Abstract
Background
Placental malaria (PM) is a major public health problem associated with adverse pregnancy outcomes such as low birth weight (LBW), preterm delivery and maternal anemia. The present study is aimed to determine the prevalence of placental malaria among asymptomatic pregnant women in Wolkite health center, Gurage zone, Southern Ethiopia.
Method
Facility-based cross-sectional study was carried out from June 2019 to August 2019. A total of 230 pregnant women were involved in the study where socio-demographic data, medical and obstetric history were collected using pretested structured questionnaires. Blood samples were collected at delivery from maternal capillary, placenta and umbilical cord for the detection of malarial parasite. Maternal hematocrit was determined to screen for anemia.
Result
In this study, the prevalence of placental malaria, peripheral malaria and umbilical cord malaria was 3.9% (9/230), 15.2% (35/230) and 2.6% (6/230) respectively. Plasmodium falciparum and Plasmodium vivax were detected by microscopy. All babies with positive umbilical cord blood films were born from a mother with placental malaria. Maternal anemia was recorded in 58.3% of the women. In univariate analysis, placental malaria was significantly associated with LBW (p < 0.001) unlike parity and maternal anemia.
Conclusion
Placental malaria among asymptomatic pregnant women is low in Wolkite health centre, Gurage zone in Southern Ethiopia. Moreover, placental malaria was strongly associated with LBW. Thus, further strengthening the existing prevention and control activities and screening of asymptomatic pregnant women as part of routine antenatal care service is very essential.
Similar content being viewed by others
Background
Malaria in pregnancy (MIP) is a major preventable cause of maternal morbidity and poor birth outcomes in Sub-Saharan Africa [1]. Globally, MIP contributes to about 10,000 maternal deaths and up to 200,000 newborn deaths annually [2]. In 2010, a study in Africa showed that 12.4 million pregnant women were exposed to infection. Of them, 11.4 million women had placental infections without pregnancy-specific protection [3]. World Health Organization (WHO) reported that MIP is responsible for 20% of stillbirths, 11% of newborn deaths and 3.3% of LBW deliveries in sub-Saharan Africa [4].
Placental malaria infection is characterized by the accumulation of parasitized red blood cells (pRBCs) in placental intervillous spaces that express unique membrane-bound proteins from the P. falciparum erythrocyte membrane protein-1 (PfEMP-1) to bind to host receptors like chondroitin sulfate A (CSA) in the placental intervillous space leading to placental malaria (PM) [5]. It is associated with increased risk of maternal anemia, LBW, preterm delivery, intrauterine growth restriction, reduced fetal anthropometric parameters, fetal anemia, and congenital malaria [6].
The symptoms and complications of malaria in pregnancy depend on the malaria transmission intensity and the individual’s level of acquired immunity [7]. Pregnant women residing in high malaria transmission areas develop acquired immunity and often have paucisymptomatic or asymptomatic P. falciparum infections. However,parasite accumulation in the placenta contributes to maternal anemia and can lead to infiltration of mononuclear cells in the placental intervillous space, which is a risk factor for LBW [8]. On the other hand, pregnant women living in low or unstable malaria transmission areas have relatively little acquired immunity to malaria and are at higher risk of developing severe malaria, which may lead to spontaneous abortion, stillbirth, prematurity, and LBW [8].
As major strategies, WHO has recommended the use of insecticide-treated mosquito nets (ITNs), effective case management of malaria and administration of intermittent preventive treatment with sulfadoxine-pyrimethamine (IPTp-SP) in African countries. Ethiopia does not support IPTp-SP due to cost-effectiveness considerations and low malaria transmission in most parts of the country [9, 10]. The most substantive malaria prevention and control measures in Ethiopia are improving prompt access to diagnostics and treatment, prioritization of ITN use by pregnant women, and enhanced social and behavior change communication activities targeting pregnant women in malaria-endemic areas [10].
Gurage Zone is a malaria-endemic area that has unstable and seasonal malaria transmission. In a previous study, the prevalence of microscopically confirmed malaria cases among symptomatic patients was 8.6% where P.vivax accounted for a higher share of 69.7% and P. falciparum accounted for 29.3% [11]. In malaria-endemic areas, asymptomatic infection with Plasmodium species is common [12] and such individuals can be the source of malaria transmission to healthy individuals posing serious challenges for malaria control and elimination [13]. We hypothesized that asymptomatic pregnant women are at risk of having undiagnosed placental malaria due to parasite sequestration in the placenta [14]. Hence, the objective of this study was to assess the prevalence of placental malaria among asymptomatic pregnant women in Wolkite health center, Gurage zone, Southern Ethiopia.
Materials and methods
Study area
This study was conducted in Wolkite health center, which is found in Wolkite town and located at 158 km southwest of Addis Ababa, the capital city of Ethiopia. Wolkite town is the capital of Gurage Zone, has latitude and longitude of 8°17'N37°47'E and an altitude of 1910- 1935 meters above sea level. Based on the 2007 Ethiopian Census, the town has a total population of 28,856, of whom 15,068 were males and 13,788 were females [15].
Wolkite health center provides service to patients coming from various districts of Gurage zone and an average of 980-1080 women give birth every year.
Study design and study population
A facility-based cross-sectional study was conducted among asymptomatic pregnant women in Wolkite health center from June 2019 to August 2019. During the study period a total of 230 pregnant women participated in the study. Pregnant women with absence of clinical symptoms mainly fever or history of fever, chills and headache within the last 24 hours, axillary temperatures ≤ 37.5°C and those who were willing to participate and signed the informed consent in the labor ward were included in the study. Participants who took antimalarial drugs, had multiple pregnancies and those with serious delivery complications that led to a referral to other hospitals and those who were unwilling to participate in the study were excluded. Women with P. falciparum were treated with Artemether-Lumefantrine (AL) and chloroquine was used for the treatment of P. vivax. Infants with umbilical cord malaria were treated with artesunate. All treatments were according to the national treatment guideline. Women who were anemic were treated with ferrous sulfate and folic acid.
Data collection method
Demographic and clinical data collection
After obtaining written informed consent, socio-demographic characteristics of the participants were collected using pre-tested structured questionnaires in the labor ward. An enrollment form containing demographic data, past medical and obstetric history was completed for every participant by trained midwives. A general clinical examination was performed for each woman with reference to antenatal care (ANC) follow up, bed net use, parity, newborn sex. Axillary temperature was measured by an electronic instrument to the nearest 0.1°C at delivery. All newborns were weighted immediately after birth using an electronic scale to the nearest 10 grams and newborn sex was recorded. Newborns were classified as normal birth weight (≥2500 g, regardless of gestational age) or LBW (<2500 g) according to the WHO guidelines [16].
Laboratory investigation
Maternal peripheral, placental and umbilical cord blood sample collection
At delivery, placental blood films were prepared by the incision of approximately 1.5 cm of the placenta on the maternal side and a drop of blood was placed on a slide. The umbilical cord blood samples were obtained by wiping away excess blood from a clamped cord to avoid contamination with maternal blood. Then, a small cut was made with a lancet and a drop of blood was taken to prepare blood film. Maternal capillary blood samples were collected for blood film and hematocrit determination. All specimens were correctly labeled and sent to the hematology laboratory for hematocrit analysis.
Hematocrit determination
Packed cell volume (PCV) of maternal peripheral blood collected after delivery was determined using the standard method. Based on their PCV value women were considered as anemic if PCV is < 33% and were classified as mild (PCV 27–32.9%), moderate (21.0–26.9%) and severe (PCV < 21%) [17].
Blood film preparation and examination
Even though placental histology is a gold standard method to diagnose placental malaria [14] due to limited resources and expertise, Giemsa stained blood smear microscopy method was employed.
Both thick and thin smears of the maternal peripheral blood, placental blood and umbilical cord blood were prepared. After proper labeling and air-drying, thin films were fixed with absolute methanol for 5 s while both thin and thick films were stained with freshly prepared 3% Giemsa solution diluted with buffered water (PH 7.1–7.2) for 30 min and examined under 100X magnification [18]. Thick blood films were used to detect the presence of asexual stages of Plasmodium species while thin blood films were used for species identification when thick blood films were positive. Blood films were examined individually by two experienced laboratory technologists. Any discordant results were resolved by a third reader, a senior parasitologist from Wolkite University.
According to WHO, parasite density estimation from parasites counted against high power field (HPF) (100× objective and 10× eyepieces) on the thick film was calculated as [19];
The standard volume of blood per HPF is assumed to be 0.002. A negative blood film was reported if no parasite were identified after 100 HPFs were observed.
Data management and quality control
Data quality was ensured using standardized data collection materials, pre-tested questionnaires, properly trained data collectors and intensive supervision during data collection. Pre-analytical, analytical and post-analytical stages of quality assurance that are incorporated in standard operating procedures (SOPs) were strictly followed while performing laboratory analysis.
Data analysis
The collected data were entered and analyzed using the Statistical Package for Social Sciences (SPSS) version 20 software. Univariate analysis was performed to determine the association of placental malaria with pregnancy and neonatal outcomes. Pearson Chi-square test or Fisher’s exact test was used for categorical variables whereas Student’s t-test was used for continuous variables. A p-value <0.05 was considered statistically significant.
Results
Characteristics of the study population
Two hundred forty-two women visited Wolkite health center for delivery from June 2019 to August 2019. Of these, 12 were excluded due to prenatal death, twin delivery and referral to a different hospital due to delivery complications. From 230 normal deliveries, 53.9% of the babies were females and 46.1% were males. The age of the participants ranged from 18 to 40 years old with a mean (SD) age of 24.7 (5.1) years. The majority (72.2%) of participants were in the age group of 18–27 years. Sixty-four percent of the participants resided in Wolkite town and more than 90% of the women reported using bed nets during their pregnancy and attended ANC (Table 1).
Prevalence of malaria at the time of delivery
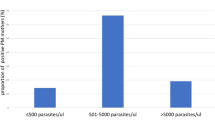
The prevalence of placental, peripheral and umbilical cord malaria was 3.9% (9/230), 15.2% (35/230) and 2.6% (6/230) respectively. P. falciparum and P. vivax were both detected in the blood films by microscopy. Mixed infection was not detected in the blood films. P. falciparum was detected in 1.7% of placental blood films, 5.2% from peripheral blood films and 1.7% from umbilical cord blood films. P. vivax was identified in 2.2% of placental blood films, 10% from peripheral blood films and 0.9% from umbilical cord blood films. The range of parasitemia/μl determined from positive placental, peripheral and cord blood films was 2500–9000/ μl, 2500–13,500/ μl and 2000–7500/ μl (Table 2).
Association of placental malaria with maternal and umbilical cord parasitemia
Of the 9 women with placental malaria, 6 had peripheral parasitemia and all babies with positive umbilical cord blood films were born from a mother with placental malaria. Placental parasitemia was strongly associated with peripheral and umbilical cord blood film parasitemias (p < 0.001) (Table 3).
Association of Placental malaria with pregnancy and neonatal outcomes
The mean birth weight (SD) among women with placental malaria was 2.2 (0.2) as compared to those without malaria 3.4 (0.4) and the difference was statistically significant (p = 0.04). Of 230 babies delivered, 7.4% (17) had LBW (< 2.5 kg) and 66.7% (6/9) women with placental malaria had LBW babies. The difference was statistically significant (p < 0.001).
The mean hematocrit (HCT) among women was 31.0 (6.2) and anemia (HCT < 33%) was recorded in 57.8% (133) of the women. Of these, mild anemia was seen in 61% (81) of the women, moderate anemia in 31.5% (42) women and severe anemia was seen in 7.5% (10) of the women. From 9 women with placental malaria, 8 (88.9%) of them were anemic and among 221 women without placental malaria, 125 (56.6%) of them were anemic. The difference was not statistically significant (p = 0.05) (Table 4).
Discussion
In this study, the prevalence of placental malaria among asymptomatic pregnant women who delivered at Wolkite health center was 3.9%. This is comparable to the previous finding in unstable malaria transmission areas of Ethiopia which was 2.5% [20] but, lower than other studies reported in Nigeria (65.2%) and Sudan (58.9%) using placental blood microscopy [21, 22]. This prevalence variation might be due to differences in geographic location and malaria transmission intensity. According to the 2015 malaria indicator survey in Ethiopia, 73.9% of pregnant women own an ITN in areas below 2000 m [10]. Malaria endemic areas of Gurage zone had reported the higher utilization of ITN by pregnant women (75%) which might be attributed to low prevalence in the study area [23].
In the present study, the prevalence of maternal peripheral malaria was 15.2% which was comparable to a pooled prevalence among pregnant women in Ethiopia (12.7%) and abroad Guinea (15.8%) [24, 25]. There were 32 discordant results between placental and peripheral malaria: 29 infected women had peripheral parasitemia without placental malaria while 3 had placental malaria without peripheral parasitemia. This was consistent with other studies [26, 27]. Malaria infection may be restricted to periphery due to lack of variant surface antigen expression that can cause placental sequestration [28] or in multigravida, women produce antibodies to VAR2CSA that inhibit the binding of pRBCs to CSA which prevent placental parasitemia [29]. In some cases, women may be infected with variants that adhere to other host receptors, including CD36 and ICAM-1, leading to sequestration in other vascular beds. Thus, these variants may not be detected in placental smears [30]. On the other hand, submicroscopic malaria infections are common during pregnancy and more sensitive approaches like placental histology are needed to detect sub-microscopic infections [31].. A certain level of host immunity may also contribute to microscopically undetectable densities [32].
The prevalence of umbilical cord parasitemia in the present study was 2.6% and all positive babies were born from a mother with both peripheral and placental malaria. There was a strong association of placental malaria with umbilical cord parasitemia (p< 0.001). This prevalence was in agreement with the previous study done in unstable area of Ethiopia (1.6%) and abroad Burkina Faso (1.4%) [20, 33] but lower than studies done by Bassey et al. and Fehintola et al. which reported malaria prevalences of 50.8% and 40% in Nigeria [21, 34]. Congenital malaria is generally considered infrequent in endemic areas. This might be due to several reasons. The placenta acts as a physical barrier preventing infected erythrocytes from reaching the fetal circulation. In the fetal circulation the presence of fetal hemoglobin (HbF) and low free-oxygen tension cause a poor environment for parasite replication [35]. Further, congenital malaria may be acquired antenatally through placental damage during delivery or premature separation of the placenta resulting in the contamination of fetal blood with infected maternal blood at delivery [36].
The prevalence of LBW in the present study was 7.4% which was comparable with studies in other African countries, including Nigeria 6.7% [21] and Tanzania 6.3% [37]. However, it was lower than other studies in Papua New Guinean 56.4% [38], Sudan 56.4% [22], Malawi 12.2% [39] and Burkino faso 11.6% [40]. Differing results in these studies may be due to transmission intensity, ITN use which was reportedly high in this study and the diagnostic methodology used to diagnose PM.
In our finding, placental malaria was strongly associated with LBW (p<0.001). LBW can be caused by either preterm delivery and/or intrauterine growth restriction. It has been explained in the literature that the sequestration of pRBCs, monocytes in the placenta induce altered cytokine profiles and complement activation that cause placental tissue injury that leads to placental insufficiency, fetal growth restriction which results in LBW [41, 42].
The prevalence of anemia was 58.3% in this study with the majority of women having mild anemia. In the present study, there was no statistically significant association of placental malaria with maternal anemia. The prevalence of anemia in this study was higher than studies conducted by Bassey et al. (31.9%) [21] and Cisse et al. (21.7%) [40]. Even though most of the participants attended ANC where they received oral iron supplementation, the prevalence of anemia was still high. The cause of anemia during pregnancy is multi-factorial which might be nutritional (iron and folic acid deficiency) and/or non-nutritional factors like parasitic infection and HIV infection. Another mechanism is that Plasmodium species affect the iron status through reducing absorption, directly consuming iron for survival or sequestrating iron within the malarial pigment hemozoin [6].The present study has some limitations. Placental histology is the “gold standard” for the diagnosis of placental malaria, as placental histology is considerably more sensitive than microscopy. Further, placental histology can differentiate between acute, chronic, and past malaria infections based on the presence of parasites and/or pigment in the placental intervillous space. Without histology it is not possible to ascertain the duration of placental infections.
Conclusion
The prevalence of placental malaria among asymptomatic pregnant women was low and all babies with positive umbilical cord blood films were born from a mother with placental malaria. Placental malaria was significantly associated with LBW. Thus, further strengthening the existing prevention and control activities and screening of asymptomatic pregnant women as part of routine ANC is essential.
Moreover, future studies with more sensitive diagnostic techniques including placental histology and PCR should be conducted to determine the burden and effect of submicroscopic infection in pregnant women in high transmission areas.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LBW:
-
Low birth weight
- LLIN:
-
Long-lasting insecticidal nets
- MIP:
-
Malaria in pregnancy
- PCV:
-
Packed cell volume
- PM:
-
Placental malaria
- PRBCs:
-
Parasitized red blood cells
- SOPs:
-
Standard Operating Procedures
- WHO:
-
World Health Organization
- IPTp-SP:
-
Intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine
- HPFs:
-
High power fields
- ANC:
-
Antenatal care
- CSA:
-
Chondroitin sulphate A
- HbF:
-
Fetal hemoglobin
References
World Health Organization. Malaria in pregnancy. WHO. https://www.who.int/malaria/mpac/mpac-sept2015-erg-mip-report. Accessed 23 Sept 2019.
Schantz-Dunn J, Nour NM. Malaria and pregnancy: a global health perspective. Rev Obstet Gynecol. 2009;2(3):186.
Walker PGT, FO tK, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health. 2014;2:e460–7.
World Health Organization. Implementing malaria in pregnancy programs in the context of World Health Organization recommendations on antenatal care for a positive pregnancy experience. .https://www.who.int/reproductivehealth/publications/implementing-malaria-pregnancy-programmes-brief/en/. Accessed 23 Sep 2019.
Odongo CO, Odida M, Wabinga H, Obua C, Byamugisha J. Burden of placental malaria among pregnant women who use or do not use intermittent preventive treatment at Mulago hospital, Kampala. Malar Res Treat. 2016;2016:1839795. https://doi.org/10.1155/2016/1839795.
Uneke CJ. Impact of placental Plasmodium falciparum malaria on pregnancy and perinatal outcome in sub-Saharan Africa: part III: placental malaria, maternal health, and public health. Yale J Biol Med. 2008;81(1):1–7.
Yaya S, Uthman O, Amouzou A, Bishwajit G. Use of intermittent preventive treatment among pregnant women in sub-Saharan Africa: evidence from malaria indicator surveys. Trop Med Infect Dis. 2018;3(1):18.
Rogerson SJ. Management of malaria in pregnancy. Indian J Med Res. 2017;146(3):328.
World Health Organization. WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). Geneva: World Health Organization; 2013.
President’s Malaria Initiative. Ethiopia. Malaria operational plan FY 2019. 2019.
Solomon A, Kahase D, Alemayehu M. Trend of malaria prevalence in Wolkite health center: an implication towards the elimination of malaria in Ethiopia by 2030. Malar J. 2020;19(1):1–8.
Feleke DG, Adamu A, Gebreweld A, Tesfaye M, Demisiss W, Molla G. Asymptomatic malaria infection among pregnant women attending antenatal care in malaria endemic areas of north-Shoa, Ethiopia: a cross-sectional study. Malar J. 2020;19(1):1–6.
World Health Organization. World malaria report 2018. Geneva: World health organization; 2018. https://wwwwhoint/malaria/publications/world-malaria-report-2018/en/ Accessed 30 Jul 2019.
Fried M, Muehlenbachs A, Duffy PE. Diagnosing malaria in pregnancy: an update. Expert Rev Anti-Infect Ther. 2012;10(10):1177–87. https://doi.org/10.1586/eri.12.98.
Federal Democratic Republic of Ethiopia Population Census Commission. Summary and statistical report of the 2007 population and housing census: population size by age and sex. Addis Ababa: Central Statistics Agency; 2012.
World Health Organization. International statistical classification of diseases and related health problems, tenth revision. 2nd ed. Geneva: World Health Organization; 2004.
Dim CC, Ugwu EO, Dim NR, Anyaehie UB. Hematocrit, anemia, and arm preference for blood sample collection: a cross sectional study of pregnant women in Enugu, south eastern, Nigeria. Ann Med Health Sci Re. 2015;5(1):36–41.
Cheesbrough M. District laboratory practice in tropical countries. New York: Cambridge University Press; 2006.
Research Malaria Microscopy Standards Working Group. Microscopy for the detection, identification and quantification of malaria parasites on stained thick and thin films. Geneva: World Health Organization; 2015.
Newman RD, Hailemariam A, Jimma D, Degifie A, Kebede D, Rietveld AE, Nahlen BL, Barnwell JW, Steketee RW, Parise ME. Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopia during a non-epidemic year. J Infect Dis. 2003;187(11):1765–72.
Bassey G, Nyengidiki TK, John CT. Prevalence of placenta Plasmodium parasitemia and pregnancy outcome in asymptomatic patients at delivery in a university teaching Hospital in Nigeria. Niger J Clin Pract. 2015;18(1):27–32.
Omer SA, Idress HE, Adam I, Abdelrahim M, Noureldein AN, Abdelrazig AM, Elhassan MO, Sulaiman SM. Placental malaria and its effect on pregnancy outcomes in Sudanese women from Blue Nile state. Malar J. 2017;16(1):374.
Girum T, Hailemikael G, Wondimu A. Factors affecting prevention and control of malaria among endemic areas of Gurage zone: an implication for malaria elimination in South Ethiopia, 2017. Trop Dis Travel Med Vaccin. 2017;3:17.
Tegegne Y, Asmelash D, Ambachew S, Eshetie S, Addisu A, JejawZeleke A. The prevalence of malaria among pregnant women in Ethiopia: a systematic review and meta-analysis. J Parasitol Res. 2019;2019:8396091. https://doi.org/10.1155/2019/8396091.
Touré AA, Doumbouya A, Diallo A, Loua G, Cissé A, Sidibé S, Beavogui AH. Malaria-associated factors among pregnant women in Guinea. J Trop Med. 2019;2019:3925094.
Ezebialu IU, Eke AC, Ezeagwuna DA, Nwachukwu CE, Ifediata F, Ezebialu CU. Prevalence, pattern, and determinants of placental malaria in a population of southeastern Nigerian parturients. Int J Infect Dis. 2012;16(12):e860–5.
Cisse M, Diallo AH, Somé DA, Poda A, Awandare AG, Guiguemdé TR. Association of placental Plasmodium falciparum parasitaemia with maternal and newborn outcomes in the periurban area of Bobo-Dioulasso, Burkina Faso. Parasitol Open. 2016;2:e15.
Cohee LM, Kalilani-Phiri L, Mawindo P, Joshi S, Adams M, Kenefic L, Jacob CG, Taylor TE, Laufer MK. Parasite dynamics in the peripheral blood and the placenta during pregnancy-associated malaria infection. Malar J. 2016;15(1):483.
Babakhanyan A, Fang R, Wey A, Salanti A, Sama G, Efundem C, Leke RJ, Chen JJ, Leke RG, Taylor DW. Comparison of the specificity of antibodies to VAR2CSA in Cameroonian multigravidae with and without placental malaria: a retrospective case–control study. Malar J. 2015;14(1):480.
Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41(4):1370–4.
Cohee LM, Kalilani-Phiri L, Boudova S, Joshi S, Mukadam R, Seydel KB, Mawindo P, Thesing P, Kamiza S, Makwakwa K, Muehlenbachs A. Submicroscopic malaria infection during pregnancy and the impact of intermittent preventive treatment. Malar J. 2014;13(1):274.
Omer S, Khalil E, Ali H, Sharief A. Submicroscopic and multiple Plasmodium falciparum infections in pregnant Sudanese women. N Am J Med Sci. 2011;3(3):137.
Ouédraogo A, Tiono AB, Diarra A, Bougouma EC, Nébié I, Konaté AT, Sirima SB. Transplacental transmission of Plasmodium falciparum in a highly malaria endemic area of Burkina Faso. J Trop Med. 2012;2012:109705.
Fehintola AO, Fehintola FO, Loto OM, Fasubaa OB, Bakare B, Ogundele O. Pregnancy and fetal outcome of placental malaria parasitemia in Ile-Ife, Nigeria. Trop J Obstet Gynaecol. 2016;33(3):310.
Uneke CJ. Impact of placental Plasmodium falciparum malaria on pregnancy and perinatal outcome in sub-Saharan Africa: part II: effects of placental malaria on perinatal outcome; malaria and HIV. Yale J Biol Med. 2007;80:95–103.
Mukhtar MY, Lesi FE, Iroha EU, Egri-Okwaji MT, Mafe AG. Congenital malaria among inborn babies at a tertiary Centre in Lagos, Nigeria. J Trop Pediatr. 2006;52(1):19–23.
Sarr D, Marrama L, Gaye A, Dangou JM, Niang M, Mercereau-Puijalon O, Lehesran JY, Jambou R. High prevalence of placental malaria and low birth weight in Sahelianperiurban area. Am J Trop Med Hyg. 2006;75(1):171–7.
Lufele E, Umbers A, Ordi J, Ome-Kaius M, Wangnapi R, Unger H, Tarongka N, Siba P, Mueller I, Robinson L, Rogerson S. Risk factors and pregnancy outcomes associated with placental malaria in a prospective cohort of Papua New Guinean women. Malaria J. 2017;16(1):427.
Rantala AM, Taylor SM, Trottman PA, Luntamo M, Mbewe B, Maleta K, Kulmala T, Ashorn P, Meshnick SR. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J. 2010;9:269.
Cisse M, Sangare I, Lougue G, Bamba S, Bayane D, Guiguemde RT. Prevalence and risk factors for Plasmodium falciparum malaria in pregnant women attending antenatal clinic in Bobo-Dioulasso (Burkina Faso). BMC Infect Dis. 2014;14(1):631.
Mohammed AH, Salih MM, Elhassan EM, Mohmmed AA, Elzaki SE, El-Sayed BB, Adam I. Submicroscopic Plasmodium falciparum malaria and low birth weight in an area of unstable malaria transmission in Central Sudan. Malar J. 2013;12(1):172.
Perrault SD, Hajek J, Zhong K, Owino SO, Sichangi M, Smith G, Shi YP, Moore JM, Kain KC. Human immunodeficiency virus co-infection increases placental parasite density and transplacental malaria transmission in Western Kenya. Am J Trop Med Hyg. 2009;80(1):119–25.
Acknowledgments
We would like to thank Wolkite University Research coordinator, director and the Vice President for providing a female grant to conduct the research. Our deepest gratitude goes to Wolkite Zonal Health Office, Wolkite health center for supporting us during data collection. We would like to thank data collectors in the midwifery department for their irreplaceable effort and we would like to extend our gratitude to the study participants.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to conception and study design, data analysis, reviewed the paper critically then gave final approval to be published and agreed to be responsible for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics and review committee of Wolkite University. A letter of permission to conduct the study was obtained from Gurage zone health bureau and Wolkite health center. Then after a brief explanation of all the benefits and the risk of the procedure in the understandable local language, written informed consent was taken in the labor ward from all pregnant women. Confidentiality was assured for all the information gathered from the participant by giving a unique ID number.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Solomon, A., Kahase, D. & Alemayhu, M. Prevalence of placental malaria among asymptomatic pregnant women in Wolkite health center, Gurage zone, Southern Ethiopia. Trop Dis Travel Med Vaccines 6, 20 (2020). https://doi.org/10.1186/s40794-020-00121-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40794-020-00121-3