Abstract
Background
Pichia pastoris is one of the most important cell factories for production of industrial enzymes and heterogenous proteins. The genome-scale metabolic model of high quality is crucial for comprehensive understanding of the P. pastoris metabolism.
Methods
In this paper, we upgraded P. pastoris genome-scale metabolic model based on the combination of latest genome annotations and literatures. Then the performance of the new model was evaluated using the Cobra Toolbox v2.0.
Results
Compared with the recently published model iMT1026, the reaction number in the new model iRY1243 was increased from 2035 to 2407 and the metabolite number was increased from 1018 to 1094. Accordingly, the unique ORF number was increased from 1026 to 1243. To improve the metabolic functions of P. pastoris genome-scale metabolic model, the biosynthesis pathways of vitamins and cofactors were carefully added. iRY1243 showed good performances when predicting the growth capability on most of the reported carbon and nitrogen sources, the metabolic flux distribution with glucose as a sole carbon source, the essential and partially essential genes, and the effects of gene deletion or overexpression on cell growth and S-adenosyl-l-methionine production.
Conclusion
iRY1243 is an upgraded P. pastoris genome-scale metabolic model with significant improvements in the metabolic coverage and prediction ability, and thus it will be a potential platform for further systematic investigation of P. pastoris metabolism.
Similar content being viewed by others
Background
Pichia pastoris (P. pastoris), one important cell factory, has been widely used to express more than 200 kinds of industrial enzymes and heterogenous proteins (Cereghino and Cregg 2000; Ahmad et al. 2014) because of its many interesting properties: the availability of well-established protocols and techniques for gene operation (Bhataya et al. 2009; Nocon et al. 2014; Morozkina et al. 2016), the ability of post-translational modification (De Schutter et al. 2009), the ease of establishing high-cell density culture on simple and defined media, and the simple separation and purification processes (Macauley-Patrick et al. 2005).
To improve the protein production levels by P. pastoris, comprehensive understanding of its metabolism is necessary. Genome-scale metabolic model (GSMM) has been one of the most widely used tools in system biology (Kim et al. 2012) and has shown its potential for predicting cell phenotypes under certain conditions (Famili et al. 2003; Fong et al. 2003), integrating multiple omic data (Saha et al. 2014; Angione et al. 2016), discovering metabolic network characteristics (Feist and Palsson 2008), and predicting engineering target for improving the titer of industrial cell factories.
The earliest metabolic network model of P. pastoris is the small model of central carbon metabolism network model (Maaheimo et al. 2001), which is used to calculate the 13C metabolic flux distribution (Sola et al. 2004). The gene sequencing of P. pastoris has laid the foundation for GSMM construction (Mattanovich et al. 2009; Kuberl et al. 2011). There are different P. pastoris GSMM models. Sohn et al. (2010), Chung et al. (2010), and Caspeta et al. (2012) established the PpaMBEL1254, iPP668, and iLC915 models, respectively. The model iMT1026 was reconstructed by merging PpaMBEL1254, iPP668, and iLC915 models, and adding the synthesis and decomposition pathway of fatty acid, sphingolipid biosynthesis pathway, oxidative phosphorylation, and glycosylation pathway (Tomas-Gamisans et al. 2016). Compared with the latest Saccharomyces cerevisiae GSMMs, the quality of these P. pastoris models still needs to be improved at least in three aspects: the balance of each reaction in mass and electrical charge, the model metabolic coverage, and the correction in GPRs. The biomass components of P. pastoris (Carnicer et al. 2009) and the utilization of energy (Chung et al. 2010) have been reported. With the availability of new gene annotations and literatures about P. pastoris, it is necessary to update the P. pastoris GSMM models to improve the model performance.
In this paper, based on the latest gene annotations and the newly published literatures, we reconstructed a new genome-scale metabolic network model of P. pastoris. The new model iRY1243 contained 1243 annotated genes, 2407 reactions, 1740 metabolites, and 9 reaction locations. At the same time, we updated the cell components of P. pastoris based on the latest literatures. The model performance was evaluated by the data from RNA-Seq, chemostat experiments, and 13C labeling experiments. Model iRY1243 was then used to predict the secretion of products and essential genes on synthetic medium.
Methods
Procedures for model reconstruction
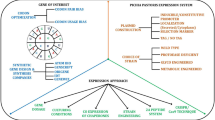
The procedures for reconstruction of P. pastoris GSMM could be divided into two phases (Fig. 1). Firstly, by collating and evaluating the existing models, we upgraded the GSMM based on the existing iPP668, PpaMBEL1254, iLC915, and iMT1026 models and re-annotated the information of all metabolites by referring to the databases of KEGG and BiGG, to ensure that each metabolite had correct structure and charge in pH 7.2. Then, each reaction in models was manually checked and corrected, thus making it comparable with the published GSMM model.
Subsequently, to update the P. pastoris GSMM systematically, the genome annotation information from mainly three databases (KEGG, UniProtKB, IMG) was gathered and merged (Table 1). To establish the new gene–protein–reaction associations (GPRs), the genes were collected from each database. With the aid of KEGG and Enzyme databases, the relationship between proteins and reactions were further checked to ensure the high quality of GPRs in the new P. pastoris GSMM. Next, using the gapFind function of the Cobra Toolbox v2.0 to identify dead-end metabolites, essential reactions from KEGG and other databases were added to reduce the number of dead-end metabolites to improve the function of the metabolic network.
Lastly, the cell components and the energy parameter were updated. Compared with the primordial model, some new components of cell, like the cofactors including folate and thiamin, were added to ensure the function of the corresponding metabolic pathways. With the fitting of the data from chemostat cultivations, the non-growth-associated maintenance (NGAM) in the model was adjusted from 3.7 to 2.52 mmol ATP/gDCW h.
Experimental procedures
Strains
The Pichia pastoris strains GS115 and G1HL were used to evaluate the model performance.
Medium and culture conditions
In this study, 50 mL YPG medium as the seed medium was taken in 500-mL flask (yeast extract 10%, peptone 20%, glycerol 20%). The composition of the medium used in chemostat cultivations was as follows: 10 g/L glucose, 9.1 g/L K2SO4, 0.46 g/L CaSO4, 7.5 g/L MgSO4·7H2O, 2.06 g/L KOH, 13.4 mL/L H3PO4, and 6 mL/L PTM1. The components of PTM1 were described by Baumann et al. (2008).
The culture conditions for seed culture and chemostat culture were described by Nie et al. (2014). Chemostat cultivation was carried out in a 5-L bioreactor (National Engineering Research Center for Biotechnology, Shanghai, China) with a working volume of 3 L. With glucose as the sole carbon source, the initial aeration rate was set to 1 vvm, controlled by a mass flow controller (Sevenstar, Beijing, China). The dissolved oxygen (DO) was maintained above 30% by adjusting the agitation and the aeration to ensure fully aerobic conditions. The O2 and CO2 concentrations in the off-gas were measured online using a process mass spectrometer (MAX300-LG, Extrel, America). Pressure, pH, and temperature were controlled at 0.04 MPa, pH 5.5 (with adding NH3·H2O), and 30 °C, respectively. The specific growth rate of the cells was controlled by the dilution ratio (D).
Determination of the concentration of cell, glucose, and other extracellular metabolites
The cell concentration was measured from the optical density at 600 nm. For dry cell weight (DCW) measurement, 10 mL broth was filtered using a pre-dried and weighted microporous membrane (Shanghai Diqing Filtration Technology CO., LTD Shanghai, China). After being washed 3 times, the filter paper was dried at 105 °C for 3 h. The glucose kits (Shanghai Kexin Biotechnology Institute, China) were used for residual glucose concentration analysis. Extracellular metabolite measurements (such as ethanol, acetic acid, propionic acid, formic acid, pyruvic acid, and acetaldehyde) were performed as described in Nie et al. (2014).
Sensitivity analysis
During the sensitivity analysis of the GSMM, the specific glucose consumption rate was changed from 0 to 2.0 mmol/gDCW h. In each simulation, only one of the following model parameters was evaluated, including the lipid composition (6.2–16.2%), protein composition (37–47%), RNA composition (6.6–16.6%), DNA composition (0.1–1.0%), carbohydrate composition (26.9–36.9%), and NGAM value (1.26–3.78 mmol ATP/gDCW h). The specific biomass growth rate and oxygen uptake rate were simulated to evaluate the effects of the changes of parameters on the model prediction ability.
Calculation of energy parameters
The ATP required for cell survival can be calculated by chemostat experiments. The generated ATP can be divided into two forms: non-growth-associated ATP maintenance (NGAM) and growth-associated ATP maintenance (GAM). GAM required for biomass synthesis (i.e., precursor biosynthesis and polymerization) can be obtained from literature. NGAM, used for cell maintenance, is an independent reaction in the model, which can be calculated from chemostat data.
NGAM and GAM are added to calculate the total amount of ATP required for cell growth, as shown in the following equation:
where Y xATP and m ATP represent the GAM and NGAM, respectively, μ is the specific growth rate, and r ATP represents the total amount of ATP consumed by P. pastoris.
Biomass composition
As the biomass composition has obvious effects on the model validation and the strain improvement, we updated the cell composition of P. pastoris. The biomass is composed of macromolecular substances (i.e., protein, lipid, DNA, RNA, carbohydrates, small-molecule pool). The details on the biomass components of P. pastoris were referred from Verduyn et al. (1991), Carnicer et al. (2009), De Schutter et al. (2009) and Tomas-Gamisans et al. (2016). The detailed information of biomass composition is described in the supporting information (see Additional file 2).
Constraint-based FBA
Constraint-based flux-balanced analysis (FBA) (Bordbar et al. 2014) was widely used in the reconstruction of GSMM model, analysis of strain capabilities under different environmental and genetic perturbations, and prediction of strain phenotype (Orth et al. 2010). Generally, FBA predicts metabolic fluxes based on maximization of objective functions (typically the specific growth rate or the target metabolite production rate) (Feist and Palsson 2010; Sanchez et al. 2012), and the basic framework of FBA is composed of variables, objectives, and constraints. The constraints used in FBA include the balance of intracellular metabolites (Eq. 2), the reversibility and the demarcation line of reactions, as well as the extracellular metabolite exchange rates (Eq. 3). In this study, all FBA was conducted with the cell growth rate as the objective function (Eq. 1). Each reaction had an upper and a lower bound on the flux it can carry. For reversible reaction, the upper and lower bounds were set at −1000 and 1000, respectively, while for irreversible reaction the lower bound was set at 0. FBA was conducted using Cobra Toolbox v2.0 (Schellenberger et al. 2011) and Gurobi 6 solver based on Matlab.
where S is a matrix of m*n and m and n are the numbers of metabolites and reactions, respectively. In Eq. 1, C T represents the coefficient of metabolites in the objective function. In Eq. 3, v is the rate of all reactions. lb and ub defined the lower and upper bounds of the flux for each reaction, respectively.
During the simulation with iRY1243, NGAM and GAM were set at 2.52 mmol ATP/gDCW h and 20.4 mmolATP/gDCW, respectively (see Additional file 3). The lower bound of exchange reactions except for glucose, ammonia, oxygen, sulfur, phosphorus, and Fe2+ was set at 0. To predict the specific growth rate (μ), the exchange rates of glucose were set at −0.478, −0.692, −0.942, −1.294, and −1.846 mmol/gDCW h, respectively. The exchange rates of other substrates and by-products were set at the corresponding values at each dilution rate.
Prediction of growth-supporting carbon and nitrogen sources
To investigate the prediction capability of iRY1243, 30 carbon and 21 nitrogen sources for P. pastoris were collected. FBA was used to analyze the growth capability on each carbon or nitrogen source. For prediction of carbon utilization, only NH4 + was set as the nitrogen source, while the phosphorus and sulfur sources were maintained sufficient. At the same time, the metabolites of the other exchange reactions containing carbons were set as zero. For prediction of nitrogen utilization, glucose was used as the only carbon source. The target carbon or nitrogen source was considered growth supporting if the predicted specific growth rate was above zero.
Prediction of essential genes
The prediction of essential genes and partially essential genes was conducted using the singleGeneDeletion function based on Cobra Toolbox. During the essential gene analysis, according to the matrix of GPRs and the Boolean rule definition, the fluxes of reactions containing the relating genes were set at zero, while the bounds of other reactions were maintained constant. Based on the simulated specific growth rate, the genes were classified into essential genes (the calculated specific growth rate was 0), partially essential genes (the calculated specific growth rate of P. pastoris was in the range from 0 to the maximum specific growth rate), and non-essential genes (the calculated specific growth rate was the same as the maximum specific growth rate). The synthetic medium, which was made up of glucose, oxygen, ammonia, sulfur, and phosphorus, was used to predict the essential genes for P. pastoris growth.
Transcriptome analysis
The strain P. pastoris G1HL was used. The medium and procedures for seed culture and chemostat cultivations were described in Nie et al. (2014) with glucose as the only carbon source.
During the stable phase of P. pastoris G1HL culture, about 20 mL broth was taken into three 50-mL centrifuge tubes. Samples were centrifuged at 4000 rpm for 10 min at 4 °C and supernatant was discarded. The harvested cells were washed three times with 0.9% NaCl and dried with a filter paper. The samples were stored in a −80 °C refrigerator. The RNA extraction and sequence, read mapping, quantification, and data analysis were accomplished by KangChen Bio-tech Inc. (Shanghai, China).
Results and discussion
Reconstruction of the P. pastoris GSMM iRY1243
The update of the P. pastoris GSMM was based on the iPP668, PpaMBEL1254, iLC915, and iMT1026 models. The procedure for reconstruction of P. pastoris is shown in Fig. 1. First, the elements and charge in all reactions were balanced based on the metabolites’ annotation information. Gap analysis was also conducted and gaps were reduced by adding essential reactions from KEGG and other databases. To overcome the deficiencies of these models, P. pastoris gene annotation information from three common databases (KEGG, UniProtKB, and IMG) was sorted and compared. Among them, KEGG was the largest metabolic network database.
Comparison of iRY1243 with the other models
As shown in Table 2, iRY1243 contains 2407 reactions and 1740 metabolites, which is more than that in the model of iMT1026 (Tomas-Gamisans et al. 2016), indicating that this upgrade enlarged the scale of the P. pastoris GSMM. The ORF number in iRY1243 is 1243, which is also more than that in iMT1026. Based on the latest gene annotations, the model iRY1243 contains more genes compared to the previous model iMT1026, such as the genes relating to the metabolism of porphyrin and chlorophyll, biotin, the membrane transport reactions, etc. The biomass contains the products of these pathways, which are crucial for the prediction of essential genes. iRY1243 is an upgraded P. pastoris GSMM with significant improvements in the metabolic coverage. In conclusion, the P. pastoris GSMM was comprehensively improved based on gene annotations from different databases and literatures (Additional file 1).
Accurate cell composition and NGAM value were important for improving the metabolic functions of GSMM. By referring to the latest GSMM of P. pastoris and Saccharomyces cerevisiae, the essential cell trace components were added, including the small-molecule pool (see Additional file 2). The NGAM was calculated by chemostat data (see Additional file 3).
Validation of GSMM
Model verification by RNA-Seq
The gene in the newly updated P. pastoris GSMM iRY1243 was firstly verified with the latest RNA sequence data. The RNA-Seq results showed that the expression of 2393 genes could be determined in the sampling condition. The iRY1243 contains 1243 genes and the expression of most genes (895 genes, 73.42%) could be verified according to the results of RNA-seq (Fig. 2a) when glucose was used as the sole carbon source. Removing the exchange reactions, among the remaining 1773 reactions, the expression of genes from 14.33% (254 reactions) of these reactions was not measured (Fig. 2b). After removing the exchange reactions and reactions without the corresponding annotated genes, there are 1042 and 471 reactions associated with single and multiple genes, respectively. Based on the transcriptome analysis, about 79.37% of the single-gene reactions and 91.93% of the multi-gene reactions were verified (Fig. 2c, d), indicating that the existence of most reactions in the model iRY1243 was reliable.
Verification of iRY1243 by RNA-seq data. Proportions of expressed and unexpressed genes to total genes involved in iRY1243 (a), proportion of verified reactions, unverified reactions, and no-gene annotation reactions in remained 1784 reactions (removing the exchange and transport reactions) (b), proportion of single-gene reactions with and without gene expression (c), and proportion of multi-gene reactions with and without gene expression (d)
Sensitivity analysis of iRY1243
During sensitivity analysis, the influence of different biomass components (lipid, protein, RNA, DNA, carbohydrate) and the energy parameters (NGAM) on the prediction accuracy of the strain phenotype was comprehensively investigated. As shown in Fig. 3, the specific growth rate (µ) and the specific oxygen uptake rate (qo2) were hardly affected by the changes of the protein, RNA, DNA, and carbohydrate contents. However, as the content of lipid increased, µ decreased. The reason may be that the synthesis of fatty acids requires more ATP and NADPH. Different from protein, RNA, DNA, carbohydrate, and the predicted µ and qo2 were more obviously affected by NGAM changes. As NGAM increased, the predicted µ decreased rapidly, while the qo2 increased accordingly as shown in Fig. 3f.
Effects of each parameter change on specific growth rate and specific oxygen uptake rate for sensitivity analysis with iRY1243. The simulations were performed by varying the lipid content (6.2–16.2%) (a1, a2), protein content (37–47%) (b1, b2), RNA content (6.6–16.6%) (c1, c2), DNA content (0.1–1%) (d1, d2), carbohydrate content (26.9–36.9%) (e1, e2), and the NGAN (1.26–3.78 mmol ATP/gDCW h) (f1, f2). Red represents the simulated results for the maximum values of the input parameters, and black represents the lower content in all cases
Model verification by growth capabilities on different carbon and nitrogen sources
To comprehensively evaluate the prediction ability of the model iRY1243, P. pastoris phenotype data reported from 1998 to 2017 were collected (Menendez et al. 1998; Hsieh et al. 2010; Klompmaker et al. 2010; Sohn et al. 2010; Zhang et al. 2015), together with physiological data from our lab. P. pastoris could grow on a series of carbon sources and nitrogen sources. The growth-supporting 30 carbon and 21 nitrogen sources can be found in Tables 3 and 4. The in silico growth capabilities of P. pastoris on these carbon and nitrogen sources were checked using iRY1243. As shown in Tables 3 and 4, growth on 30 carbon and 21 nitrogen sources could be predicted using iRY1243. Compared to model iMT1026 (Chung et al. 2010; Sohn et al. 2010; Caspeta et al. 2012; Nocon et al. 2014; Irani et al. 2016; Tomas-Gamisans et al. 2016), the prediction ability of iRY1243 was better.
Model verification by physiological growth parameters
The maximization of cell growth rate usually served as the objective function in FBA. Thus, a comparison between the measured and predicted μ values could show the quality of iRY1243. The reported NGAM of P. pastoris was 2.26 mmolATP/gDCW h (Chung et al. 2010), while, according to our calculation based on 13C labeled experiment, the NGAM was 9.48 mmolATP/gDCW h for P. pastoris G1HL (Nie et al. 2014). Due to the limitations of the central carbon metabolic network model used to calculate the fluxes of pathways, the model cannot contain all the ATP reactions. As the NGAM had an obvious effect on the specific growth rate, the NGAM in the iRY1243 was firstly calculated using P. pastoris G1HL chemostat cultivation data without by-product formation. We further compared the predicted and measured values with a series of chemostat cultivations using P. pastoris G1HL producing beta-galactosidase with the new NGAM value (2.52 mmol ATP/gDCW h). As shown in Fig. 4, iRY1243 could predict μ, qo2, qco2, and RQ under a range of the specific glucose consumption rate inputs.
To compare the function of each model, the chemostat data published by Carnicer et al. (2012) were used to verify the predictive function of the models (see Additional file 4). FBA was used to compare the predictive ability of each model with the maximization of cell growth rate as the objective function. The results showed that iRY1243 and iMT1026 could predict P. pastoris specific growth rate and CO2 specific consumption rate. However, the model iMT1026 predicted some unreasonable by-products (citric acid, etc.).
Model verification by in vivo 13C fluxes
To further evaluate the prediction performance of iRY1243, the simulated fluxes using pFBA were compared with the calculated metabolic flux from 13C labeled experiments (Nie et al. 2014). As shown in Fig. 5, the correlation coefficient between the simulated fluxes by GSMM and the calculated 13C fluxes for G1HL was 0.88, initially showing the good performance of iRY1243. However, there are still significant differences at some points, which may be caused due to the following reasons. Firstly, the algorithms used to calculate the fluxes were totally different between FBA and 13C central flux analysis. Secondly, there is a great difference in the size of the two models for the 13C flux model only containing the central carbon metabolism pathways. Generally, the 13C fluxes were more accurate as there are more constraints from 13C labeled metabolite information. On the whole, the high consistency between the simulated fluxes and the calculated 13C fluxes validated the good prediction performance of iRY1243. The PPP pathway, EMP pathway, and TCA cycle flux distribution can be well predicted (see Additional file 5).
Consistent changes in fluxes can be predicted using iRY1243 compared with 13C metabolic fluxes. The growth physiological parameters used for pFBA calculation, as well as the calculated 13C fluxes of P. pastoris G1HL, were described in previous research (Nie et al. 2014)
Prediction of essential genes
Based on the relationship of genes, proteins, and reactions in the genome-scale metabolic network model, the relationship between genotype and phenotype can be predicted (Thiele and Palsson 2010). The singleGeneDeletion function in Cobra Toolbox v2.0 can be used to predict the essential genes, partially essential genes, and non-essential genes (Schellenberger et al. 2011). On the synthetic medium, 123 essential genes were found, which were related to cofactor metabolism, TCA cycle, lipids biosynthesis, etc. (Fig. 6). At the same time, 169 partially essential genes were obtained (Additional file 6), which may be vital for subsequent in silico strain design (Pan and Hua 2012), considering the fact that the yield of target metabolite production may increase with the slight slowdown of growth.
To further evaluate the potential of the new P. pastoris GSMM in rational strain design, data of some engineered P. pastoris (the specific growth rate and S-adenosyl-l-methionine production) were collected from our lab and previously published studies. Then iRY1243 was used to simulate the effects of the corresponding gene insertion, deletion, or overexpression. The high consistency between the experimental and simulated results (see Additional file 7) indicated that iRY1243 can be used to design a more efficient P. pastoris for production of S-adenosyl-l-methionine or other heterogenous proteins.
Conclusion
Pichia pastoris GSMM was systematically upgraded based on the latest gene annotations and literatures. The resulting P. pastoris GSMM, iRY1243, accounts for 1243 ORFs and contains 2407 reactions and 1094 metabolites. The RNA-Seq data confirmed that the existence of most reactions in iRY1243 was reliable. iRY1243 showed good performance when predicting the cell growth physiological parameters, the growth capability on the reported carbon and nitrogen sources, the metabolic flux distribution with labeled glucose as the sole carbon source, the essential and partially essential genes, and the effects of gene deletion or overexpression on cell growth and S-adenosyl-l-methionine production. The significant improvement in the metabolic coverage and prediction ability make iRY1243 a potential platform for further systematic investigation of P. pastoris metabolism.
Abbreviations
- P. pastoris :
-
Pichia pastoris
- GSMM:
-
genome-scale metabolic network model
- GPRs:
-
gene–protein–reaction associations
- NGAM:
-
non-growth-associated maintenance
- GAM:
-
growth-associated ATP maintenance
- D:
-
dilution ratio
- DO:
-
dissolved oxygen
- DCW:
-
dry cell weight
- FBA:
-
constraint-based flux-balanced analysis
- μ :
-
specific growth rate
References
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol 98(12):5301–5317
Angione C, Conway M, Lio P (2016) Multiplex methods provide effective integration of multi-omic data in genome-scale models. BMC Bioinform 17:83
Baumann K, Maurer M, Dragosits M, Cos O, Ferrer P, Mattanovich D (2008) Hypoxic fed-batch cultivation of Pichia pastoris increases specific and volumetric productivity of recombinant proteins. Biotechnol Bioeng 100(1):177–183
Bhataya A, Schmidt-Dannert C, Lee PC (2009) Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem 44(10):1095–1102
Bordbar A, Monk JM, King ZA, Palsson BO (2014) Constraint-based models predict metabolic and associated cellular functions. Nat Rev Genet 15(2):107–120
Carnicer M, Baumann K, Toplitz I, Sanchez-Ferrando F, Mattanovich D, Ferrer P, Albiol J (2009) Macromolecular and elemental composition analysis and extracellular metabolite balances of Pichia pastoris growing at different oxygen levels. Microb Cell Fact 8:65
Carnicer M, ten Pierick A, van Dam J, Heijnen JJ, Albiol J, van Gulik W, Ferrer P (2012) Quantitative metabolomics analysis of amino acid metabolism in recombinant Pichia pastoris under different oxygen availability conditions. Microb Cell Fact 11:83
Caspeta L, Shoaie S, Agren R, Nookaew I, Nielsen J (2012) Genome-scale metabolic reconstructions of Pichia stipitis and Pichia pastoris and in silico evaluation of their potentials. BMC Syst Biol 6:24
Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24(1):45–66
Chung BKS, Selvarasu S, Andrea C, Ryu J, Lee H, Ahn J, Lee H, Lee DY (2010) Genome-scale metabolic reconstruction and in silico analysis of methylotrophic yeast Pichia pastoris for strain improvement. Microb Cell Fact 9:50
De Schutter K, Lin YC, Tiels P, Van Hecke A, Glinka S, Weber-Lehmann J, Rouze P, de Peer YV, Callewaert N (2009) Genome sequence of the recombinant protein production host Pichia pastoris. Nat Biotechnol 27(6):561–566
Famili I, Forster J, Nielson J, Palsson BO (2003) Saccharomyces cerevisiae phenotypes can be predicted by using constraint-based analysis of a genome-scale reconstructed metabolic network. Proc Natl Acad Sci USA 100(23):13134–13139
Feist AM, Palsson BO (2008) The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli. Nat Biotechnol 26(6):659–667
Feist AM, Palsson BO (2010) The biomass objective function. Curr Opin Microbiol 13(3):344–349
Fong SS, Marciniak JY, Palsson BO (2003) Description and interpretation of adaptive evolution of Escherichia coli K-12 MG1655 by using a genome-scale in silico metabolic model. J Bacteriol 185(21):6400–6408
Hsieh LS, Yeh CS, Pan HC, Cheng CY, Yang CC, Lee PD (2010) Cloning and expression of a phenylalanine ammonia-lyase gene (BoPAL2) from Bambusa oldhamii in Escherichia coli and Pichia pastoris. Protein Expr Purif 71(2):224–230
Irani ZA, Kerkhoven EJ, Shojaosadati SA, Nielsen J (2016) Genome-scale metabolic model of Pichia pastoris with native and humanized glycosylation of recombinant proteins. Biotechnol Bioeng 113(5):961–969
Kim TY, Sohn SB, Bin Kim Y, Kim WJ, Lee SY (2012) Recent advances in reconstruction and applications of genome-scale metabolic models. Curr Opin Biotechnol 23(4):617–623
Klompmaker SH, Kilic A, Baerends RJ, Veenhuis M, van der Klei IJ (2010) Activation of a peroxisomal Pichia pastoris d-amino acid oxidase, which uses d-alanine as a preferred substrate, depends on pyruvate carboxylase. FEMS Yeast Res 10(6):708–716
Kuberl A, Schneider J, Thallinger GG, Anderl I, Wibberg D, Hajek T, Jaenicke S, Brinkrolf K, Goesmann A, Szczepanowski R, Puhler A, Schwab H, Glieder A, Pichler H (2011) High-quality genome sequence of Pichia pastoris CBS7435. J Biotechnol 154(4):312–320
Maaheimo H, Fiaux J, Cakar ZP, Bailey JE, Sauer U, Szyperski T (2001) Central carbon metabolism of Saccharomyces cerevisiae explored by biosynthetic fractional C-13 labeling of common amino acids. Eur J Biochem 268(8):2464–2479
Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22(4):249–270
Mattanovich D, Callewaert N, Rouze P, Lin YC, Graf A, Redl A, Tiels P, Gasser B, De Schutter K (2009) Open access to sequence: browsing the Pichia pastoris genome. Microb Cell Fact 8:53
Menendez J, Delgado J, Gancedo C (1998) Isolation of the Pichia pastoris PYC1 gene encoding pyruvate carboxylase and identification of a suppressor of the pyc phenotype. Yeast 14(7):647–654
Morozkina EV, Vavilova EA, Zatcepin SS, Klyachko EV, Yagudin TA, Chulkin AM, Dudich IV, Semenkova LN, Churilova IV, Benevolensky SV (2016) Engineering of a system for the production of mutant human alpha-fetoprotein in the methylotrophic yeast Pichia pastoris. Appl Biochem Microbiol 52(2):170–175
Nie YS, Huang MZ, Lu JJ, Qian JC, Lin WL, Chu J, Zhuang YP, Zhang SL (2014) Impacts of high beta-galactosidase expression on central metabolism of recombinant Pichia pastoris GS115 using glucose as sole carbon source via C-13 metabolic flux analysis. J Biotechnol 187:124–134
Nocon J, Steiger MG, Pfeffer M, Sohn SB, Kim TY, Maurer M, Russmayer H, Pflugl S, Ask M, Haberhauer-Troyer C, Ortmayr K, Hann S, Koellensperger G, Gasser B, Lee SY, Mattanovich D (2014) Model based engineering of Pichia pastoris central metabolism enhances recombinant protein production. Metab Eng 24:129–138
Orth JD, Thiele I, Palsson BO (2010) What is flux balance analysis? Nat Biotechnol 28(3):245–248
Pan PC, Hua Q (2012) Reconstruction and in silico analysis of metabolic network for an oleaginous yeast, Yarrowia lipolytica. PLoS ONE 7(12):e51535
Saha R, Chowdhury A, Maranas CD (2014) Recent advances in the reconstruction of metabolic models and integration of omics data. Curr Opin Biotechnol 29:39–45
Sanchez CEG, Garcia CAV, Saez RGT (2012) Predictive potential of flux balance analysis of Saccharomyces cerevisiae using as optimization function combinations of cell compartmental objectives. PLoS ONE 7(8):e43006
Schellenberger J, Que R, Fleming RMT, Thiele I, Orth JD, Feist AM, Zielinski DC, Bordbar A, Lewis NE, Rahmanian S, Kang J, Hyduke DR, Palsson BO (2011) Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox v2.0. Nat Protoc 6(9):1290–1307
Sohn SB, Graf AB, Kim TY, Gasser B, Maurer M, Ferrer P, Mattanovich D, Lee SY (2010) Genome-scale metabolic model of methylotrophic yeast Pichia pastoris and its use for in silico analysis of heterologous protein production. Biotechnol J 5(7):705–715
Sola A, Maaheimo H, Ylonen K, Ferrer P, Szyperski T (2004) Amino acid biosynthesis and metabolic flux profiling of Pichia pastoris. Eur J Biochem 271(12):2462–2470
Thiele I, Palsson BO (2010) A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat Protoc 5(1):93–121
Tomas-Gamisans M, Ferrer P, Albiol J (2016) Integration and validation of the genome-scale metabolic models of Pichia pastoris: a comprehensive update of protein glycosylation pathways, lipid and energy metabolism. PLoS ONE 11(1):e0148031
Verduyn C, Stouthamer AH, Scheffers WA, van Dijken JP (1991) A theoretical evaluation of growth yields of yeasts. Antonie Van Leeuwenhoek 59(1):49–63
Zhang X, Liu J, Yu X, Wang F, Yi L, Li Z, Liu Y, Ma L (2015) High-level expression of human arginase I in Pichia pastoris and its immobilization on chitosan to produce l-ornithine. BMC Biotechnol 15:66
Authors’ contributions
RY conducted the experiments and wrote the manuscript. MH provided advice on the experiment design and data analysis. All authors read and approved the final manuscript.
Acknowledgements
This work was financially supported by the National Basic Research Program of China (No. 2013CB733600), the National Natural Science Foundation of China (No. 201276081), and the National Key Technology Support Program of China (No. 2011BAF02B05).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The dataset supporting the conclusions of this article is available.
Funding
National Basic Research Program of China (No. 2013CB733600). National Natural Science Foundation of China (No. 201276081). National Key Technology Support Program of China (No. 2011BAF02B05).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
40643_2017_152_MOESM7_ESM.docx
Additional file 7. Measured and simulated influences of gene deletion or overexpression on the growth and S-adenosyl-l-methionine production by P. pastoris.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ye, R., Huang, M., Lu, H. et al. Comprehensive reconstruction and evaluation of Pichia pastoris genome-scale metabolic model that accounts for 1243 ORFs. Bioresour. Bioprocess. 4, 22 (2017). https://doi.org/10.1186/s40643-017-0152-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-017-0152-x