Abstract
Background
To prove the feasibility of integrating CT urography (CTU) into 68Ga-PSMA-11 PET/CT and to analyze the impact of CTU on assigning focal tracer accumulation in the ureteric space to either ureteric excretion or metastatic disease concerning topographic attribution and diagnostic confidence.
Methods
Ten prostate cancer patients who underwent 68Ga-PSMA-11 PET/CT including CTU because of biochemical relapse or known metastatic disease were retrospectively analyzed. CTU consisted of an excretory phase 10 min after injection of 80 mL iodinated contrast material. Ureter opacification at CTU was evaluated using the following score: 0, 0% opacification; 1, < 50%; 2, 50–99%; 3, 100%. Topographic attribution and confidence of topographic attribution of focal tracer accumulation in the ureteric space were separately assessed for 68Ga-PSMA-11 PET/CT without and with CTU. Diagnostic confidence was evaluated using the following score: 0, < 25% confidence; 1, 26–50%; 2, 51–75%; 3, 76–100%.
Results
At CTU, mean ureter opacification score was 2.6 ± 0.7. At 68Ga-PSMA-11 PET/CT without CTU, mean confidence of topographic attribution of focal tracer accumulation was 2.5 ± 0.7 in total and 2.6 ± 0.7 for metastatic disease. At 68Ga-PSMA-11 PET/CT with CTU, mean confidence of topographic attribution of focal areas of tracer accumulation was significantly higher with 2.9 ± 0.2 in total and 2.7 ± 0.9 for metastatic disease (p < 0.001). In 4 of 34 findings (12%) attribution to either ureteric excretion or metastatic disease was discrepant between 68Ga-PSMA-11 PET/CT without and with CTU (n.s).
Conclusions
Integration of CTU into 68Ga-PSMA-11 PET/CT is feasible and increases diagnostic confidence of assigning focal areas of tracer accumulation in the ureteric space to either metastatic disease or ureteric excretion.
Similar content being viewed by others
Background
Prostate Cancer is the most common malignancy in men and the third most frequent cause of cancer-associated death [1, 2]. With the introduction of prostate-specific antigen (PSA) screening, many patients are diagnosed with localized disease, but still a subset of patients develops high-risk or metastatic disease. After radical treatment of localized disease, biochemical relapse according to PSA elevation occurs in approximately 30% of prostate cancer patients [3].
Accurate diagnosis of prostate cancer relapse, identification of involved anatomical sites, and assessment of tumor load are crucial for treatment stratification and metastases-directed therapies such as salvage lymph node dissection. Early detection of prostate cancer relapse is, however, a major challenge for conventional imaging methods including computed tomography (CT) and magnetic resonance imaging (MRI) [4]. Functional imaging techniques targeting the prostate-specific membrane antigen (PSMA) using positron emission tomography (PET) have shown great potential for detection of prostate cancer and its metastases [5]. A key step in PET imaging with PSMA ligands was the development of 68Ga-labelled PSMA (68Ga-PSMA-11) [6]. 68Ga-PSMA-11 binds to the extracellular part of the PSMA receptor and is then internalized into the prostate cancer cell.
68Ga-PSMA-11 is eliminated via the renal pathway, so that detection of disease with PET/CT using 68Ga-PSMA-11 may be limited by topographic proximity of tumor sites with the urinary tract or by superimposition of tumor sites and ureteric tracer accumulation [7]. Furthermore, the low-dose CT scan of a conventional 68Ga-PSMA-11 PET/CT may be limited in regards of anatomical landmarking and, thus, may have limited diagnostic confidence to correctly classify a focal tracer accumulation as tumor uptake or ureteric tracer excretion. Hence, improving visualization of the upper urinary tract using CT urography (CTU) as the first choice imaging technique to depict the urinary tract may have added value for 68Ga-PSMA-11 PET/CT if conducted after the regular PET/CT scan.
The aim of this retrospective study was to evaluate the integration of CTU into 68Ga-PSMA-11 PET/CT. We hypothesized that availability of a CTU scan improves diagnostic confidence of attributing focal areas of tracer accumulation in the ureteric space to either ureteric tracer excretion or tumor uptake at 68Ga-PSMA-11 PET/CT.
Methods
Study design and study population
This retrospective single-center exploratory study was approved by the local institutional review board (S-321/2012) and conducted in agreement with the Declaration of Helsinki and its later amendments. In 10 consecutive patients, in which 68Ga-PSMA-11 PET/CT was performed for standard clinical indications, 68Ga-PSMA-11 PET/CT was supplemented by CTU at the discretion of the attending nuclear medicine physician. Clinical data was extracted from the electronical medical records.
Imaging
All patients underwent imaging on a Biograph mCT Flow scanner (Siemens, Erlangen, Germany). FlowMotion was used to acquire PET in 3D mode (matrix 200 × 200). Correction for randoms, scatter and decay was performed for the emission data. Images were reconstructed with an ordered subset expectation maximization (OSEM) algorithm with two iterations/21 subsets and Gauss-filtered to a transaxial resolution of 5 mm at full-width at half-maximum (FWHM). Attenuation was corrected using unenhanced low-dose CT reconstructed to a slice thickness of 5 mm with an increment of 3–4 mm.
CTU was performed during the same 68Ga-PSMA-11 PET/CT session on the same scanner. All patients received a bolus of 80 mL intravenous iodine-based contrast agent (iopromide, 300 mg per mL, Ultravist, Bayer, Leverkusen, Germany) via injection. The excretory phase of CTU was finally performed 600 s after injection (slice thickness, 2.0 mm; reconstruction increment, 1.6 mm; tube voltage, 120 kVp; reference tube current time product, 190 mAs). Additional means to intensify ureteric opacification at CTU, such as furosemide injection, were not undertaken.
Image analysis
Two independent analyses of image data sets were performed: First, contrast opacification of the lumen of the ureters at CTU was assessed to investigate the quality of CTU. Second, the confidence of attributing a PET-positive finding in the ureteric space to metastatic disease or ureteric tracer excretion was determined.
In order to assess the contrast enhancement of the ureters two blinded board-certified readers with more than 10 years of experience in abdominal radiology each (FLG, TFW) reviewed CTU images independently on an imaging workstation (Centricity, GE Healthcare, Chalfont St. Giles, Great Britain). The ureters were divided into a proximal segment above the iliac vessels and a distal segment below the iliac vessels. The readers were advised to assign an opacification score to each section according to the CTU analyses published by other groups before [8]. The opacification scores were the following: 0, 0% opacification; 1, < 50% opacification; 2, 50–99% opacification; 3, 100% opacification.
In order to assess the confidence of attributing a PET-positive finding in the ureteric space to tumor uptake or ureteric tracer excretion a two-step analysis was performed by one reader (TFW). First, fused unenhanced 68Ga-PSMA-11 PET/CT images were reviewed without consideration of the CTU scan (confidence read 1). Focal areas of tracer accumulation in the ureteric space were identified and attributed either to tumor uptake or ureteric tracer excretion. Diagnostic confidence of assignment was recorded using the following confidence score: 0, < 25% confidence; 1, 26–50% confidence; 2, 51–75% confidence; 3, 76–100% confidence. Second, four weeks after confidence read 1 the examinations were read again including the CTU scan (confidence read 2). PET-positive findings identified at confidence read 1 were anatomically correlated to the CTU scan. Again, diagnostic confidence of attributing focal areas of tracer accumulation in the ureteric space to either tumor uptake or ureteric tracer excretion was scored for each finding.
Statistics
Interreader agreement of the assessment of ureter opacification was calculated by using the intraclass coefficient (ICC) employing a two-way consistency model indicating that the absolute differences were neglected. The average-measures ICC is given with 95% confidence interval. 0.00 to 0.20 indicates slight agreement, 0.21 to 0.40 fair agreement, 0.41 to 0.60 moderate agreement, 0.61 to 0.80 substantial agreement, and 0.81 to 1.00 almost perfect or perfect agreement. Differences in anatomical attribution of PET-positive findings and differences in diagnostic confidence of anatomical attribution were analyzed using Wilcoxon signed-rank tests for paired samples.
Results
Patients
The study group consisted of 10 men with a median age of 70 years (range, 63–81). Indication for 68Ga-PSMA-11 PET/CT was biochemical relapse after radical prostatectomy in 8 patients, staging prior to treatment in 1 patient, and re-staging during hormonal therapy in 1 patient, respectively. For 5 patients, long-term oncological follow-up data was available. Of these patients, 4 had confirmed advanced/metastatic disease diagnosed at 68Ga-PSMA-11 PET/CT, and tumor-specific therapy was initiated. 1 patient had no morphological sign of disease recurrence despite PSA progression but developed recurrent tumor later on during the course of disease. For the other 5 patients, PSMA CT revealed recurrent advanced diseases in 3 patients, and in 2 cases no tumor activity was detected. Clinical characteristics are summarized in Table 1.
Opacification score
In total, 40 ureter segments (10 patients, 4 segments per patient) have been evaluated. Ureter opacification was scored by reader 1 with a mean score of 2.5 ± 0.6 and by reader 2 with a mean score of 2.7 ± 0.7. The total opacification score averaged over both readers was 2.6 ± 0.7. Both readers demonstrated near perfect agreement for all ratings taken together (ICC, 0.86 (0.79–0.91)). Table 2 shows the mean score for each segment by each reader and the interreader agreement per segment.
Reader 1 rated opacification of 34 of 40 segments (85%) with a score of at least 2. Reader 2 rated opacification of 33 of 40 segments (82.5%) with a score of at least 2. The quantitative distribution of ureteric opacification scores is given in Table 3.
Diagnostic confidence
In confidence read 1, considering fused images of the unenhanced PET/CT alone, 34 focal areas of tracer accumulation in the ureteric space were identified. Of these, 14 were attributed to tumor uptake. Mean confidence of attribution in read 1 was 2.5 ± 0.7 in total and 2.6 ± 0.7 for tumor uptake.
In confidence read 2, considering fused images of the unenhanced PET/CT and CTU together, attribution of focal areas of tracer accumulation to tumor uptake or ureteric excretion was discrepant from confidence read 1 in 4 of 34 findings (12%) or 2 of 10 patients (20%), respectively. Three of these discrepancies occurred in one patient and consisted of attribution of tracer accumulation to ureteric excretion rather than to tumor uptake (Fig. 1). In this patient, other focal areas of tracer accumulation attributed to tumor uptake in both reads were present as well. The remaining discrepancy consisted of attribution of tracer accumulation to tumor uptake rather than to ureteric excretion in a different patient (Fig. 2). In this patient, other focal areas of tracer accumulation attributed to tumor uptake were not present. The confidence score differed from read 1 in 15 findings (44%). In 14 of these 15 findings the confidence score in read 2 was at least 1 grade higher than in read 1. Mean confidence of attribution in read 2 was 2.9 ± 0.2 in total and 2.7 ± 0.9 for tumor uptake.
Discrepancies for 68Ga-PSMA-11 PET/CT without and with CTU in anatomical assignment of tracer accumulation in a patient with multifocal retroperitoneal nodal relapse. a shows focal tracer accumulation in the right iliac retroperitoneum that was assigned to tumor uptake in 68Ga-PSMA-11 PET/CT alone (arrow in a, confidence score 1) and to right ureteric excretion in 68Ga-PSMA-11 PET/CT with CTU (arrow heads in b refer to both ureters, confidence score 3). c again shows focal tracer accumulation in the right iliac retroperitoneum that was assigned to tumor uptake in 68Ga-PSMA-11 PET/CT alone (arrow in c, confidence score 1) and to right ureteric excretion in 68Ga-PSMA-11 PET/CT with CTU (arrow heads in b refer to both ureters, confidence score 3). There is another focal tracer accumulation that was assigned to metastatic disease in 68Ga-PSMA-11 PET/CT without and with CTU (curved arrow in c and d, both confidence score 3)
Discrepancies for 68Ga-PSMA-11 PET/CT without and with CTU in anatomical assignment of tracer accumulation in a patient with unifocal retroperitoneal nodal relapse according to 68Ga-PSMA-11 PET/CT with CTU. a shows a focal tracer accumulation in the left retroperitoneum at the the level of the ureteric crossing of the iliac vessels with anatomical assignment to the left ureter with confidence score 1. Using 68Ga-PSMA-11 PET/CT with CTU, the focus was assigned to a small periureteric soft tissue mass (circle in b) between the left ureter (arrow head in b) and the left iliac vessels with confidence score 3
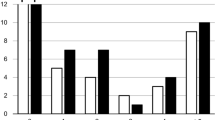
Anatomic attribution of focal areas of tracer accumulation to either tumor uptake or ureteric excretion did not differ significantly between confidence read 1 and 2 in total (p = 0.375). The confidence of attribution was significantly higher for the combined read of PET/CT and CTU than for PET/CT alone (p < 0.001) (Fig. 3).
Increase of diagnostic confidence by integrating CTU into 68Ga-PSMA-11 PET/CT with CTU. a shows a focal tracer accumulation in the right iliac retroperitoneum (arrow in a) with anatomical assignment to the right ureter with confidence score 1. Anatomical assignment is hampered by proximity of the focus to small intestine and iliac crossing of the right ureter. Using 68Ga-PSMA-11 PET/CT with CTU and delineation of both ureters (arrow heads in b), the focus was assigned to right ureteric excretion with confidence score 3
CTU radiation exposure
The mean CT volume dose index (CTDIvol) and dose length product (DLP) of CTU were 15 ± 6 mGy and 694 ± 334 mGycm.
Discussion
The present analysis demonstrates that integration of CTU into 68Ga-PSMA-11 PET/CT imaging of prostate cancer patients is feasible and increases diagnostic confidence of attribution of focal areas of tracer accumulation in the ureteric space to either tumor uptake or ureteric excretion. In a considerable amount of focal areas of tracer accumulation integration of CTU changed the assessment of anatomic allocation (12%) and, thus, may impact on cancer patient management in select cases.
Nowadays, 68Ga-PSMA-11 PET/CT plays an important role in management of prostate cancer patients [9]. Especially in the event of biochemical relapse, early detection of recurrent disease is of major importance to identify individuals that are eligible for curative treatment options including salvage lymphadenectomy. In a study of 319 patients with biochemical failure after curatively intended therapy more than 80% of patients had at least one positive lesion at 68Ga-PSMA-11 PET/CT [10]. The probability of a positive 68Ga-PSMA-11 PET/CT increases with the blood level of PSA: For PSA levels between 0.5 and 1.0 ng/mL it is almost 60% and rises rapidly with increasing PSA levels [10, 11]. In a recent meta-analysis, overall sensitivity and specificity were about 86% for per-patient analysis and 80% and 97% for per-lesion analysis, respectively [11].
A ligand-specific challenge is evaluation of tumor foci in close proximity to ureter and bladder because of urinary excretion of 68Ga-PSMA-11 [7, 12]. A focal tracer accumulation in the retroperitoneum and pelvis can be misinterpreted because the low-dose scan typically included in standard PET/CT imaging may not offer sufficient anatomical detail to correctly identify the underlying cause of accumulation, especially for the inexperienced reader. Thus, urinary excretion of 68Ga-PSMA-11 may influence data on false positive and false negative lymph node assessment at 68Ga-PSMA-11 PET/CT [13,14,15]. As the problem of identification of local recurrences in the prostate bed, that may be obscured by tracer activity within the bladder, was considered not to be addressed by CTU integration, the ability of detecting local recurrences beneath the ureter orifices was not subject of this study.
Although uncertainty of anatomical attribution of focal areas of tracer accumulation may be present only in a minority of cases, the impact of over- or understaging disease on patient management may be tremendous and may justify integration of an additional CTU scan at least in select cases with equivocal findings at standard 68Ga-PSMA-11 PET/CT. Due to the small size of our study cohort and lack of long-term clinical data in half of our patients, impact of CTU integration on specific oncological treatment decisions and patient outcome cannot be derived from the results.
The standard CTU protocol consists of unenhanced, nephrographic phase, and excretory phase imaging. Common general indications for CTU include hematuria, staging and follow-up of urothelial cancer, urinary tract obstruction, and anomalies of urinary tract anatomy [16]. It does not only allow detailed assessment of the urinary tract but also facilitates evaluation of adjacent abdominal and pelvic structures. The urography protocol used in this study was a single-bolus technique with administration of 80 ml of contrast material and excretory phase imaging 10 min after injection. Although the frequently recommended amount of contrast material for CTU is 100 to 150 ml, opacification of the urinary tract was sufficient in our study even in distal ureter segments [17]. As anatomic delineation of the ureter and not evaluation of the upper urinary tract for urothelial masses was the intention for CTU, the reduction of the amount of contrast material was justified. For the same reason we did not use oral hydration, intravenous saline infusion, or furosemide injection to intensify ureter distention - techniques that have been suggested to improve assessment of the upper urinary tract [18,19,20].
Because a standard dose contrast enhanced CT scan during nephrographic or portal venous phase was not performed in our study, it remains unclear if such an acquisition would provide similar results to CTU regarding anatomic attribution of focal tracer accumulations. A nephrographic or portal venous phase was not acquired because sole visceral metastases are rare in prostate cancer patients, the excretory phase is considered to be superior for exact urinary tract depiction, and it would have added even more additional radiation exposure to the diagnostic procedure. Of note, using a split bolus technique for CTU, simultaneous acquisition of a nephrographic and excretory phase has been introduced as an alternative one-stop-shop CTU technique with reduced radiation dose [8, 21]. CTU was done in our study with standard abdominal scanning parameters with 120 kVp and image reconstruction using filtered back projection. Studies have shown that iterative image reconstruction or reduction of tube voltage to 100 kVp may allow significant dose reduction without decline of CTU image quality [22, 23]. Iterative reconstruction is already available on modern PET/CT scanners and may be preferred over filtered back projection for diagnostic contrast enhanced dose reduced body scans. An alternative to CTU could be to increase ureteric clearance of 68Ga-PSMA-11 by injection of furosemide prior to the delayed PET scan and adding another unenhanced low-dose scan of the abdomen and pelvis for anatomical correlation. Derlin et al. have shown that forced diuresis with furosemide injection may reduce linear and focal tracer accumulation in standard 68Ga-PSMA-11 PET/CT and, thus, may improve image quality without the need of intravenous contrast administration and CTU scanning [24]. However, correct timing of furosemide injection seems to be crucial in this approach as too early furosemide injection may even deteriorate image quality.
Today, several PSMA ligands aside from 68Ga-PSMA-11 are available: 68Ga-PSMA-I + T, 68Ga-PSMA-617 as well as 18F–DCFBC, 18F–DCPyL, and, most recently, 18F–PSMA-1007. All of these PSMA-ligands have similar characteristics in regards of tumor uptake and tracer clearance except for 18F–PSMA-1007 [12, 25,26,27,28]. This ligand is cleared primarily via the hepatobiliary route and not via the urinary tract and may improve diagnostic confidence particular in these suspicious areas [12].
Limitations
This retrospective study is limited by small sample size and, thus, can only be considered hypothesis-generating. It cannot be derived from our data how often integration of CTU into 68Ga-PSMA-11 PET/CT may actually change oncological patient management and influence long-term clinical outcome. Larger and prospectively generated cohorts are to be studied to fully assess the clinical benefit of our method. As histological correlation of imaging findings was not undertaken, a gold standard to estimate the diagnostic accuracy of 68Ga-PSMA-11 PET/CT with and without CTU including rates of false positive and false negative findings is not available. Data on interrater reliability of the improvement of diagnostic confidence are not available.
Conclusions
Integration of CTU into 68 Ga-PSMA-11 PET/CT may help the imaging specialist in anatomical attribution of questionable findings and enhance confidence in interpretation of 68Ga-PSMA-11 PET/CT scans in select cases.
Abbreviations
- CT:
-
Computed tomography
- CTDIvol:
-
CT volume dose index
- CTU:
-
CT urography
- DLP:
-
Dose length product
- FWHM:
-
Full-width at half-maximum
- ICC:
-
Intraclass coefficient
- MRI:
-
Magnetic resonance imaging
- OSEM:
-
Ordered subset expectation maximization
- PET:
-
Positron emission tomography
- PSA:
-
Prostate-specific antigen
- PSMA:
-
Prostate-specific membrane antigen
References
Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–23.
Kosuri S, Akhtar NH, Smith M, Osborne JR, Tagawa ST. Review of salvage therapy for biochemically recurrent prostate cancer: the role of imaging and rationale for systemic salvage targeted anti-prostate-specific membrane antigen radioimmunotherapy. Adv Urol. 2012;2012:921674.
Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K, Haberkorn U. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J Nucl Med. 2016;57:79S–89S.
Eder M, Neels O, Muller M, Bauder-Wust U, Remde Y, Schafer M, Hennrich U, Eisenhut M, Afshar-Oromieh A, Haberkorn U, Kopka K. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel). 2014;7:779–96.
Freitag MT, Radtke JP, Afshar-Oromieh A, Roethke MC, Hadaschik BA, Gleave M, Bonekamp D, Kopka K, Eder M, Heusser T, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging. 2017;44:776–87.
Maheshwari E, O'Malley ME, Ghai S, Staunton M, Massey C, Split-bolus MDCT. Urography: upper tract opacification and performance for upper tract tumors in patients with hematuria. AJR Am J Roentgenol. 2010;194:453–8.
Kratochwil C, Afshar-Oromieh A, Kopka K, Haberkorn U, Giesel FL. Current status of prostate-specific membrane antigen targeting in nuclear medicine: clinical translation of chelator containing prostate-specific membrane antigen ligands into diagnostics and therapy for prostate cancer. Semin Nucl Med. 2016;46:405–18.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, Eisenhut M, Boxler S, Hadaschik BA, Kratochwil C, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, Bolton D, Lawrentschuk N. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–37.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, Kesch C, Tolstov Y, Singer S, Grabe N, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–88.
Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, Gildehaus FJ, Stief CG, Gratzke C, Fendler WP. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70:553–7.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, Wester HJ, Heck M, Kubler H, Beer AJ, et al. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–43.
Budaus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, Graefen M, Steuber T, Rosenbaum C. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69:393–6.
Silverman SG, Leyendecker JR, Amis ES, Jr.: What is the current role of CT urography and MR urography in the evaluation of the urinary tract? Radiology 2009, 250:309–323.
Van Der Molen AJ, Cowan NC, Mueller-Lisse UG, Nolte-Ernsting CC, Takahashi S, Cohan RH. Radiology CTUWGotESoU: CT urography: definition, indications and techniques. A guideline for clinical practice. Eur Radiol. 2008;18:4–17.
McTavish JD, Jinzaki M, Zou KH, Nawfel RD, Silverman SG. Multi-detector row CT urography: comparison of strategies for depicting the normal urinary collecting system. Radiology. 2002;225:783–90.
Kemper J, Regier M, Stork A, Adam G, Nolte-Ernsting C. Improved visualization of the urinary tract in multidetector CT urography (MDCTU): analysis of individual acquisition delay and opacification using furosemide and low-dose test images. J Comput Assist Tomogr. 2006;30:751–7.
Nolte-Ernsting CC, Wildberger JE, Borchers H, Schmitz-Rode T, Gunther RW, Multi-slice CT. Urography after diuretic injection: initial results. Rofo. 2001;173:176–80.
Chow LC, Kwan SW, Olcott EW, Sommer G, Split-bolus MDCT. Urography with synchronous nephrographic and excretory phase enhancement. AJR Am J Roentgenol. 2007;189:314–22.
Lee S, Jung SE, Rha SE, Byun JY. Reducing radiation in CT urography for hematuria: effect of using 100 kilovoltage protocol. Eur J Radiol. 2012;81:e830–4.
van der Molen AJ, Miclea RL, Geleijns J, Joemai RM, Survey A. Of radiation doses in CT urography before and after implementation of iterative reconstruction. AJR Am J Roentgenol. 2015;205:572–7.
Derlin T, Weiberg D, von Klot C, Wester HJ, Henkenberens C, Ross TL, Christiansen H, Merseburger AS, Bengel FM. 68Ga-PSMA I&T PET/CT for assessment of prostate cancer: evaluation of image quality after forced diuresis and delayed imaging. Eur Radiol. 2016;26:4345–53.
Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, Antonarakis ES, Fan H, Dannals RF, Chen Y, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–74.
Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, Holland-Letz T, Hadaschik BA, Giesel FL, Debus J, Haberkorn U. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20.
Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, Endres CJ, Dannals RF, Sgouros G, Lodge M, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–91.
Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, Eisenhut M, Kubler W, Holland-Letz T, Giesel FL, et al. The Theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–705.
Acknowledgements
Not applicable
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
LW acquisition of the data, interpretation of results, writing parts of the manuscript, and critically reviewing the manuscript. FLG conception and design of the study, interpretation of the data, responsible for attaining ethical approval, and critically reviewing the manuscript. MTF statistical analysis and interpretation of the results, and critically reviewing the manuscript. CK, and CK acquisition of PET-CT scans, AKB, WM, KK, SAK, HR, CK, CK, HUK, and UH interpretation of the data, and critically reviewing the manuscript. TFW writing parts of the manuscript, study design, interpretation of results, and critically reviewing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study has been approved as a retrospective study by the local ethics committee of Heidelberg (S-321/2012). For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Will, L., Giesel, F.L., Freitag, M.T. et al. Integration of CT urography improves diagnostic confidence of 68Ga-PSMA-11 PET/CT in prostate cancer patients. Cancer Imaging 17, 30 (2017). https://doi.org/10.1186/s40644-017-0132-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-017-0132-6