Abstract
Background
Spinocerebellar ataxia type 3/Machado-Joseph disease (SCA3/MJD) is an autosomal dominant inherited neurodegenerative disorder. Several post-mortem and imaging studies have shown cerebellar and brainstem atrophy. A number of studies have used volumetric regional information to investigate the relationship between neurodegeneration and the ataxia severity. However, regional analysis can obscure the specific location in which the degenerative process is affecting the brain tissue, which can be crucial for the development of new target treatments for this disease.
Here we explored the relationship between the gray matter degeneration and the ataxia severity on a cohort of SCA3 patients using a voxel-wise approach.
Methods
Seventeen patients with molecular diagnose of SCA3 and 17 matched healthy controls participated in this study. Magnetic resonance imaging (MRI) brain images were acquired and voxel-based morphometry was used to obtain the grey matter volume of each participant. Ataxia severity in the patient group was evaluated using the scale for the assessment and rating of ataxia (SARA).
Results
Group comparison revealed significant atrophy in SCA3 including bilateral cerebellum, vermis, brainstem, and occipital cortex. Significant negative correlations between gray matter volume and SARA scores were found in the cerebellum and the cingulate gyrus.
Conclusions
These findings highlight the specific contribution of the cerebellum and the cingulate cortex to the ataxia deficits among the other regions showing neurodegeneration in SCA3 patients.
Similar content being viewed by others
Background
Spinocerebellar ataxia type 3/Machado-Joseph disease (SCA3/MJD) is an autosomal dominant inherited neurodegenerative disorder with a wide range of clinical manifestations [1]. SCA3/MJD is caused by an unstable CAG trinucleotide repeat expansion within the coding region of a gene located on chromosome 14q32.1 [2]. The predominant symptoms are progressive ataxia, ophthalmoplegia, spastic gait and peripheral neuropathy [1,2,3]. Previous morphometric SCA3/MJD MRI analysis revealed significant atrophy of the cerebellar hemispheres, cerebellar vermis, pontine base, middle cerebellar peduncle, medulla oblongata, cervical spinal cord and enlargement of the fourth ventricle [3,4,5,6,7,8,9]. However, only few studies have analyzed the possible relationship between brain degeneration and the ataxia severity in this disease [7,8,9]. In those reports, researchers have used manually predefined anatomical landmarks to segment and calculate the total volume of different anatomical structures [7], automated region segmentation to calculate cortical thickness and volume [8] or atlas predefined regions to later group them into one single region [9]. Those analyses led to broad correlations between volume of the cerebellum, brainstem and other cortical regions and the ataxia impairment.
However, a more accurate mapping of the disease-related degeneration and its association with the symptoms is key for the development of possible treatments/therapies for this specific type of patients. Here, we assessed the ataxia severity and gray matter degeneration in a cohort of SCA3/MJD patients by using the scale for the assessment and rating of ataxia (SARA) and whole brain voxel based-morphometry (VBM) to find voxel-wise associations between brain atrophy and motor impairment.
Methods
Participants
The patient group consisted of seventeen patients with a molecular diagnosis of SCA3/MJD (10 female; right-handed; mean age/SD, 40.1/11.9 years, for more detailed information look at Table 1). Motor impairment was measured using SARA [10], which has eight items, including tests of gait, stance, sitting, and speech, as well as a finger-chase test, finger-nose test, fast alternating movements, and heel-shin test. The control group consisted of 17 healthy volunteers that were age and gender matched. All participants gave written, informed consent before entering the study. The procedures carried out were in accordance with the ethical standards of the committees on human experimentation of the Universidad Nacional Autonoma de Mexico.
Image acquisition
All images were acquired using a 32-channel quadrature head coil in a 3.0-T Achieva MRI scanner (Phillips Medical Systems, Eindhoven, The Netherlands). Foam-rubber cushion was used for fixing the head of the subject in place, so as to minimize any head movements. The high-resolution anatomical acquisition consisted of a 3-D T1 Fast Field-Echo sequence, with TR/TE of 8/3.7 ms, FOV of 256 × 256 mm, flip angle 25° and an acquisition and reconstruction matrix of 256 × 256, resulting in an isometric resolution of 1 × 1 × 1 mm.
Voxel-based morphometry
Gray matter volume (GMV) measurements were performed using voxel based morphometry (VBM) as implemented on FSL (FMRIB, Oxford, UK) following the standard procedure as previously reported [11]. Using the FSL randomise tool, a two-sample t test was performed between the SCA3 group and controls. Significance was defined as p < 0.05 after correcting for multiple comparisons using the randomized permutation method (TFCE). For the SCA3 group, whole-brain correlation maps were created by calculating the Pearson’s partial correlation between the GMV and SARA scores including age in the analysis. Partial correlation maps were corrected for multiple comparisons by using false discovery rate (FDR) with a p value < 0.05.
Results
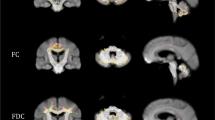
VBM analysis revealed gray matter atrophy in SCA3 patients compared to the control group (Fig. 1a) involving bilateral cerebellum, vermis, brainstem and the occipital cortex (Table 2). Four significant negative correlations were found between GMV and SARA scores in the SCA3 group (Fig. 1b) in regions of the cerebellum and the cingulate gyrus (Table 3).
Discussion
In this study, we analyzed the relationship between gray matter loss and SARA scores in SCA3 patients. As expected, significant negative correlations between SARA scores and GMV were found in the cerebellum, but also in the cingulate cortex.
Previous reports have shown the close relationship between the extent of the brain atrophy, predominately in the cerebellum, and a variety of symptoms in different SCA subtypes [7,8,9, 12, 13]. Accordingly, our analysis showed a significant negative correlation between GMV and the SARA score in the bilateral lobule VI, extending to lobule V, which are involved in sensorimotor processing as suggested by deficits in stroke patients [14, 15]. Lobule IX, which its GMV also correlated with SARA, is not only considered essential for visual guidance of movement [14], but its damage has been related to gait and balance impairment [15].
The only extra-cerebellar region where the GMV correlated with SARA score was the dorsal anterior cingulate, known to be critically involved in motor functions [16]. A previous report showed a significant degeneration in SCA3 in this area [6], however, our analysis also showed a negative correlation between GMV and the ataxia score, corroborating the functional relevance of this deterioration as shown by patients with lesions in this area, whom often show deficits in spontaneous initiation of movement and speech, as well as inability to suppress externally triggered motor subroutines [16].
Abbreviations
- FDR:
-
False Discovery Rate
- GMV:
-
Gray Matter Volume
- MDJ:
-
Machado-Joseph Disease
- MRI:
-
Magnetic Resonance Imaging
- SARA:
-
Scale for Assessing and Rating of Ataxia
- SCA3:
-
Spinocerebellar Ataxia 3
- VBM:
-
voxel-Based Morphometry
References
Matilla T, McCall A, Subramony SH, Zoghbi HY. Molecular and clinical correlations in spinocerebellar ataxia type 3 and Machado‐Joseph disease. Ann Neurol. 1995;38(1):68–72.
Kawaguchi Y, Okamoto T, Taniwakiz M, Aizawa M. CAG expansions in a novel gene for Machado-Joseph disease at. Nat Genet. 1994;8(3):221–8.
Bürk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, Didierjean O, Brice A, Klockgether T. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119(5):1497–505.
Lukas C, Schöls L, Bellenberg B, Rüb U, Przuntek H, Schmid G, Köster O, Suchan B. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: a voxel-based morphometry study. Neurosci Lett. 2006;408(3):230–5.
Goel G, Pal PK, Ravishankar S, Venkatasubramanian G, Jayakumar PN, Krishna N, Purushottam M, Saini J, Faruq M, Mukherji M, Jain S. Gray matter volume deficits in spinocerebellar ataxia: an optimized voxel based morphometric study. Parkinsonism Relat Disord. 2011;17(7):521–7.
Guimarães RP, D'Abreu A, Yasuda CL, França MC, Silva BH, Cappabianco FA, Bergo FP, Lopes‐Cendes IT, Cendes F. A multimodal evaluation of microstructural white matter damage in spinocerebellar ataxia type 3. Mov Disord. 2013;28(8):1125–32.
Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, Schoels L, Timmann D, van de Warrenburg B, Dürr A, Pandolfo M. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage. 2010;49(1):158–68.
Rezende TJ, D'abreu A, Guimaraes RP, Lopes TM, Lopes‐Cendes I, Cendes F, Castellano G, França MC. Cerebral cortex involvement in Machado − Joseph disease. Eur J Neurol. 2015;22(2):277–e24.
D’Abreu A, Franca Jr MC, Yasuda CL, Campos BA, Lopes‐Cendes I, Cendes F. Neocortical Atrophy in Machado‐Joseph Disease: A Longitudinal Neuroimaging Study. J Neuroimaging. 2012;22(3):285–91.
Schmitz-Hübsch T, Du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B. Scale for the assessment and rating of ataxia Development of a new clinical scale. Neurology. 2006;66(11):1717–20.
Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6):805–21.
Hernandez-Castillo CR, Galvez V, Diaz R, Fernandez-Ruiz J. Specific cerebellar and cortical degeneration correlates with ataxia severity in spinocerebellar ataxia type 7. Brain Imaging Behav. 2016;10(1):252–7.
Reetz K, Lencer R, Hagenah JM, Gaser C, Tadic V, Walter U, Wolters A, Steinlechner S, Zühlke C, Brockmann K, Klein C. Structural changes associated with progression of motor deficits in spinocerebellar ataxia 17. The Cerebellum. 2010;9(2):210–7.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44.
Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain. 2008;131(11):2913–27.
Paus TS. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–24.
Acknowledgements
We want to thank all the patients and their caregivers for their participation in this study.
Funding
This study was founded in part by CONACYT 220871, PAPIIT IN214716 to JFR, and CONACYT 222009 to ACR.
Availability of data and materials
Please contact the corresponding author for data requests.
Authors’ contributions
CRHC designed the study Analyze the data and produced the first draft of the manuscript. JFR designed the study and provided expertise in brain imaging. RD and ACR were in charge of logistics as well as supervised and acquired data from patients. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All participants gave written, informed consent before entering the study. The procedures carried out were in accordance with the ethical standards of the committees on human experimentation of the Universidad Nacional Autonoma de Mexico.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hernandez-Castillo, C.R., Diaz, R., Campos-Romo, A. et al. Neural correlates of ataxia severity in spinocerebellar ataxia type 3/Machado-Joseph disease. cerebellum ataxias 4, 7 (2017). https://doi.org/10.1186/s40673-017-0065-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40673-017-0065-7