Abstract
Background
Nivolumab is a monoclonal antibody against programmed death 1 and has become a standard treatment of advanced melanoma because of its durable response and survival benefits. In this report, we present a case of severe intestinal perforation after nivolumab immunotherapy for malignant melanoma.
Case presentation
A 73-year-old man with stage IV malignant melanoma underwent nivolumab therapy. The patient presented to our hospital because of a progressing abdominal pain. Radiological evaluation revealed evidence of free intraperitoneal air. Therefore, we diagnosed the patient as having an intestinal perforation, which was successfully resolved after surgical treatment.
Conclusion
Although intestinal perforation after nivolumab immunotherapy is rare, it can be severe and requires early diagnosis and emergency surgery to ensure a favorable prognosis.
Similar content being viewed by others
Background
Immune checkpoint blockade is a new therapy that uses cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) inhibitors to treat melanoma and squamous cell lung cancer. In the last decade, studies have clarified the integral role of the immune regulatory pathway involving PD-1 (a receptor expressed in activated T and B cells) and PD-1 ligands (PD-L1 and PD-L2) in the downregulation of antitumor immunity. Thus, inhibition of this immune regulatory pathway by using blocking monoclonal antibodies (mAbs) against PD-1 or PD-L1 is emerging as an effective therapy for achieving tumor regression in patients with advanced disease [1]. Nivolumab is an anti-PD-1 mAb that provides a durable response in various advanced malignancies [2, 3]. For example, in cases of melanoma, nivolumab provides 1- and 2-year overall survival rates of 62 and 43%, respectively [4]. Thus, nivolumab has recently become a standard treatment for patients with advanced melanoma [5].
However, the use of immune checkpoint inhibitors can lead to novel autoimmune-related adverse events, including interstitial pneumonia, colitis, vitiligo, autoimmune hepatitis, and endocrine dysfunction [4, 6]. In this report, we describe a case of advanced melanoma with intestinal perforation that developed shortly after the start of nivolumab therapy. The patient underwent surgical treatment for the intestinal perforation and medical management of sepsis and recovered successfully without complications. The patient provided informed consent for publishing this report.
Case presentation
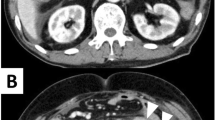
A malignant melanoma in the anal canal was treated with curative surgery when the patient was 72 years of age. Five months after surgery, curative lymphadenectomy was performed owing to the recurrence of metastases in the right inguinal and right lateral lymph nodes. Fourteen months after the initial curative surgery, when the patient was 73 years of age, multiple metastases were observed in the lungs, liver, and bones, thyroid gland, and subcutaneous tissue; we accordingly initiated nivolumab treatment. We treated the patient with intravenous nivolumab therapy (2 mg/kg every 3 weeks), but he subsequently developed abdominal distension and progressive diffuse abdominal pain within a week after receiving his third dose of nivolumab. Abdominal and pelvic computed tomography revealed free air near the small intestine (Fig. 1). Thus, the patient was hospitalized under the diagnosis of intestinal perforation. His medical history included diabetes mellitus, pericarditis, and previous surgery (laparoscopic abdominoperineal resection melanoma in the anal canal, and resection of lateral and inguinal lymph node metastases).
Initial evaluation revealed dehydration and tachycardia (97 beats/min), a low-grade fever, abdominal distension, tenderness on palpation, no peristalsis, rebound phenomenon, and muscle stiffness. Laboratory testing revealed the following results: white blood cells, 7100/μL; hemoglobin, 13.7 g/dL; platelets, 190,000/μL; creatinine, 0.55 mg/dL; and C-reactive protein, 0.05 mg/L. Based on these findings, he underwent emergency surgery, during which purulent free fluid, no necrosis of the colon and small intestine, and extensive adhesion of the small intestine with perforation of the small intestine were found. The perforation was found in the ileum, 240 cm away from the Treitz ligament. The patient had generalized peritonitis and underwent an emergency operation in a poor general condition; partial resection of the small intestine including the perforation area was performed. We decided not to perform intestinal anastomosis but to perform ileostomy after considering the risk of sutural insufficiency. Therefore, we performed small intestinal resection with ileostomy and cavity lavage. During the surgery, extensive adhesion caused by past surgery was found in the abdominal cavity of the patient. The intestinal wall distal from the perforation area was injured during surgery; partial resection of the small intestine including the injured area was required. We accordingly resected 50 cm of the small intestine. No surgical complications occurred during the perioperative period, although the patient was admitted to the intensive care unit because of hemodynamic instability. During the postoperative period, the patient exhibited signs of systemic inflammation and was prescribed several antibiotics, which included vancomycin hydrochloride. The patient required mechanical ventilation on the fifth postoperative day, and the patient’s hemodynamic status remained stable without the need for vasopressors. Thus, we maintained the intermediate therapy. The patient had a bowel movement on the tenth postoperative day, and oral administration of water was initiated, which he tolerated well. The patient was discharged at 42 days after the surgery, with no complications, with adequate gastrointestinal function, and with the ability to tolerate an oral diet.
Macroscopic evaluation of the intestinal mucosal membrane revealed a brown, white, and moss-like appearance on the serosal side. We also identified a perforation site that was 14 cm from the oral margin and 35 cm from the anal margin. Histopathological examination revealed neutrophil infiltration and fibrinous deposits near the perforation site (Fig. 2). Inflammations were more severe in the stromal areas around the perforation than in other areas. The non-perforated section of the intestinal tract had a normal luminal structure, and we did not detect any tumorous lesions on mucous membrane, or blood clots. We did not recognize the intestinal metastasis before nivolumab therapy by CT and PET examination.
Discussion
In recent years, biological and molecular targeted drugs have been developed and implemented for the treatment of malignant melanoma. These drugs include vemurafenib (a selective BRAF V600 kinase inhibitor) [7], ipilimumab (an antibody preparation that targets CTLA-4 and inhibits T-cell activation) [8], and nivolumab (a mAb to PD-1) [9]. PD-1 is an inhibitory and co-stimulatory factor that is expressed on activated T cells and was first identified by Ishida et al. in 1992 as a protein that is upregulated during T cell apoptosis [10]. In this context, PD-L1 and PD-L2 are expressed in cancer cells, where they bind to PD-1 and deliver inhibitory signals to T cells. Thus, nivolumab is a novel immune checkpoint inhibitor that exerts its anti-tumor effect by inducing PD-L1/PD-L2 binding and maintaining T cell function [11]. Clinical studies have confirmed that nivolumab is effective for treating malignant melanoma [12, 13], as it provides a 1-year survival rate of 72.9%, compared with 42.1% among patients who received dacarbazine monotherapy [4]. Currently, nivolumab therapy is indicated for patients who are refractory to conventional chemotherapy, which includes dacarbazine, and is typically administered intravenously at a dose of 2 mg/kg every 3 weeks. Treatment time with nivolumab is short because it does not require premedication to prevent the occurrence of nausea, and outpatient treatment is available, as no serious cytopenia occurs after treatment.
Nivolumab has several side effects, which are often considered relatively mild (e.g., malaise, pruritus, and nausea) [4, 5, 12, 13]. However, nivolumab is also associated with several severe side effects, which include interstitial pulmonary disease, liver dysfunction, thyroid deficiency, diarrhea, colitis, and infusion reactions [4, 13]. To the best of our knowledge, this is the first English report on a case of intestinal perforation after nivolumab therapy. The pathological mechanism of immunoreaction resulting in intestinal perforation is similar to that of an inflammatory reaction [14]. In a previous report on inflammatory colitis with metastatic melanoma, colonoscopic biopsy specimens demonstrated interstitial lymphoid infiltration after administration of anti-programmed death-1 antibody [15]. In the resected specimen obtained from our patient, we observed similar inflammatory findings (Fig. 3). An immunologic reaction may have a potential to influence intestinal perforation, and in the present case, we cannot deny that an immunologic reaction influenced the development of intestinal perforation. We consider that the perforation in our case was associated with the autoimmune mechanism of nivolumab, which would indicate that this drug has unique properties that differentiate it from other anti-tumor drugs. However, the mechanism of gastrointestinal perforation due to nivolumab is not understood, and there is no past evidence regarding this. While it has been previously reported that using nivolumab could cause colitis, there is also the risk that the gastrointestinal perforation could appear during treatment; therefore, physicians should be aware of the risk of gastrointestinal perforation when using nivolumab.
Nivolumab therapy has potential efficacy for malignant melanoma refractory to existing pharmaceutical therapies, such as in the present case. Moreover, nivolumab therapy could improve overall survival and quality of life among patients with malignant melanoma, and is expected to become a central or combination therapy for patients with unresectable malignant melanoma. However, nivolumab therapy can cause serious side effects (e.g., intestinal perforation in the present case), and patients who receive nivolumab therapy should be monitored for the onset of possible side effects. Nevertheless, the accumulation of additional cases or large-scale studies are needed to validate our findings.
Conclusions
We treated a patient with a malignant melanoma, who subsequently developed intestinal perforation due to nivolumab therapy.
Abbreviations
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen-4
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
References
Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13.
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolmab. J Clin Oncol. 2014;32:1020–30.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30.
Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499–507.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;362:2507–16.
Hodi FS, O’day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, et al. In vitro characterization of the anti-PD-1 antibody nivolumab. BMS 936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–56.
Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1 a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–7.
Weber JS, SP D’A, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermontt DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Lankes K, Hundorfean G, Harrer T, Pommer AJ, Agaimy A, Angelovska I, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;18; 5(6):e1128611.
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75.
Acknowledgements
I would like to show my appreciation to Dr. Koji Murono, Dr. Manabu Kaneko, and Dr. Kazuhito Sasaki by clinical contribution. And I would like to show my greatest appreciation to Dr. Watanabe Toshiaki.
Funding
None
Author information
Authors and Affiliations
Contributions
KY designed the study and analyzed the data, and drafted the manuscript. TT participated in the design of the study and helped to draft the manuscript. SI, KO, TN, TK, KK, KH, and HN revised the manuscript critically. YM participated in the design of the study. YS helped to draft the manuscript about pathological findings. TW conceived of the study and given final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
The patient provided informed consent for the publication of this case report.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yasuda, K., Tanaka, T., Ishihara, S. et al. Intestinal perforation after nivolumab immunotherapy for a malignant melanoma: a case report. surg case rep 3, 94 (2017). https://doi.org/10.1186/s40792-017-0370-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-017-0370-7