Abstract
Background
Preoperative accurate assessment of endometrial cancer can assist in the planning of additional surgical options, and in predicting the prognosis. The aim of the present study was to evaluate the diagnostic potential of non-contrast PET/MRI with 18F-fluorodeoxyglucose (18F-FDG) for assessment in preoperative staging of endometrial cancer.
Methods
Thirty-six patients with biopsy-proven endometrial cancer underwent preoperative 18F-FDG PET/MRI, contrast-enhanced CT (ceCT) and pelvic dynamic contrast-enhanced MRI (ceMRI) for initial staging. The diagnostic performance of 18F-FDG PET/MRI and ceMRI for assessing the extent of the primary tumor (T stage), and 18F-FDG PET/MRI and ceCT for assessing nodal (N stage) and distant (M stage) metastasis, was evaluated by two experienced readers. Histopathological and follow-up imaging results were used as the gold standard. The McNemar test was employed for statistical analysis.
Results
Accuracy for T status was 77.8 and 75.0% for 18F-FDG PET/MRI and ceMRI, respectively. Patient-based accuracy for detecting regional nodal and distant metastasis was 91.3 and 81.8% for 18F-FDG PET/MRI, and 87.0 and 81.8% for ceCT. None of these parameters was statistically significant (p > 0.05). Lesion-based sensitivity, specificity and accuracy for detecting regional nodal metastasis were 100, 96.9 and 97.0% for 18F-FDG PET/MRI, and 14.3, 97.6 and 93.3% for ceCT; sensitivity was statistically significant (p < 0.05).
Conclusions
Non-contrast 18F-FDG PET/MRI, which combines the individual advantages of PET and MRI, offers a high diagnostic value equivalent to that of ceMRI for assessment of the primary tumor, and equivalent to that of ceCT for the assessment of nodal and distant metastatic staging, in patients with endometrial cancer. These findings suggest that 18F-FDG PET/MRI might provide an alternative diagnostic strategy to conventional imaging modalities in the preoperative staging of endometrial cancer.
Similar content being viewed by others
Introduction
Endometrial cancer is the most common gynecological malignancy in developed countries, and its incidence continues to increase. Because endometrial cancer is staged postoperatively, the primary treatment is determined by predicting the FIGO classification [1]. Imaging examinations are thus indispensable in planning optimal treatment, for advanced disease as well as early-stage disease confined to the uterus; however, the FIGO classifications do not currently include imaging findings.
Standard treatment for early-stage disease is surgical resection, including hysterectomy and bilateral salpingo-oophorectomy. However, accurate assessment of the primary tumor, lymph nodes and distant metastasis can assist in the planning of additional surgical options such as pelvic or para-aortic lymphadenectomy or radical hysterectomy, and in predicting the prognosis [2].
In these evaluations of local disease, magnetic resonance imaging (MRI) offers better diagnostic performance than transvaginal ultrasonography (US) and could be considered the reference standard [3]. In particular, contrast-enhanced MRI (ceMRI) could provide additional diagnostic value particularly regarding myometrial invasion [4]. Positron emission tomography (PET), particularly with 18F-fluorodeoxyglucose (18F-FDG) as a tracer that reflects cellular metabolism, has been shown to be worth consideration alongside conventional imaging modalities. For the detection of lymph node and distant metastasis, 18F-FDG PET/computed tomography (CT) could be more useful than conventional imaging such as CT or MRI [5]; however, limited data are available for assessment of local disease [6].
The new integrated modality of 18F-FDG PET/MRI provides high soft-tissue contrast along with functional imaging of 18F-FDG uptake, without using gadolinium-based contrast agent, and has shown potentially better diagnostic performance than 18F-FDG PET/CT for gynecologic cancers [7,8,9]. In endometrial cancer, fusion of PET and MRI has revealed significantly higher accuracy for T staging compared with 18F-FDG PET/CT, and comparable accuracy for N staging compared with 18F-FDG PET/CT, suggesting that integrated PET/MRI has a possible diagnostic role to play, whereas integrated PET/MRI has not been well studied as yet [10].
Therefore, the aim of our study was to evaluate the diagnostic value of non-contrast 18F-FDG PET/MRI for whole-body tumor staging of patients with endometrial cancer, and to compare the diagnostic accuracy of 18F-FDG PET/MRI with that of ceCT and ceMRI.
Material and methods
Patients
We retrospectively reviewed the medical records of 53 patients with pathologically proven endometrial cancer who were treated at our institution between February 2016 and November 2018. Of these, 36 patients (mean age, 61.2 years; age range, 38–86 years) who had undergone 18F-FDG PET/MRI, ceCT, and pelvic dynamic ceMRI for initial staging based on the Japanese Imaging Guidelines of the Japan Radiological Society were included in the present study [11, 12]. Written informed consent was obtained from all patients prior to the imaging procedures. The characteristics of excluded patients are listed in Supplementary Table 1. All patients had completed 18F-FDG PET/MRI, ceCT and ceMRI within 3 months prior to treatment. The maximum interval among 18F-FDG PET/MRI, ceCT and ceMRI was 70 days (mean, 13.6 days; range, 1–70 days). Of the 36 patients, 33 underwent total abdominal or laparoscopic hysterectomy and 3 underwent radical hysterectomy; 31 patients underwent bilateral and 2 underwent unilateral salpingo-oophorectomy; 3 patients underwent bilateral salpingectomy; 23 patients underwent pelvic and 14 underwent para-aortic lymphadenectomy; 11 patients underwent partial omentectomy; and 1 patient underwent neoadjuvant chemotherapy. Patients were followed up for at least 1 year and 10 months after treatment based on the guidelines for treatment of uterine body neoplasm of the Japan Society of Gynecologic Oncology [12, 13]. Of the 36 patients, 6 had recurrent disease such as liver or lung metastasis, intra-pelvic masses, or carcinomatous peritonitis that was the cause of death in 2 of these 6 patients. Recurrent disease was suspected based on the subjective symptoms or elevated tumor markers, and diagnosed using CT, 18F-FDG PET/CT or PET/MRI.
18F-FDG PET/MRI
Whole-body PET/MRI
Patients fasted for at least 4 h prior to intravenous injection of 200 MBq of 18F-FDG. Fifty minutes after the injection, patients were transferred to a whole-body 3.0-T PET/MR scanner (Signa PET/MR, GE Healthcare, Waukesha, WI, USA). Anatomical coverage was from the vertex to the mid-thigh. PET acquisition was performed in 3D mode with 5.5 min/bed position (89 slices/bed) in 5–6 beds with a 24-slice overlap. A 2-point Dixon 3D volumetric interpolated T1-weighted fast spoiled gradient echo sequence was acquired at each table position and was used to generate MR attenuation correction (MR-AC) maps. Dixon-based MR-AC classifies body tissues into soft tissue, fat, and air. PET data were reconstructed with ordered subset expectation maximization (OSEM), selecting 14 subsets and 3 iterations, and post-smoothing with a 3-mm Gaussian filter. Reconstructed images were then converted to semiquantitative images corrected by the injected dose and the subject’s body weight (= standardized uptake value [SUV]).
Pelvic PET/MRI
After whole-body scanning and a brief break for urination, the patients were repositioned in the PET/MR scanner. The pelvic PET scan was performed as a 3D acquisition in list mode with 10 min/bed position (89 slices/bed) in 1–2 beds with a 24-slice overlap. The regional PET data were reconstructed with OSEM selecting 16 subsets and 4 iterations, and post-smoothing with a 4-mm Gaussian filter. The reconstructed images were then converted to SUV images. For pelvic MRI, T2-weighted images were acquired in the sagittal, transaxial and coronal planes, using the following T2-weighted image parameters: TR 4000–7000 ms, TE 90 ms, section thickness 4 mm, section overlap 0 mm, flip angle 100°, FOV 240 × 240 mm, matrix 384 × 384, two excitations, and bandwidth 83.3 kHz. High-resolution DW images were then obtained in the sagittal or transaxial plane with b-values of 0 and 800 s/mm2. A 2D RF excitation pulse and 180° refocusing pulse were used to reduce the FOV in the phase-encoding direction while simultaneously suppressing signal from fat. The imaging parameters were as follows: TR 4000 ms, TE 62.8 ms, section thickness 4 mm, section spacing 0 mm, flip angle 90°, FOV 240 × 120 mm (phase FOV = 0.5), matrix 96 × 128, 8 excitations, and bandwidth 250 kHz.
Dynamic Contrast-Enhanced (DCE) MRI
Pelvic MRI was performed using a 3-T clinical scanner (Discovery MR750, GE Healthcare, Waukesha, WI) in 27 patients. To delineate the anatomy of the pelvis prior to pelvic DCE-MRI, T2-weighted imaging was performed in the sagittal, transaxial, and coronal planes. The following T2-weighted image parameters were used: TR 3200–6000 ms, TE 60–85 ms, section thickness 4 mm, interval 1 mm, flip angle 111°, FOV 240 × 240 mm, matrix 320 × 224, two excitations, echo train length 10, and bandwidth 62.5 kHz. For DCE-MRI, a sagittal 3D fast spoiled-gradient-recalled T1-weighted sequence using the Dixon method with fat suppression (LAVA Flex, GE Healthcare) was used with the following parameters: TR 5.0 ms, TE 1.3 ms, section thickness 3 mm, flip angle 12°, FOV 260 × 260 mm, matrix 320 × 192, 1 excitation, and bandwidth 166.7 kHz. After non-contrast images were acquired, 0.1 mmol/kg of gadolinium-diethylenetriamine pentaacetic acid were injected at a rate of 2 ml/s using a contrast injector, followed by a 20-ml saline flush. Image sets were acquired at multiple phases, at 45, 80 and 120 s after initiation of the injection. In nine patients, DCE-MRI was performed at other institutes using 1.5-T clinical scanners (Magnetom Aera, Siemens Healthineers, or Signa HDe, GE Healthcare).
Contrast-Enhanced (CE) CT
CT examinations covering the chest, abdomen and pelvis were performed using a 64-slice multidetector CT scanner (Discovery CT 750HD; GE Medical Systems, Milwaukee, WI) before and after intravenous administration of nonionic iodinated contrast material (iopamidol, Iopamiron 300; Schering, Berlin, Germany).
Image interpretation
Images were analyzed on a dedicated workstation (Advantage Workstation 4.6, GE). Two board certificated radiologists/nuclear medicine physicians, each with double certifications and specialized in gynecological imaging, evaluated the 18F-FDG PET/MRI, ceCT and ceMRI images retrospectively and in consensus. The images were evaluated for the following: (a) presence of the primary tumor; (b) tumor extension into the myometrium, cervical stroma, uterine serosa or adnexa, vagina or parametrium, urinary bladder or rectum mucosa; (c) pelvic or para-aortic lymph nodes; (d) distant metastasis. Both readers were blinded to the results of other imaging studies, histopathologic findings and clinical data. Each dataset was reviewed with the consensus of the two readers after a minimum interval of 3 weeks to avoid any decision threshold bias due to reading-order effects. For MRI interpretation, several previous standard criteria related to primary tumor and nodal or distant metastatic staging of endometrial cancer were used as the reference criteria [3]. Swollen lymph nodes larger than 1 cm in short-axis diameter were graded as malignant. For 18F-FDG PET/MRI interpretations, the classification of lymph nodes as cancer-positive was based on the presence of focally appreciable metabolic activity above that of normal muscle; or asymmetric metabolic activity greater than that of normal-appearing lymph nodes at the same level in the contralateral pelvis, in a location that corresponded to the lymph node chains on the CT or MRI images, with reference to a previous report [7, 8]. Furthermore, the presence of a central unenhanced area suggesting central necrosis or peripheral low attenuation suggesting a fatty hilum within lymph nodes was considered a benign sign. Tumor invasion of neighboring structures was decided primarily on the basis of the CT or MRI findings, with reference to the 18F-FDG PET findings.
Reference standard
Histopathological correlation regarding locoregional extension of the primary tumor was available in all 36 patients, and was used as the standard of reference for T staging. For nodal staging, the histopathological results (n = 22) were used as the gold standard. Because clinical standards of patient management did not require surgery or sampling of the detected lesions, we used a modified reference standard for patients without histopathological sampling, which took into account all prior and follow-up imaging. A decrease in size under treatment (n = 1, interval: 2 months) was regarded to indicate malignancy. For M staging, the histopathological results were used (n = 11).
Statistical analysis
The McNemar test was used to determine the statistical significance of differences in the accuracy of T, N and M staging as determined by PET/MRI, ceCT and ceMRI. Statistical analysis was performed with PRISM software (Versions 6.0; GraphPad, San Diego, CA). Differences at p < 0.05 were considered to be statistically significant.
Results
Patient characteristics
According to the revised International Federation of Gynecology and Obstetrics (FIGO) criteria [1], the T stage was classified as atypical endometrial hyperplasia in 2 patients, pT1a in 17, pT1b in 7, pT2 in 4, pT3a in 3 and pT3b in 3. The histopathologic types of the primary tumors were atypical endometrial hyperplasia (n = 2), endometrioid adenocarcinoma (Grade 1 (n = 18), Grade 2 (n = 5), and Grade 3 (n = 3)), serous carcinoma (n = 6) and carcinosarcoma (n = 2). The N stage was classified as N0 in 21 patients, N1 in 2 and N2 in 1. The M stage was classified as M0 in 8 patients and M1 in 3 including the omentum and inguinal lymph nodes. The demographic data for the 36 patients are listed in Table 1.
Primary tumor detection
PET/MRI and ceMRI detected 97.2% (35/36) of the primary tumors (no significant difference, p = 1).
T staging
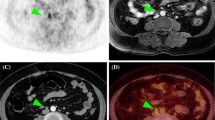
The overall accuracy of T staging for PET/MRI and ceMRI was 77.8% (28/36) and 75.0% (27/36), respectively (Table 2; no significant difference, p = 1). PET/MRI overstaged the actual T stage in three patients (8.3%) and understaged it in five (13.9%), whereas ceMRI overstaged it in four patients (11.1%) and understaged it in five (13.9%). PET/MRI incorrectly classified three T1a tumors as T1b, whereas ceMRI incorrectly classified two T1a tumors as T1b and two T1a tumors as T2. Moreover, PET/MRI incorrectly classified one T1b tumor as T1a, one T2 tumor as T1a, one T3a tumor as T2 and two T3b tumors as T1a and T1b, whereas ceMRI incorrectly classified one T1b tumor as T1a, one T2 tumor as T1a, one T3a tumor as T2 and two T3b tumors as T1b and T2. The sensitivity, specificity and accuracy for detecting invasion of the myometrium were 92.9, 86.4 and 88.9% for PET/MRI, and 92.9, 81.8 and 86.1% for ceMRI, respectively (no significant difference, p = 1). The sensitivity, specificity and accuracy for cervical stroma were 85.7, 100 and 97.2% for PET/MRI and 71.4, 93.1 and 88.9% for ceMRI, respectively (no significant difference, p = 0.248). The accuracy for detecting invasion of the uterine serosa, adnexa, vagina and parametrium were 100, 91.7, 97.2 and 97.2%, respectively, for each of PET/MRI and ceMRI. No invasion of the urinary bladder or rectum mucosa was detected. Figure 1 shows representative images for T staging.
A 51-year-old woman with endometrial cancer invading 50% or more of the myometrium and cervical stroma (pT2). a. Sagittal T2-weighted MRI shows a large mass occupying the uterine cavity. b. Sagittal T2-weighted PET/MRI shows invasion of 50% or more of the myometrium (arrow) and cervical stroma (arrowhead). c. Sagittal T1-weighted dynamic contrast-enhanced MRI in the early phase shows the invasion of 50% or more of the myometrium without sub-endometrial enhancement (arrow) and cervical stroma (arrowhead). Histopathological examination of the surgical specimen was consistent with the imaging findings
N staging
The overall accuracy of N staging for PET/MRI and ceCT was 91.3% (21/23) and 87.0% (20/23), respectively (no significant difference, p = 1; Table 2). PET/MRI overstaged the actual N stage in two patients (8.7%), whereas ceCT overstaged it in one patient (4.3%), and understaged it in two (8.7%). PET/MRI incorrectly classified two N0 lymph nodes as N1, and ceCT incorrectly classified one N0 lymph node as N1, one N1 lymph node as N0 and one N2 lymph node as N1. Patient-based sensitivity, specificity and accuracy for detecting pelvic lymph node metastasis were 100, 90.5 and 91.3% for PET/MRI, and 50.0, 95.2 and 91.3% for ceCT, respectively (no significant difference, p = 0.480). Patient-based accuracy for detecting para-aortic lymph node metastasis was 100% for PET/MRI and 92.9% for ceMRI (no significant difference, p = 1). Lesion-based sensitivity, specificity and accuracy for detecting pelvic and para-aortic nodal metastasis were 100, 96.9 and 97.0% for 18F-FDG PET/MRI, and 14.3, 97.6 and 93.3% for ceCT, respectively. Sensitivity was statistically significant (p = 0.041 < 0.05), but specificity and accuracy were not significant (p = 1 and p = 0.182, respectively) (Table 3).
M staging
The sensitivity, specificity and accuracy of M staging were 33.3, 100 and 81.8% for PET/MRI, and 66.7, 87.5 and 81.8% for ceCT, respectively (no significant difference, p = 0.480; Table 2). PET/MRI understaged the actual M stage in two patients (18.2%), whereas ceCT overstaged it in one patient (9.1%) and understaged it in another (9.1%). PET/MRI incorrectly classified two M1 tumor nodes in the omentum as M0, whereas ceCT incorrectly classified one M1 tumor in the omentum as M0 and one M0 tumor in the omentum as M1. Both PET/MRI and ceCE could detect inguinal lymph node metastasis. Figure 2 shows representative images for M staging, including metastasis to an inguinal lymph node and the omentum.
A 68-year-old woman with endometrial cancer and metastasis to an inguinal lymph node and the omentum (M1). a. Axial T2-weighted PET/MRI shows swelling of the right inguinal lymph node and high FDG uptake (arrow). b. The diameter of the node is > 10 mm (arrow) on contrast-enhanced CT. c. Axial T2-weighted PET/MRI shows omental dissemination with high FDG uptake (arrowhead) d. The diameter of the omental dissemination is > 10 mm (arrowhead) on contrast-enhanced CT. Histopathologic examination confirmed cancer involvement in the lymph node and omental nodule
Discussion
To the best of our knowledge, this is the first study to investigate the diagnostic value of non-contrast 18F-FDG PET/MRI, which has the diagnostic performance of both PET and MRI, for the staging of endometrial cancer in comparison with conventional imaging modalities such as ceMRI and ceCT. For T, N and M staging, the accuracy of 18F-FDG PET/MRI was equivalent to those of ceMRI and ceCT. This finding suggests that 18F-FDG PET/MRI might provide an alternative diagnostic strategy to conventional imaging modalities in the preoperative staging of endometrial cancer.
To optimize decision-making for additional surgical options (such as pelvic or para-aortic lymphadenectomy or radical hysterectomy) in the treatment in endometrial cancer it is important to identify the extent of disease prior to surgery. MRI is useful for evaluating the extent of local disease and its overall staging accuracy has been reported to be 85–95%. In evaluating myometrial invasion, the addition of dynamic ceMRI to T2WI has been reported to improve accuracy, and is considered the reference standard for preoperative detection of deep myometrial invasion [4]. However, contrast agents may induce anaphylactic shock or nephrogenic systemic fibrosis and cannot be used for patients with impaired renal function or allergy, which suggests that an alternative diagnostic strategy is needed for these patients. 18F-FDG PET/CT was comparable to ceMRI for predicting myometrial invasion, and was superior to that modality for identifying cervical invasion in a study of 318 patients with endometrial cancer [6]. In terms of PET/MRI, Kitajima et al. reported that fusion of PET and MRI had significantly higher accuracy for T staging compared with 18F-FDG PET/CT [10]. In the present study, the accuracy for identifying myometrial and cervical invasion was 88.9 and 97.2% for integrated 18F-FDG PET/MRI, and 86.1 and 88.9% for ceMRI. None of these parameters was statistically significant (p > 0.05), which suggests that integrated 18F-FDG PET/MRI might be an alternative modality to ceMRI.
In terms of the assessment of adnexae, a study of 58 patients with endometrial cancer and ovarian malignancy found that preoperative pelvic MRI had sensitivity of 51.7%, which is far below its specificity of 99.9% [14]. In that study, 43.1% of the lesions were occult carcinomas that could barely be identified by preoperative imaging or surgical exploration. 18F-FDG PET/CT offered low diagnostic value in differentiating ovarian malignancies due to low 18F-FDG uptake, leading to false-negative results [15]. Nevertheless, the usefulness of 18F-FDG PET/MRI has been reported: fused 18F-FDG PET/MRI showed higher sensitivity (94%, and specificity of 100%) for the characterization of ovarian tumors compared with those of MRI and 18F-FDG PET/CT [16]. In terms of the assessment of vaginal and parametrial invasion, there have been no reports in endometrial cancer. In cervical cancer, MRI provides rather low sensitivity for the assessment of vaginal and parametrial invasion, but high specificity compared with CT and clinical assessment. Also for cervical cancer, 18F-FDG PET/CT has been reported to have lower accuracy (53.3%) [17], whereas 18F-FDG PET/MRI correctly identified the T stage (85%) [18]. In the present study, both 18F-FDG PET/MRI and ceMRI showed low sensitivity and high specificity for detecting the adnexal, vaginal and parametrial invasion due to the presence of micro-invasion and these results were consistent with those of previous studies. None of these parameters was statistically significant (p > 0.05), which suggests that18F-FDG PET/MRI may also be applied to endometrial cancer with ovarian malignancies, or vaginal and parametrium invasion, in place of ceMRI.
The FIGO classification system divides lymph node metastasis into pelvic lymph node metastases and para-aortic lymph node metastases. In a systematic review of the Cochrane database, lymphadenectomy did not decrease the risk of death or recurrence, and appeared to increase the risk of surgical-related complications in women with low risk of recurrence; however, in patients at intermediate or high risk of recurrence, combined pelvic and para-aortic lymphadenectomy may improve overall survival [19]. Accurate preoperative detection of lymph node metastasis as well as local disease may thus be necessary to determine the optimal treatment and to improve prognosis. A previous study found that for the detection of lymph node metastasis, 18F-FDG PET/CT had a slightly low sensitivity of ~ 70% and high specificity of > 90%, and may be superior to CT or MRI [5]. In the assessment of N staging in endometrial cancer using fused 18F-FDG PET/MRI, Kitajima et al. reported that patient-based sensitivity, specificity and accuracy for detecting pelvic nodal metastasis were 100, 96.3 and 96.7% for both fused 18F-FDG PET/MRI and 18F-FDG PET/ceCT, and 66.7, 100 and 96.7% for MRI, respectively [10]. In addition, Shih et al. reported that SUVmax using integrated 18F-FDG PET/MRI was significantly higher in tumors with lymph node metastasis [20]. In terms of M staging, for the detection of distant metastasis such as to supraclavicular or mediastinal lymph nodes or bone (IVB), a previous study reported sensitivity and specificity of more than 90% for 18F-FDG PET/CT, which indicates its value compared with conventional imaging [21]. These results suggest that 18F-FDG PET/MRI can be superior to ceCT and comparable to 18F-FDG PET/CT in the assessment of N and M staging. However, in the present study, there were no significant differences in N and M staging between 18F-FDG PET/MRI and ceCT, although previous reports have suggested the superiority of 18F-FDG PET/MRI compared with the conventional modalities in these situations of other cancers [18, 22]. The possible reason for the lack of difference could partly be due to the small number of events and samples in our study. Further studies with a larger sample size are needed.
On the basis of these previous studies, 18F-FDG PET/MRI has a higher diagnostic value than 18F-FDG PET/CT and is comparable to ceMRI for the assessment of T staging, whereas 18F-FDG PET/MRI has a higher diagnostic value than ceCT and is comparable to 18F-FDG PET/CT for the assessment of N or M staging. In the present study, 18F-FDG PET/MRI showed comparable diagnostic value to ceMRI and ceCT for the staging of endometrial cancer: the accuracy of T, N and M staging by18F-FDG PET/MRI was 77.8, 91.3 and 81.8%; and the accuracy of T staging by ceMRI and that of N and M staging by ceCT was 75.0, 87.9 and 81.8%, respectively. The diagnostic accuracy of our results is slightly lower than in previous studies because there were high rates of micro-invasion or micro-metastasis in the present patients, and the total sample number was small. Taking these facts into consideration, our study demonstrates the potential diagnostic value of non-contrast 18F-FDG PET/MRI for the staging of endometrial cancer.
This study had several limitations. First, it was a retrospective study, and not all MRI examinations were performed at our institution. However, our readers re-evaluated the images from other hospitals and were blinded to the initial imaging findings. Second, the sample size of our study was relatively small, and further prospective studies are needed. Third, we could not evaluate the histopathological correlation with imaging in 13 (36.1%) patients who had not undergone lymphadenectomy. Thus, we performed node-specific comparison between imaging and histopathology in all other patients.
Conclusion
For the assessment of primary, nodal and metastatic staging in patients with endometrial cancer, integrated 18F-FDG PET/MRI without a gadolinium-based contrast agent shows potential diagnostic value compared with the conventional modalities of ceCT, ceMRI or 18F-FDG PET/CT. 18F-FDG PET/MRI might provide an alternative diagnostic strategy to conventional imaging modalities in the preoperative staging of endometrial cancer, particularly for patients with severe renal dysfunction or allergy to contrast agents.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ceMRI:
-
Contrast-enhanced magnetic resonance imaging
- ceCT:
-
Contrast-enhanced computed tomography
- US:
-
Ultrasonography
- 18F-FDG PET:
-
18F-fluorodeoxyglucose Positron emission tomography
- SUV:
-
Standardized uptake value
References
Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–4.
Lewin SN, Herzog TJ, Barrena Medel NI, Deutsch I, Burke WM, Sun X, Wright JD. Comparative performance of the 2009 international federation of gynecology and obstetrics' staging system for uterine corpus cancer. Obstet Gynecol. 2010;116(5):1141–9.
K. Kinkel, R. Forstner, F.M. Danza, L. Oleaga, T.M. Cunha, A. Bergman, J.O. Barentsz, C. Balleyguier, B. Brkljacic, J.A. Spencer, I. European Society of Urogenital. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol 19(7) (2009) 1565–1574.
Sala E, Crawford R, Senior E, Shaw A, Simcock B, Vrotsou K, Palmer C, Rajan P, Joubert I, Lomas D. Added value of dynamic contrast-enhanced magnetic resonance imaging in predicting advanced stage disease in patients with endometrial carcinoma. Int J Gynecol Cancer. 2009;19(1):141–6.
Bollineni VR, Ytre-Hauge S, Bollineni-Balabay O, Salvesen HB, Haldorsen IS. High diagnostic value of 18F-FDG PET/CT in endometrial Cancer: systematic review and meta-analysis of the literature. J Nucl Med. 2016;57(6):879–85.
Antonsen SL, Jensen LN, Loft A, Berthelsen AK, Costa J, Tabor A, Qvist I, Hansen MR, Fisker R, Andersen ES, Sperling L, Nielsen AL, Asmussen J, Hogdall E, Fago-Olsen CL, Christensen IJ, Nedergaard L, Jochumsen K, Hogdall C. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer - a multicenter prospective comparative study. Gynecol Oncol. 2013;128(2):300–8.
Queiroz MA, Kubik-Huch RA, Hauser N, Freiwald-Chilla B, von Schulthess G, Froehlich JM, Veit-Haibach P. PET/MRI and PET/CT in advanced gynaecological tumours: initial experience and comparison. Eur Radiol. 2015;25(8):2222–30.
Beiderwellen K, Grueneisen J, Ruhlmann V, Buderath P, Aktas B, Heusch P, Kraff O, Forsting M, Lauenstein TC, Umutlu L. [(18)F] FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: initial results. Eur J Nucl Med Mol Imaging. 2015;42(1):56–65.
Tsuyoshi H, Yoshida Y. Diagnostic imaging using positron emission tomography for gynecological malignancy. J Obstet Gynaecol Res. 2017;43(11):1687–99.
Kitajima K, Suenaga Y, Ueno Y, Kanda T, Maeda T, Takahashi S, Ebina Y, Miyahara Y, Yamada H, Sugimura K. Value of fusion of PET and MRI for staging of endometrial cancer: comparison with (1)(8)F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur J Radiol. 2013;82(10):1672–6.
Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C, E.-E.-E.E.C.C.W. Group. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int J Gynecol Cancer. 2016;26(1):2–30.
Lalwani N, Dubinsky T, Javitt MC, Gaffney DK, Glanc P, Elshaikh MA, Kim YB, Lee LJ, Pannu HK, Royal HD, Shipp TD, Siegel CL, Simpson L, Wahl AO, Wolfson AH, Zelop CM, R. American College of Radiology. ACR Appropriateness Criteria(R) pretreatment evaluation and follow-up of endometrial cancer. Ultrasound Q. 2014;30(1):21–8.
Sartori E, Pasinetti B, Chiudinelli F, Gadducci A, Landoni F, Maggino T, Piovano E, Zola P. Surveillance procedures for patients treated for endometrial cancer: a review of the literature. Int J Gynecol Cancer. 2010;20(6):985–92.
Xu X, Li N, Chen Y, Ouyang H, Zhao X, Zhou J. Diagnostic efficacy of MRI for pre-operative assessment of ovarian malignancy in endometrial carcinoma: A decision tree analysis. Magn Reson Imaging. 2019;57:285–92.
Kitajima K, Suzuki K, Senda M, Kita M, Nakamoto Y, Onishi Y, Maeda T, Yoshikawa T, Ohno Y, Sugimura K. FDG-PET/CT for diagnosis of primary ovarian cancer. Nucl Med Commun. 2011;32(7):549–53.
Fiaschetti V, Calabria F, Crusco S, Meschini A, Nucera F, Schillaci O, Simonetti G. MR-PET fusion imaging in evaluating adnexal lesions: a preliminary study. Radiol Med. 2011;116(8):1288–302.
Kitajima K, Suenaga Y, Ueno Y, Kanda T, Maeda T, Deguchi M, Ebina Y, Yamada H, Takahashi S, Sugimura K. Fusion of PET and MRI for staging of uterine cervical cancer: comparison with contrast-enhanced (18)F-FDG PET/CT and pelvic MRI. Clin Imaging. 2014;38(4):464–9.
Sarabhai T, Schaarschmidt BM, Wetter A, Kirchner J, Aktas B, Forsting M, Ruhlmann V, Herrmann K, Umutlu L, Grueneisen J. Comparison of (18)F-FDG PET/MRI and MRI for pre-therapeutic tumor staging of patients with primary cancer of the uterine cervix. Eur J Nucl Med Mol Imaging. 2018;45(1):67–76.
Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2015;2015(9):CD007585.
Shih IL, Yen RF, Chen CA, Chen BB, Wei SY, Chang WC, Sheu BC, Cheng WF, Tseng YH, Chen XJ, Chen CH, Wei LH, Chiang YC, Torng PL, Yen ML, Shih TT. Standardized uptake value and apparent diffusion coefficient of endometrial cancer evaluated with integrated whole-body PET/MR: correlation with pathological prognostic factors. J Magn Reson Imaging. 2015;42(6):1723–32.
Kakhki VR, Shahriari S, Treglia G, Hasanzadeh M, Zakavi SR, Yousefi Z, Kadkhodayan S, Sadeghi R. Diagnostic performance of fluorine 18 fluorodeoxyglucose positron emission tomography imaging for detection of primary lesion and staging of endometrial cancer patients: systematic review and meta-analysis of the literature. Int J Gynecol Cancer. 2013;23(9):1536–43.
Morsing A, Hildebrandt MG, Vilstrup MH, Wallenius SE, Gerke O, Petersen H, Johansen A, Andersen TL, Hoilund-Carlsen PF. Hybrid PET/MRI in major cancers: a scoping review. Eur J Nucl Med Mol Imaging. 2019;46(10):2138–51.
Acknowledgements
The authors thank Hiroshi Oikawa and other staff of the Biomedical Imaging Research Center and doctors in the Department of Obstetrics and Gynecology, University of Fukui, for technical and clinical support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HT, and YY designed the study. TT, and HO contributed to data acquisition. HT, and SY contributed to the data analysis and interpretation. HT, and SY performed the statistical analysis. HT, TT, SY, HO, and YY together contributed to drafting of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of this retrospective study was approved by the Ethics Committee of University of Fukui, Faculty of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Supplementary Table 1.
Characteristics of patients excluded from the present study
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tsuyoshi, H., Tsujikawa, T., Yamada, S. et al. Diagnostic value of 18F-FDG PET/MRI for staging in patients with endometrial cancer. Cancer Imaging 20, 75 (2020). https://doi.org/10.1186/s40644-020-00357-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-020-00357-4