained + curcumin supplementation (TC). The experimental protocol lasted 20 weeks. Animals were housed collectively (four animals per cage) and kept in conventional conditions (22–24 °C and 12-h light-dark cycle) with unlimited access to water and food, which was a pelletized AIN-93G diet [30]. Food intake was monitored three times a week. Body weight was measured weekly. Blood glucose was measured every 14 days using a drop of tail blood and a glucometer (FreeStyle Lite–Abbott). Mice were considered diabetic when their blood glucose level was higher than 250 mg/dL. Diabetic animals that lost more than 25% of their body weight were killed before 20 weeks. All procedures were reviewed and approved by the Ethics Committee on Animal Use in Research (CETEA-FMRP, protocol no. 190/2014) in compliance with the Ethical Principles in Animal Research adopted by the National Council for the Control of Animal Experimentation (CONCEA) and following the guidelines for the care and use of laboratory animals [31].
Sample Size
Sample size was calculated based on the primary outcome in this study, which was immune cell infiltration into pancreatic islets (insulitis). As insulitis is a nominal qualitative variable, the sample size was determined using the following equation:
Considering that each section of pancreas has approximately 10 islets and that in control groups, approximately 60% have some level of insulitis even in the absence of T1D [32], sample size was established to reach statistical significance when the applied interventions reduced the number of islets with some level of insulitis at least by half (30%).
Curcumin Supplementation
The AIN-93 diet was prepared twice a week, protected from light, and kept frozen. The food offered to the CUR and TC groups had curcumin added (500 mg/kg). Curcumin was purchased from Sigma-Aldrich (St. Louis, MO, USA). Considering the daily food intake of the mice, the curcumin dose reached approximately 65 mg/kg per day.
Exercise Training Protocol
All mice from the T and TC groups performed a maximal incremental running test at the beginning of the experimental protocol. This test began at 10 m/min and increased by 1.7 m/min every 2 min until exhaustion (determined when the animal touched the bottom of the bay five times within 1 min). The speed at which exhaustion occurred was considered 100%. Adjustment of exercise load was done every 4 weeks by repeating the same incremental test. According to the animals’ performance in the incremental test, they were divided into three running groups (best, middle, and lower) to guarantee that exercise intensity was maintained and to respect the differences in mouse performance.
Exercise training was carried out for 20 weeks, 5 days a week. All exercise sessions were performed in the morning. The exercise session consisted of a 5-minute warm-up followed by 60 min of running at training intensity and a 2-min cool down. Training intensity varied during week sessions. On Monday, Wednesday, and Friday, the mice ran at 60% of their maximal speed reached in the incremental test; on Tuesday and Thursday, mice had recovery sessions running at 30% of the maximal speed.
Cytokine and Insulin Concentration
Following overnight fasting, the animals were euthanized by decapitation 48 h after the last exercise session, without previous anesthetic. The serum obtained was used to determine glucose concentration by the colorimetric method (Labtest, Lago Santa, Brazil), and frozen samples (−70 °C) were used to measure insulin, TNF-α, and IL-6 using Luminex xMAP® technology with a Milliplex® kit (Millipore Corporation) analyzed on MAGPIX®; the standard curve of all analytes had R 2 = 1.
A homeostatic model assessment of insulin resistance (HOMA-IR) was calculated to estimate insulin resistance as previously described [33].
Insulitis
Pancreas was fixed in formalin (10%), embedded in paraffin, and stained with hematoxylin-eosin. Images were taken on an optical microscope (Olympus BX61VS). Three sections of each pancreas were analyzed. Islets were counted as previously described [34] and labeled as: No Insulitis (absence of immune cells), Pre-Insulitis (few immune cells around the islet), and Insulitis (>25% of cells infiltrated and destruction of islet architecture). Counts were performed manually by two independent researchers (blinded). Insulitis extension was calculated as previously described [35]:
Statistics
Data are presented as the means ± standard deviation. Analysis of variance (one-way ANOVA) followed by a Tukey post hoc test was performed, except for HOMA-IR and IL-6, which did not have a normal distribution and were analyzed using a Kruskal-Wallis test. Weekly blood glucose was analyzed using a repeated measure ANOVA. A log-rank test was used to compare diabetes incidence. A Chi-square test was used to compare insulitis distribution. The level of significance adopted was p < 0.05.
Results
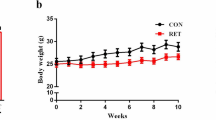
Although the daily food intake was approximately 10% greater in the trained group than in the control group, body weight gain was approximately 41% lower in the trained mice after 20 weeks. Only four animals developed T1D, two each in groups C and CUR. Insulin levels tended to be lower in CUR and T, but the difference only reached a statistical level of significance in the TC group. HOMA-IR was not modified by curcumin or exercise training (Table 1). Blood glucose was similar among groups during the 20 weeks, as seen in Fig. 1.
Despite the low incidence of T1D, insulitis was seen in all groups but in different levels. The trained and trained + curcumin groups had 50% less immune cell infiltration in pancreatic islets than the sedentary groups (Fig. 2a). In addition, insulitis extension was also reduced with exercise training (Fig. 2b). Regarding cytokine levels, IL-6 and TNF-α were not modified by curcumin or exercise training (Fig. 3).
Discussion
To the best of our knowledge, this study is the first to show that exercise training has the potential to protect pancreatic β cells against an immune response in vivo. However, curcumin supplementation failed to attenuate insulitis in NOD mice.
Curcumin is a polyphenolic compound that exhibits low bioavailability [36]. The route of administration and the dose of curcumin used in this study were established based on previous studies that aimed to simulate a rich polyphenol diet [36–39]. Although chronic oral curcumin (500 ppm in diet) reached detectable concentrations in plasma (0.035 μg/ml) and in brain tissue (0.469 μg/ml) in a dose-dependent study [37], our study indicates that this concentration was not enough to prevent an immune response against pancreatic β cells. However, intraperitoneal curcumin supplementation (25 mg/kg body weight) inhibited leucocyte infiltration in accelerated murine models of T1D [40]. These data suggest that the concentration of curcumin required to modulate immune function could not be reached using dietary strategies.
The low incidence of T1D observed was expected because animals were fed a gluten-free diet and were not in a germ-free environment [41, 42]. However, NOD mice present ~60% immune cell infiltration in islets even in the absence of T1D [32], which is consistent with our findings. Exercise training lowered this rate to 30%.
The effect of exercise training in diminishing body weight gain is well documented and was observed in the present study, showing the importance of physical exercise for caloric balance. Lower body weight gain could be one mechanism that explains the reduction of insulitis in the T and TC groups. Since obesity induces chronic inflammation [43] and the Overload Hypothesis proposes that environment factors, such as obesity, increase T1D risk [12], this line of thought is strengthened.
Another possible mechanism to explain the effects of exercise training is through dendritic cells modulation. It is well known that dendritic cells modulate both innate and adaptive immune responses, and the role of these cells on the development of T1D was recently demonstrated [44]. Several studies have shown that exercise training decreases the number of dendritic cells or diminishes their response [45–48]. Thus, exercise could mitigate autoimmunity by shaping dendritic cell activation.
Regarding cytokine signaling, IL-6 has been recognized as one of the myokines produced during exercise training [49]. In 2010, exercise training was documented as an anti-inflammatory approach, which is able to prevent type 2 diabetes, cardiovascular diseases, cancer, and dementia [50]. Although no significant differences were seen in cytokine levels, the CUR, T, and TC groups exhibited an IL-6 concentration more than twofold higher than the C group (p = 0.07). In an ex vivo study, the pancreas of trained animals had fewer apoptosis biomarkers than sedentary animals. When an IL-6 blocker was added to the trained animals’ pancreas, the apoptosis biomarkers rose to sedentary levels. The authors concluded that the benefit of exercise training on pancreatic β cell survival is through the IL-6 pathway [51]. However, the results of the present study cannot reinforce the role of IL-6 in preventing insulitis because the CUR group had the same insulitis markers as the C group, which suggests that the effect of exercise training is due to other mechanisms.
Conclusions
In conclusion, moderate intensity exercise training has the potential to protect pancreatic β cells against an immune response in NOD mice. The limitation of the present study is a lack of mechanisms that establish a causal effect, as well as the lack of an evaluation of other inflammatory markers, and both pancreatic and blood oxidative stress, epinephrine levels, and the characterization of infiltrating immune cells. Those measurements are an important area of future research. Therefore, additional and prospective studies are needed to uncover the mechanisms that explain the link between exercise training and autoimmunity.
Abbreviations
- AIN:
-
American Institute of Nutrition
- ANOVA:
-
Analysis of variance
- C:
-
Control
- CONCEA:
-
Conselho Nacional de Controle de Exeperimentação Animal
- CUR:
-
Curcumin
- FMRP:
-
Faculdade de Medicina de Ribeirão Preto
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- IL:
-
Interleukin
- NOD:
-
Non-obese diabetic
- T:
-
Trained
- T1D:
-
Type 1 diabetes
- TC:
-
Trained + curcumin
- TNF:
-
Tumor necrosis factor
References
Wållberg M, Cooke A. Immune mechanisms in type 1 diabetes. Trends Immunol. 2013;34:583–91.
Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–25.
The autoimmune diseases coordinating committee (ADCC). Progress in Autoimmune Diseases Research Progress. Bethesda: National Institutes of Health; 2005. p. 1–129.
Forlenza GP, Rewers M. The epidemic of type 1 diabetes: what is it telling us? Curr Opin Endocrinol Diabetes Obes [Internet]. 2011;18:248–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21844707.
Egro FM. Why is type 1 diabetes increasing? J Mol Endocrinol. 2013;51:R1–13.
Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127–36.
Bach JF, Chatenoud L. The hygiene hypothesis: an explanation for the increased frequency of insulin-dependet diabetes. Cold Spring Harb Perspect Med. 2012;2:1–10.
Ludwig DS. Relation between consumption of sugar sweetened drinks and childhood obesity: a prospective observational analysis. Lancet. 2001;357:505–8.
Ayer J, Charakida M, Deanfield JE, Celermajer DS, Ng M, Fleming T, et al. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J. 2015;36:1371–6.
Downing KL, Hnatiuk J, Hesketh KD. Prevalence of sedentary behavior in children under 2 years: a systematic review. Prev Med (Baltim). 2015;78:105–14.
Noutsios GT, Floros J. Childhood asthma: causes, risks, and protective factors; a role of innate immunity. Swiss Med Wkly. 2014;144:w14036.
Dahlquist G. Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia. 2006;49:20–4.
Bonaccio M, Pounis G, Cerletti C, Donati MB, Iacoviello L, de Gaetano G. Mediterranean diet, dietary polyphenols and low-grade inflammation: results from the moli-sani study. Br J Clin Pharmacol. 2016;83:107–13.
Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phyther Res. 2014;28:633–42.
Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. BioFactors. 2013;39:69–77.
Srivastava RM, Singh S, Dubey SK, Misra K, Khar A. Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol. 2011;11:331–41.
Ji LL, Zhang Y. Antioxidant and anti-inflammatory effects of exercise: role of redox signaling. Free Radic Res. 2013;48:1–9.
Nielsen S, Pedersen BK. Skeletal muscle as an immunogenic organ. Curr Opin Pharmacol. 2008;8:346–51.
Wasinski F, Gregnani MF, Ornellas FH, Bacurau AVN, Câmara NO, Araujo RC, et al. Lymphocyte glucose and glutamine metabolism as targets of the anti-inflammatory and immunomodulatory effects of exercise. Mediators Inflamm. 2014;2014:1–10.
Ringeis R, Eder K, Mooren FC, Kruger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. 2015;21:58–68.
Driver JP, Serreze DV, Chen Y-G. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol. 2011;33:67–87.
Pearson JA, Wong FS, Wen L. The importance of the non obese diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88.
Ventura-Oliveira D, Vilella CA, Zanin ME, Castro GM, Moreira Filho DC, Zollner RL. Kinetics of TNF-alpha and IFN-gamma mRNA expression in islets and spleen of NOD mice. Brazilian J Med Biol Res. 2002;35:1347–55.
Wang JL, Qian X, Chinookoswong N, John L, Chow G, Theill LE, et al. Polyethylene glycolated recombinant TNF receptor I improves insulitis and reduces incidence of spontaneous and cyclophosphamide-accelerated diabetes in nonobese diabetic mice. Endocrinology. 2002;143:3490–7.
Kägi D, Ho A, Odermatt B, Zakarian A, Ohashi PS, Mak TW. TNF receptor 1-dependent beta cell toxicity as an effector pathway in autoimmune diabetes. J Immunol. 1999;162:4598–605.
Pakala SV, Chivetta M, Kelly CB, Katz JD. In autoimmune diabetes the transition from benign to pernicious insulitis requires an islet cell response to tumor necrosis factor alpha. J Exp Med. 1999;189:1053–62.
Chee J, Angstetra E, Mariana L, Graham KL, Carrington EM, Bluethmann H, et al. TNF receptor 1 deficiency increases regulatory T cell function in nonobese diabetic mice. J Immunol. 2011;187:1702–12.
Fu Z, Yuskavage J, Liu D. Dietary flavonol epicatechin prevents the onset of type 1 diabetes in nonobese diabetic mice. J Agric Food Chem. 2013;61:4303–9.
Fu Z, Zhen W, Yuskavage J, Liu D. Epigallocatechin gallate delays the onset of type 1 diabetes in spontaneous non-obese diabetic mice. Br J Nutr. 2011;105:1218–25.
Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51.
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US); 2011. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54050/. doi:10.17226/12910.
Reddy S, Chai RCC, Rodrigues JA, Hsu TH, Robinson E. Presence of residual beta cells and co-existing islet autoimmunity in the NOD mouse during longstanding diabetes: a combined histochemical and immunohistochemical study. J Mol Histol. 2008;39:25–36.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Zhang C, Todorov I, Lin C-L, Atkinson M, Kandeel F, Forman S, et al. Elimination of insulitis and augmentation of islet beta cell regeneration via induction of chimerism in overtly diabetic NOD mice. Proc Natl Acad Sci USA. 2007;104:2337–42.
Signore A, Annovazzi A, Giacalone P, Beales PE, Valorani MG, Vestri AR, et al. Reduced cumulative incidence of diabetes but not insulitis following administration of chimeric human IL-15-murine IgG2b in NOD mice. Diabetes Metab Res Rev. 2003;19:464–8.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB, Anand P, Kunnumakkara AB, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18.
Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326:196–208.
Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053–64.
Zhang D-W, Fu M, Gao S-H, Liu J-L. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013;2013:1–6.
Castro CN, Barcala Tabarrozzi AE, Winnewisser J, Gimeno ML, Antunica Noguerol M, Liberman AC, et al. Curcumin ameliorates autoimmune diabetes. Evidence in accelerated murine models of type 1 diabetes. Clin Exp Immunol. 2014;177:149–60.
Marietta EV, Gomez AM, Yeoman C, Tilahun AY, Clark CR, Luckey DH, et al. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS One. 2013;8:1–9.
Hoorfar J, Buschard K, Dagnaes-Hansen F. Prophylactic nutritional modification of the incidence of diabetes in autoimmune non-obese diabetic (NOD) mice. Br J Nutr. 1993;69:597–607.
Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–7.
Pane JA, Fleming FE, Graham KL, Thomas HE, Kay TWH, Coulson BS. Rotavirus acceleration of type 1 diabetes in non-obese diabetic mice depends on type I interferon signalling. Sci Rep. 2016;6:1–12.
Morgado JM, Rama L, Silva I, Inácio De Jesus M, Henriques A, Laranjeira P, et al. Cytokine production by monocytes, neutrophils, and dendritic cells is hampered by long-term intensive training in elite swimmers. Eur J Appl Physiol. 2012;112:471–82.
Nickel T, Emslander I, Sisic Z, David R, Schmaderer C, Marx N, et al. Modulation of dendritic cells and toll-like receptors by marathon running. Eur J Appl Physiol. 2012;112:1699–708.
Deckx N, Wens I, Nuyts AH, Hens N, De Winter BY, Koppen G, et al. 12 weeks of combined endurance and resistance training reduces innate markers of inflammation in a randomized controlled clinical trial in patients with multiple sclerosis. Mediators Inflamm. 2016;2016:1–13. Hindawi Publishing Corporation.
MacKenzie B, Andrade-Sousa AS, Oliveira-Junior MC, Assumpção-Neto E, Alves-Rangel MB, Silva Renno A, et al. Dendritic cells are involved in the effects of exercise in a model of asthma. Med Sci Sport Exerc. 2016;48:1459–67.
Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34:246–51.
Pedersen BK, Brandt C. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010:1–6.
Paula FMM, Leite NC, Vanzela EC, Kurauti MA, Freitas-Dias R, Carneiro EM, et al. Exercise increases pancreatic β-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J. 2015;29:1805–16.
Funding
No financial support was received for the conduct of this study or preparation of this manuscript.
Authors’ Contributions
LKO conceived, designed the study, performed the experiments, analyzed the data, and wrote the paper. CDM and AMN conceived and designed the study, contributed to discussions, and edited the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Competing Interests
Author L.K.O., Author C.D.M., and Author A.M.N declare no conflict of interest.
Consent for Publication
Not applicable.
Ethics Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Oharomari, L.K., de Moraes, C. & Navarro, A.M. Exercise Training but not Curcumin Supplementation Decreases Immune Cell Infiltration in the Pancreatic Islets of a Genetically Susceptible Model of Type 1 Diabetes. Sports Med - Open 3, 15 (2017). https://doi.org/10.1186/s40798-017-0082-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40798-017-0082-3