Abstract
Background
Controlling blood pH during acute ventilatory failure and hypercapnia in individuals suffering from severe acute kidney injury (AKI) and undergoing continuous renal replacement therapy (CRRT) is of paramount importance in critical care settings. In this situation, the optimal concentration of sodium bicarbonate in the dialysate is still an unsolved question in critical care since high concentrations may worsen carbon dioxide levels and low concentrations may not be as effective in controlling pH.
Methods
We performed a randomized, non-blinded, experimental study. AKI was induced in 12 female pigs via renal hilum ligation and hypoventilation by reducing the tidal volume during mechanical ventilation with the goal of achieving a pH between 7.10–7.15. After achieving the target pH, animals were randomized to undergo isovolemic hemodialysis with one of two bicarbonate concentrations in the dialysate (40 mEq/L [group 40] vs. 20 mEq/L [group 20]).
Results
Hemodynamic, respiratory, and laboratory data were collected. The median pH value at CRRT initiation was 7.14 [7.12, 7.15] in group 20 and 7.13 [7.09, 7.14] in group 40 (P = ns). The median baseline PaCO2 was 74 [72, 81] mmHg in group 20 vs. 79 [63, 85] mmHg in group 40 (P = ns). After 3 h of CRRT, the pH value was 7.05 [6.95, 7.09] in group 20 and 7.12 [7.1, 7.14] in group 40 (P < 0.05), with corresponding values of PaCO2 of 85 [79, 88] mmHg vs. 81 [63, 100] mmHg (P = ns). The difference in pH after 3 h was due to a metabolic component [standard base excess −10.4 [−12.5, −9.5] mEq/L in group 20 vs. –7.6 [−9.2, −5.1] mEq/L in group 40) (P < 0.05)]. Despite the increased infusion of bicarbonate in group 40, the blood CO2 content did not change during the experiment. The 12-h survival rate was higher in group 40 (67% vs. 0, P = 0.032).
Conclusions
A higher bicarbonate concentration in the dialysate of animals undergoing hypercapnic respiratory failure was associated with improved blood pH control without increasing the PaCO2 levels.
Similar content being viewed by others
Background
As many as 25% of patients admitted to intensive care units require mechanical ventilation due to acute respiratory failure [1]. Acute kidney injury (AKI) is common in this scenario [2,3,4], resulting in nearly 20% of patients with hypercapnia and hypoxemic respiratory failure undergoing renal replacement therapy (RRT) [1].
Achieving an acceptable pH (≥7.20) through either bicarbonate infusion or RRT should mimic the physiological metabolic adaptation to respiratory acidemia [5, 6]. In chronically hypercapnic patients, renal adaptation to hypercapnia relies on elevating plasma bicarbonate and reducing serum chloride [7].
Notwithstanding, bicarbonate supplementation in patients with limited alveolar ventilation is a matter of controversy. Over 80% of the carbon dioxide (CO2) in blood exists in the form of bicarbonate, which is in equilibrium with other forms of CO2, including the dissolved portion that can be readily eliminated by the lungs [8]. Sodium bicarbonate infusions shift this equilibrium and generate more dissolved CO2. Indeed, sodium bicarbonate infusions have been associated with elevations of the partial pressure of arterial CO2 (PaCO2) [9] and deterioration of clinical status [10, 11].
Conversely, while CRRT with low-bicarbonate replacement fluids can avoid CO2 retention in patients with limited alveolar ventilation, it is less adequate at correcting acidemia. Therefore, the aim of this study was to investigate the effect of higher (40 mEq/L) and lower (20 mEq/L) dialysate concentrations of bicarbonate on blood pH in acidemic hypercapnic animals with severe AKI, which would address an unsolved question in critical care.
Methods
The swine Agroceres® from the breeder Minipig Pesquisa e Desenvolvimento (São Paulo, Brazil) with a median weight of 31 kg (range of 26–38 kg) was used. Animal care was conducted in accordance with institutional guidelines, and approval was obtained from the Animal Research Ethical Committee at the Teaching and Research Institute of Hospital Sírio Libanês, São Paulo, Brazil, under the protocol CEUA P 2013.09 (July 2013). The timeline of the experiment is shown in Additional file 1: Figure S1.
Anesthesia and instrumentation of animals
Anesthesia pre-induction was performed with intramuscular ketamine 5.0 mg/kg and midazolam 0.5 mg/kg. Anesthesia was induced using intravenous boluses of propofol 8 mg/kg and remifentanil 6 μg/kg and maintained during the entire experiment with starting doses of remifentanil at 6–12 μg/kg/h, midazolam 0.5–1.0 mg/kg/h, and propofol 2–5 mg/kg/h. Normal saline infusion at 20 ml/kg/h was maintained until the stabilization period. Additional normal saline boluses of 250 ml were administered if either the mean arterial pressure (MAP) decreased below 65 mmHg or the heart rate (HR) increased above 110 beats/min despite analgosedation.
Animals were connected to an Evita XL® mechanical ventilator (Dräger™, Lübeck, Germany) and ventilated with 8–10 ml/kg of tidal volume (VT), a positive end-expiratory pressure (PEEP) of 5 cm H2O, inspired fraction of oxygen (FiO2) to achieve a peripheral oxygen saturation between 94 and 96%, and a respiratory rate titrated to an end-tidal carbon dioxide pressure (ETCO2) between 30 and 35 mmHg. Airway pressures, VT, and ETCO2 were monitored through a NICO® system (Dixtal Biomedica Ind Com™, SP, Brazil). Electrocardiography, heart rate, oxygen saturation, and systemic pressures were monitored with a multiparametric monitor (Infinity Delta XL®, Dräger™, Lübeck, Germany).
A central line was placed in the left femoral vein for infusions, an arterial line was placed in the right femoral artery for blood sampling collection and invasive pressure measurement, a dialysis catheter (12-French, 16 cm, Arrow™, PA, USA) was placed in the right jugular vein, and a pulmonary artery catheter (Edwards Lifesciences™, Irvine, USA) was placed in the left jugular vein. The pulmonary artery pressures were monitored with a multiparametric monitor, and cardiac output was continuously measured with a Vigilance II® monitor (Edwards Lifesciences™, Irvine, USA). Via median laparotomy, we ligated the renal veins, arteries, and ureters, and a cystostomy tube was surgically placed. Analgosedation was continuously monitored via heart rate, spontaneous movements, and blood pressure. Animals were euthanized with a bolus infusion of 10 ml of potassium chloride 19.1% at the end of the experiment.
Stabilization period
After the instrumentation phase, animals underwent stabilization. The room temperature was set to 18 °C, and a Bair Hugger® external heating air blanket (3 M™, MN, USA) and Termopet® heating mattress (Styllus term™, São Paulo, Brazil) were adjusted to maintain the animal’s central temperature at 37–38 °C.
Hypercapnia induction
The tidal volume was reduced to 2/3 of the initial settings. After 1 h and every 15 min thereafter, arterial blood samples were drawn. If necessary, VT was adjusted by 0.5 ml/kg of body weight, aiming at a blood pH range of 7.10–7.15. Hypercapnia was considered stable if three consecutive arterial blood samples had a pH in the desired range and if the PaCO2 varied less than 3% in those measurements. After achieving stable hypercapnic conditions, baseline data were collected. After initiation of hypercapnia, if the MAP dropped below 65 mmHg, normal saline boluses were allowed up to 100 ml/kg, and persistent hypotension was managed with an infusion of norepinephrine.
Randomization of animals
Animals were randomly allocated using a closed box containing sealed envelopes indicating one of the two groups just before baseline data collection. The two groups consisted of a low-bicarbonate group (20 mEq/L, group 20) and a high-bicarbonate group (40 mEq/L, group 40). We arbitrarily planned an initial cohort of 18 animals. Initially, 12 animals (6:6 scheme) were randomized, after which an additional 6 animals (3:3 scheme) would be added if necessary.
Renal replacement therapy
Blood was pumped using a peristaltic portable roller pump with a flow controller (BsMed™, Guangzhou, China) at a rate of 6 ml/kg/min−, and the effluent and affluent were regulated by infusion pumps (Hospira™, II, USA) at a rate of 60 ml/kg/h in an isovolemic setting.
Affluent fluid control was checked by conducting infusion pump volume measurements and assessing the affluent weight loss using an available scale (Toledo do Brasil Ind Bal Ltda™, SP, Brazil). Compositions of the two dialysate solutions are shown in Additional file 2: Table S1. The filter used was a Fresenius F8® (Fresenius Medical Care™, MA, USA), a low-flow polysulphone filter with a K uf of 7.5 ml h−1 mmHg−1 and a surface area of 1.8 m2. The pressures in the extracorporeal system were continuously monitored in the arterial (P1), pre-filter (P2), and in the venous lines (P3) using a Dixtal 2020® multiparametric monitor (Dixtal Biomedica Ind Com™, SP, Brazil).
Anticoagulation was achieved with systemic heparin administration with a bolus of 80 IU/kg and maintenance at 20 IU kg−1 h−1. The doses of heparin were checked every 6 h and adapted to achieve an activated coagulation time with a target of 2.0–2.5 times baseline. Calcium gluconate was infused at a rate of 0.5–1.0 mg kg−1 h−1 of elementary calcium. The RRT efficacy was evaluated by the ratio of fluid urea nitrogen to blood urea nitrogen (FUN/BUN, a value of 1 denotes the most efficient clearance) [12].
Data collection and laboratory analysis
Venous and arterial blood samples were drawn for gas analysis and other laboratory tests (e.g., Na+, K+, Ca2+, Cl−, lactate, glucose, and hemoglobin). Data were collected after stabilization, during hypercapnia installation, and every hour until either death or 12 h after initiation of dialysis.
Venous blood samples for albumin, urea, creatinine, phosphate, and magnesium measurements were collected at the end of stabilization, upon hypercapnia installation, and 1, 3, 6, 9, and 12 h after dialysis initiation or until death. Dialysis effluent was sampled for the measurement of urea, creatinine, PCO2, and pH at 1, 3, 6, 9, and 12 h after dialysis initiation or until death.
Mg2+ was measured using a colorimetric technique, and phosphate was measured using an ultraviolet technique. Urea in both the blood and urine was measured with a kinetic technique, and albumin was measured with a bromocresol dye colorimetric technique. Blood gases, Na+, K+, Ca2+, Cl−, lactate, glucose, and hemoglobin, were analyzed on an OmniAnalyser (Roche Diagnostics System, F. Hoffmann-La Roche Ltd., Basel, Switzerland).
Calculated variables
-
Blood CO2 content (mL/min) [13] = (1−((0.0289 × Hb)/(3.352–0.456 × (SatbO2/100) × (8.142, pHb)))) × 2.226 × 0.0307 + (0.00057 × (37, temperature)) + (0.00002 × (37, temperature)2) × PbCO2 × (1 + 10 (pHb–6.086) + (0.042 × (7.4, pHb)) + ((38, temperature) × 0.00472 + (0.00139 × (7.4, pHb))))
-
Standard base excess (SBE–mEq/L) = 0.9287 × (HCO3−, 24.4 + 14.83 × (pH, 7.4)) [14]
Statistical analysis
The main outcome was pH changes during the 12 h of dialysis treatment. Given the lack of published data on this outcome, a sample size calculation was not performed. Animals began to expire after 4 h of CRRT; therefore, data up to the 3rd hour after CRRT initiation (baseline, 1 and 3 h) were used for comparisons. At those timepoints, the albumin, phosphate, magnesium, urea, and creatinine measurements were all available. Another variable planned for consideration in the interim analysis was cumulative survival (log-rank test) during the 12-h experiment. To avoid the unnecessary use of animals, a target P value of <0.05 for pH difference was intended in all interim analyses.
Continuous data are presented as medians [interquartile range]. Continuous data over time for both groups were analyzed using interaction analyses with a fixed-effect of a mixed generalized model using the animals as a random factor. Post hoc analyses for interactions were performed using the Mann-Whitney test for inter-group analyses and the Wilcoxon tests for intra-group analyses. P < 0.05 was considered statistically significant. Kaplan-Meier cumulative survival curves and the log-rank test were used to evaluate the survival time from CRRT initiation to either death or 12 h of CRRT. Comparisons of the data acquired the final hour before death (in an effort to understand the causes of death) were performed using the Mann-Whitney test. R-Free statistical software (Vienna, Austria, 2009) was used for analysis and graph construction [14].
Results
The study was stopped after the inclusion of the first 12 animals due to a statistically significant difference in the pH after 3 h of CRRT and in the survival between the groups. Hemodynamic, respiratory, and metabolic behavior of the animals during the PaCO2 equilibrium phase is shown in Additional file 3: Table S2. There were no differences between groups in the analyzed variables until the end of the PaCO2 equilibrium phase. After the induction of hypercapnia, we could notice that there was a drop in mean arterial pressure and pulmonary arterial pressure, and also, metabolic acidosis was induced along hypercapnic acidosis.
Figure 1 shows the pH, PaCO2, HCO3, and SBE values at baseline and after up to 12 h of CRRT. The pH upon initiating CRRT was 7.14 [7.12, 7.15] in group 20 and 7.13 [7.09, 7.14] in group 40 (P = ns). The pH value after 3 h of CRRT was 7.05 [6.95, 7.09] in group 20 and 7.12 [7.1, 7.14] in group 40 (P < 0.05). The PaCO2 was similar between the groups over time, and the variation in pH was due to the metabolic component as demonstrated by the elevated SBE in group 40 after 3 h of CRRT (−10.4 [−12.5, −9.5] mEq/L in group 20 vs. −7.6 [−9.2] in group 40) (P < 0.05). In Fig. 2 and Table 1, the metabolic components of the acid-base metabolism are described in detail, we could notice that SID was the main component of the acid-base metabolism that was different between both groups, and in Table 1, this difference in SID could be due to chloride levels which was significant higher in group 20. Additional file 4: Table S3 and Additional file 5: Table S4 show the hemodynamic and respiratory behavior of both groups from baseline to the last timepoint evaluated. The dose of norepinephrine was the only variable significantly different between groups, and higher dosage was needed in group 20, in order to keep hemodynamic targets described in the “Methods” section.
Temporal acid-base variation based on group. Panel a shows the pH changes over time (mixed model: group vs. pH interaction, P = 0.089; timepoint vs. pH interaction, P < 0.001; timepoint vs. group interaction, P = 0.003). Panel b shows the PaCO2 changes over time (mixed model: group vs. PaCO2 interaction, P = 0.913; timepoint vs. PaCO2 interaction, P = 0.311; timepoint vs. group interaction, P = 0.899). Panel c shows the SBE evolution over time (mixed model: group vs. SBE interaction, P = 0.02; timepoint vs. SBE interaction, P < 0.001, timepoint vs. group interaction, P < 0.001). Panel d shows the HCO3 changes over time (mixed model: group vs. HCO3 interaction, P = 0.401; timepoint vs. HCO3, P = 0.607; timepoint vs. group interaction, P = 0.664). SBE denotes standard base excess. Light gray boxplots were not used in analysis. *Wilcoxon post hoc analysis, P < 0.05 vs. baseline. #Mann-Whitney post hoc analysis, variable variation from baseline to 3 h; P < 0.05 vs. group 40. $Mann-Whitney post hoc analysis, P < 0.05 vs. group 40
Temporal metabolic determinants of SBE variation based on group. Panel a shows the SID changes over time (mixed model: group vs. SIDa interaction, P = 0.021; timepoint vs. SIDa interaction, P < 0.001; timepoint vs. group interaction, P < 0.001). Panel b shows the SIG changes over time (mixed model: group vs. SIG interaction, P = 0.483; timepoint vs. SIG interaction, P = 0.797; timepoint vs. group interaction; P = 0.364). Panel c shows the lactate changes over the time (mixed model: group vs. lactate interaction, P = 0.473; timepoint vs. lactate interaction, P = 0.936; timepoint vs. group interaction, P = 0.871). Panel d shows the albumin changes over time (mixed model: group vs. albumin interaction, P = 0.182; timepoint vs. albumin interaction, P = 0.833; timepoint vs. group interaction, P = 0.432). Panel e shows the phosphate changes over time (mixed model: group vs. phosphate interaction, P = 0.565; timepoint vs. phosphate interaction, P = 0.450; timepoint vs. group interaction, P = 0.531). SID denotes strong ion difference. SIG denotes strong ion gap. SBE denotes standard base excess. *Wilcoxon post hoc analysis, P < 0.05 vs. baseline. $Mann-Whitney post hoc analysis, P < 0.05 vs. group 40
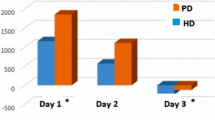
The survival rate was clearly higher in group 40, and earliest death in group 20 occurred 4 h after initiating CRRT (Fig. 3). The median time of CRRT was 420 [225, 540] min in group 20 and 720 [450, 720] min in group 40 (P = 0.062). In the last hour before death, the pH was 6.97 [6.92, 7.03] vs. 7.03 [6.95, 7.04] (P = 0.630), the SBE was −18.2 [−20.3, −15.3] vs. −9.7 [−17.5, −5.9] mEq/L (P = 0.240), and the PaCO2 was 69 [65, 80] vs. 69 [55, 105] mmHg (P = 0.818) in groups 20 vs. 40, respectively. Among the hemodynamic variables measured in the last hour, only the systemic arterial pressure was statistically lower in group 20 than in group 40 (Additional file 3: Table S2).
The CRRT efficacy variables recorded during the entire experiment are shown in Additional file 6: Table S5. CRRT efficacy variables were equals between groups, and the cumulative affluent and effluent volumes were obvious higher in group 40 due to longer CRRT duration in this arm. CO2 content in the blood from the arterial and venous lines, as well as the CO2 content in the effluent fluid, are shown in Fig. 4.
Temporal changes of CO2 content over RRT. Panel a shows the venous CO2 content evolution over the time (mixed model: group vs. venous CO2 content interaction, P = 0.230; timepoint vs. venous CO2 content interaction, P < 0.001; timepoint vs. group interaction, P < 0.001). Panel b shows the arterial CO2 content changes over time (mixed model: group vs. arterial CO2 content interaction, P = 0.231; timepoint vs. arterial CO2 content interaction, P < 0.001; timepoint vs. group interaction, P < 0.001). Panel c shows the venous-arterial CO2 gradient changes over time (mixed model: group vs. venous-arterial CO2 gradient interaction, P = 0.815; timepoint vs. venous-arterial CO2 gradient interaction, P = 0.919; timepoint vs. group interaction, P = 0.972). Panel d shows the effluent CO2 content changes over time (mixed model: group vs. effluent CO2 content interaction, P = 0.769; timepoint vs. effluent CO2 content interaction, P = 0.136; timepoint vs. group interaction, P = 0.242), for the effluent CO2 content, the baseline data were excluded. *Wilcoxon post hoc analysis, P < 0.05 vs. baseline
Discussion
This study was designed to answer the clinical dilemma of bicarbonate concentrations in the dialysate of CRRT during hypercapnia by addressing which of two concentrations—40 or 20 mEq/L—resulted in better control of blood pH in hypercapnic animals with severe AKI. Our findings support that a higher bicarbonate dialysate concentration in acidemic hypercapnic animals has a better effect on pH control as early as 3 h after CRRT initiation as shown by a median reduction of 15% in the H+ concentration from a pH of 7.05 in group 20 to a pH of 7.12 in group 40; additionally, the increased concentration had a significant impact on mortality (100% in group 20 vs. 33% in group 40). Improved pH control was due to superior metabolic compensation secondary to the effects of CRRT, which was demonstrated by a higher SBE after 3 h. Interestingly, the total blood content of CO2 was not different between the groups, showing that respiratory component did not worsen despite the increased bicarbonate concentration in the dialysate.
Although the initial pH of our study seems to be extremely low, it is in accordance with the refractory academia definition used by Meade et al. and has been observed in 7% of patients with acute respiratory failure [15]. Carbon dioxide transport in the blood occurs in three forms as follows: dissolved in plasma (~5%), as bicarbonate (~80%), or bound to hemoglobin (~15%) [16]. Thus, one could argue that elevated bicarbonate concentrations in the dialysate can potentially worsen hypercapnia, especially in patients with limited alveolar ventilation [9,18,, 17–19]. Conversely, removal of bicarbonate by CRRT with a low bicarbonate concentration in the dialysate (which has sieving and diffusing coefficients close to 1) could reduce total CO2 [20].
The blood pH of both groups decreased 1 h after initiating CRRT due to the metabolic content and was secondary to the increase in strong ion gap (SIG), which could be explained by the increase in unmeasured anions other than lactate such as pyruvate, sulphate, and citrate. In group 40, the pH returned to baseline levels 3 h after CRRT initiation, while in group 20, the pH continued to worsen. Worsening of acidemia in group 20 was mainly due to the metabolic component as indicated by the lower SBE and strong ion difference (SID) in this group. These findings support the use of CRRT with low-chloride and high-sodium bicarbonate solutions in hypercapnic animals to achieve better pH control.
Our findings could be well explained by the acid-base disturbance theory described by Peter Stewart [21], where pH and the H+ concentration are determined by the strong ion difference (SID), the total concentration of non-volatile weak acid (ATOT), and PCO2. The improved control of blood pH in the animals in group 40 was achieved by higher SID values, due to lower chloride infusion in this group.
Indeed, normally functioning kidneys exhibit similar results [7]. In healthy volunteers, CO2 retention triggers a metabolic response via renal sodium retention [22]. Rabbits exposed to high CO2 partial pressure (10%) for 52–56 h reabsorb bicarbonate through the Na+/HCO3 − co-transporter, resulting in increased serum bicarbonate and decreased serum chloride [23]. A link between hypochloremia and hypercapnia may manifest from the bicarbonate/chloride exchanger pendrin located in the apical domain of type B and non-A-non-B intercalated cells [24], and the expression of pendrin is down-regulated during hypercapnia, which provides collecting tubules the potential for increased bicarbonate reabsorption and chloride excretion [25]. Finally, these adaptations occur in acute as well as in chronic settings, with elevated urinary chloride excretion within 30 min after hypercapnia induction [7, 26, 27].
On average, the PaCO2 levels were similar between the groups. There are three non-mutually exclusive possible explanations for this observation. First, this result could be explained by Gattinoni’s “open system” theory in which CO2 clearance by the lungs attenuates the effect on blood pH of CO2 elevation secondary to sodium bicarbonate infusion; this theory contrasts the “closed system” theory in which the lungs are unable to clear CO2. In the latter situation, the lungs are unable to eliminate CO2 generated by the infusion of sodium bicarbonate; therefore, despite sodium infusion, an elevation on serum pH is not observed [28]. Acute respiratory distress syndrome, chronic obstructive pulmonary disease, and asthma are examples of “open systems” in which low-effective alveolar ventilation is associated with a preserved alveoli-capillary function [29, 30], and any increase in venous PaCO2 is associated with increased CO2 transfer in respiratory membranes [31]. In our study, we expected an increase in the total CO2 venous blood content in group 40 secondary to the influx of bicarbonate from dialysate, which would result in increased CO2 transfer in the lungs. However, neither the venous blood CO2 content nor the venous-arterial difference of CO2 was different between the groups; thus, it is difficult to ascribe the lack of PaCO2 elevation to enhanced pulmonary elimination. Second, CO2 has a large volume of distribution across different tissues and binding molecules, which might have blunted the observed effect in terms of PaCO2 (which is measured in the serum) [17, 32]. Finally, a slow infusion of bicarbonate can increase pH without significantly elevating PaCO2 [33]. Intermittent dialysis, which demands high dialysate flow, is known to be related to rising serum CO2 [10, 19]. On the other hand, a long-term CRRT with a high concentration of bicarbonate (32 mEq/L) has a non-significant effect on PaCO2 [34].
The survival was clearly different between the groups. All animals in group 20 died within the first 12 h of CRRT with a lower MAP despite the administration of high dosages of norepinephrine, nominally elevated pulmonary vascular resistance and reduced right ventricle systolic work (although these differences were not statistically significant). These findings strongly suggest that hemodynamic failure in group 20 was mainly associated with pulmonary-related right ventricle dysfunction, which could be secondary to more accentuated acidemia [35].
There were several potential limitations of our study. First, the sample size was small but in accordance with the animal research principles regarding the utilization of the lowest number of animals possible. Second, as an experimental study, the results cannot be directly extrapolated to the clinic; however, our results can serve as an important guide to building the rationale of CRRT during hypercapnia. Third, we did not assess the long-term effects of elevated PaCO2 levels on physiology and outcomes, which requires further extensive discussion [36]; this limitation would have been mitigated if we found a significant difference in PaCO2 between the groups. Fourth, we only studied healthy lungs, which, although they were severely hypoventilated, had preserved alveolocapillary membranes. Fifth, our model of kidney injury induction may cause an inflammatory response and hypercatabolism; however, this response is only found in acute and critically ill subjects. Finally, blinding was not possible because the investigators were responsible for preparing the dialysate solution.
Conclusions
Dialysate with 40 mEq/L of sodium bicarbonate is more efficient than 20 mEq/L in controlling pH during acidemia in hypercapnic animals with severe AKI. The clinical concern that respiratory acidemia may worsen because of a high bicarbonate concentration was not supported in this experimental study.
Abbreviations
- AKI:
-
Acute kidney injury
- BUN:
-
Blood urea nitrogen
- CO2 :
-
Carbon dioxide
- CRRT:
-
Continuous renal replacement therapy
- ETCO2 :
-
End-tidal carbon dioxide pressure
- FiO2 :
-
Fraction inspired of oxygen
- FUN:
-
Fluid urea nitrogen
- HCO3 − :
-
Bicarbonate
- MAP:
-
Mean arterial pressure
- Na+ :
-
Sodium
- PEEP:
-
Positive end-expiratory pressure
- SBE:
-
Standard base excess
- VT :
-
Tidal volume
References
Azevedo LC, Park M, Salluh JI, Rea-Neto A, Souza-Dantas VC, Varaschin P et al (2013) Clinical outcomes of patients requiring ventilatory support in Brazilian intensive care units: a multicenter, prospective, cohort study. Crit Care 17(2):R63
Xue JL. et al (2006) Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol. 17:1135–42
Barakat MF, McDonald HI, Collier TJ, Smeeth L, Nitsch D, Quint JK (2015) Acute kidney injury in stable COPD and at exacerbation. Int J Chron Obstruct Pulmon Dis 10:2067–77
National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT et al (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354(24):2564–75
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G et al (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338(6):347–54
Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The New England journal of medicine. 342(18):1301–8.
Alfaro V, Torras R, Ibanez J, Palacios L (1996) A physical-chemical analysis of the acid-base response to chronic obstructive pulmonary disease. Can J Physiol Pharmacol 74(11):1229–35
Cressoni M, Zanella A, Epp M, Corti I, Patroniti N, Kolobow T et al (2009) Decreasing pulmonary ventilation through bicarbonate ultrafiltration: an experimental study. Crit Care Med 37(9):2612–8
Mathieu D, Neviere R, Billard V, Fleyfel M, Wattel F (1991) Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study. Crit Care Med 19(11):1352–6
Tovbin D, Heimer D, Mashal A, Degtyar P, Avnon LS (2001) Intradialytic hypercapnic respiratory failure managed by noninvasive assisted ventilation. Am J Nephrol 21(5):383–5
Palacios CRF, Altayeh A, Qian Q (2011) Acute hypercapnic respiratory failure associated with hemodialysis. Dialysis Transplantation 40(2):83
Claure-Del Granado R, Macedo E, Chertow GM, Soroko S, Himmelfarb J, Ikizler TA et al (2011) Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol 6(3):467–75
Douglas AR, Jones NL, Reed JW (1988) Calculation of whole blood CO2 content. J Appl Physiol 65(1):473–7
Team RDC (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing
Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA: the journal of the American Medical Association. 299(6):637–45
Christiansen J, Douglas CG, Haldane JS (1914) The absorption and dissociation of carbon dioxide by human blood. J Physiol 48(4):244–71
Levraut J, Garcia P, Giunti C, Ichai C, Bouregba M, Ciebiera JP et al (2000) The increase in CO2 production induced by NaHCO3 depends on blood albumin and hemoglobin concentrations. Intensive Care Med 26(5):558–64
Sombolos KI, Bamichas GI, Christidou FN, Gionanlis LD, Karagianni AC, Anagnostopoulos TC et al (2005) pO2 and pCO2 increment in post-dialyzer blood: the role of dialysate. Artif Organs 29(11):892–8
Henrich WL, Woodard TD, Meyer BD, Chappell TR, Rubin LJ (1983) High sodium bicarbonate and acetate hemodialysis: double-blind crossover comparison of hemodynamic and ventilatory effects. Kidney Int 24(2):240–5
Geers C, Gros G (2000) Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80(2):681–715
Stewart PA (1983) Modern quantitative acid-base chemistry. Can J Physiol Pharmacol 61(12):1444–61
Longson D, Mills JN (1953) The failure of the kidney to respond to respiratory acidosis. J Physiol 122(1):81–92
Krapf R (1989) Mechanisms of adaptation to chronic respiratory acidosis in the rabbit proximal tubule. J Clin Invest 83(3):890–6
Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM et al (2002) Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283(4):F744–54
de Seigneux S, Malte H, Dimke H, Frokiaer J, Nielsen S, Frische S (2007) Renal compensation to chronic hypoxic hypercapnia: downregulation of pendrin and adaptation of the proximal tubule. Am J Physiol Renal Physiol 292(4):F1256–66
Dorman PJ, Sullivan WJ, Pitts RF (1954) The renal response to acute respiratory acidosis. J Clin Invest 33(1):82–90
Ramadoss J, Stewart RH, Cudd TA (2011) Acute renal response to rapid onset respiratory acidosis. Can J Physiol Pharmacol 89(3):227–31
Gattinoni L, Taccone P, Carlesso E (2006) Respiratory acidosis: is the correction with bicarbonate worth? Minerva Anestesiol 72(6):551–7
Gattinoni L, Pesenti A (2005) The concept of “baby lung”. Intensive Care Med 31(6):776–84
Oddo M, Feihl F, Schaller MD, Perret C (2006) Management of mechanical ventilation in acute severe asthma: practical aspects. Intensive Care Med 32(4):501–10
Park M, Costa EL, Maciel AT, Silva DP, Friedrich N, Barbosa EV et al (2013) Determinants of oxygen and carbon dioxide transfer during extracorporeal membrane oxygenation in an experimental model of multiple organ dysfunction syndrome. PLoS One 8(1):e54954
Lindinger MI, Franklin TW, Lands LC, Pedersen PK, Welsh DG, Heigenhauser GJ (1999) Role of skeletal muscle in plasma ion and acid-base regulation after NaHCO3 and KHCO3 loading in humans. Am J Physiol 276(1 Pt 2):R32–43
Abeysekara S, Zello GA, Lohmann KL, Alcorn J, Hamilton DL, Naylor JM (2012) Infusion of sodium bicarbonate in experimentally induced metabolic acidosis does not provoke cerebrospinal fluid (CSF) acidosis in calves. Can J Vet Res 76(1):16–22
Allegretti AS, Flythe JE, Benda V, Robinson ES, Charytan DM (2015) The effect of bicarbonate administration via continuous venovenous hemofiltration on acid-base parameters in ventilated patients. Biomed Res Int 2015:901590
Carvalho CR, Barbas CS, Medeiros DM, Magaldi RB, Lorenzi FG, Kairalla RA et al (1997) Temporal hemodynamic effects of permissive hypercapnia associated with ideal PEEP in ARDS. Am J Respir Crit Care Med 156(5):1458–66
Curley GF, Laffey JG, Kavanagh BP (2013) CrossTalk proposal: there is added benefit to providing permissive hypercapnia in the treatment of ARDS. J Physiol 591(Pt 11):2763–5
Acknowledgements
The authors are grateful to the Hospital Sírio Libanês Teaching and Research Institute and all the employees that contributed to the experiments.
Funding
Funding was obtained from the Hospital Sírio Libanês Teaching and Research Institute.
Availability of data and materials
Data are available by contacting the corresponding author.
Authors’ contributions
TGR, LCPA, PVM, ELCV and MP contributed to the study conception and design. TGR and MP carried out the study experiments. TGR and MP performed the data and statistical analysis. TGR and MP drafted the manuscript. TGR, LCPA, PVM, ELCV and MP wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethics approval was obtained from the Animal Research Ethical Committee of the Teaching and Research Institute at Hospital Sírio Libanês, São Paulo, Brazil, under the protocol CEUA P 2013.09 (July 2013).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Figure S1.
Timeline of the study. After the CRRT initiation, arterial blood gas analyses were collected every hour; however, the highlighted points in the figure were the analyzed timepoints. (DOCX 19 kb)
Additional file 2: Table S1.
Dialysate composition of the two solutions used in the experiment (DOCX 14 kb)
Additional file 3: Table S2.
Hemodynamics and respiratory and metabolic variables collected during the equilibrium phase. * Wilcoxon’s test for paired samples. (DOCX 18 kb)
Additional file 4: Table S3.
Hemodynamic variables categorized throughout the study according to group. Data are shown as the median [25th percentile, 75th percentile]. § Only the timepoints at baseline and at 1, 2, and 3 h after CRRT initiation were statistically analyzed. *Mixed model timepoint vs. variable interaction. #Mixed model group vs. variable interaction. $Wilcoxon post hoc analysis, P < 0.05 vs. baseline. @ Mann-Whitney post hoc analysis, P < 0.05 vs. group 40. &Mixed model timepoint vs. group interaction, P = 0.001. Mann-Whitney post hoc analysis of chloride variations from baseline to 3 h, P < 0.05 vs. group 40. %Mixed model timepoint vs. group interaction, P = 0.046. Mann-Whitney post hoc analysis of calcium variations from baseline to 3 h, P < 0.05 vs. group 40. **Mann-Whitney, P < 0.05 vs. group 40. (DOCX 25 kb)
Additional file 5: Table S4.
Respiratory variables categorized throughout the study based on the group. Data are shown as the median [25th percentile, 75th percentile]. § Only the data collected at baseline and at 1, 2, and 3 h after initiating CRRT were statistically analyzed. *Mixed model timepoint vs. variable interaction. #Mixed model group vs. variable interaction. (DOCX 21 kb)
Additional file 6: Table S5.
Hemodialysis quality assessment. Data are shown as the median [25th percentile, 75th percentile]. *Mixed model timepoint vs. variable interaction. #Mixed model group vs. variable interaction. (DOCX 21 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Romano, T.G., Azevedo, L.C.P., Mendes, P.V. et al. Effect of continuous dialysis on blood pH in acidemic hypercapnic animals with severe acute kidney injury: a randomized experimental study comparing high vs. low bicarbonate affluent. ICMx 5, 28 (2017). https://doi.org/10.1186/s40635-017-0141-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-017-0141-6