Abstract
Purpose
To compare loop elongation after 5000 cycles, loop-elongation at failure, and load at failure of the fixed-loop G-Lok device and three adjustable-loop devices (UltraButton, RigidLoop Adjustable and ProCinch RT), during testing over extended cycles under high loading.
Methods
Five devices of each type were tested on a custom-built rig fixed to an Instron machine. The testing protocol had four stages: preloading, cyclic preconditioning, incremental cyclic loading and pull-to-failure. Outcome measures were loop elongation after 5000 cycles, loop-elongation at failure, and load at failure.
Results
The loop elongation after 5000 cycles for G-Lok was 1.46 ± 0.25 mm, which was comparable to that of RigidLoop (1.51 ± 0.16 mm, p = 1.000) and ProCinch (1.60 ± 0.09 mm, p = 1.000). In comparison, the loop elongation for UltraButton was 2.66 ± 0.28 mm, which was significantly larger than all other devices (p = 0.048). The failure load for all devices ranged between 1455 and 2178 N. G-Lok was significantly stronger than all adjustable-loop devices (p = 0.048). The elongation at failure was largest for UltraButton (4.20 ± 0.33 mm), which was significantly greater than G-Lok (3.17 ± 0.33 mm, p = 0.048), RigidLoop (2.88 ± 0.20 mm, p = 0.048) and ProCinch (2.78 ± 0.08 mm, p = 0.048). There was no significant difference in elongation at failure for the rest of the devices.
Conclusions
Our study has shown that the G-Lok fixed-loop device and the three adjustable-loop devices (UltraButton, RigidLoop Adjustable and ProCinch RT) all elongated less than 3 mm during testing over an extended number of cycles at high loads, nonetheless, the fixed loop device performed best in terms of least elongation and highest load at failure.
Similar content being viewed by others
Background
Symptomatic knee instability after anterior cruciate ligament (ACL) injury may require reconstruction of the ACL with an auto- or allo- graft, which is fixed to the tibia and femur using interference screws, transfixation pins or cortical suspension loop devices [15, 20, 22, 36]. The most suitable femoral fixation technique is debatable, but cortical suspension fixed-loop devices give good, reproducible results [1].
The more recent cortical suspension adjustable-loop devices have several advantages: (1) they are easier to use in short femoral tunnels, with placement through the antero-medial arthroscopic portal; (2) they allow more of the femoral tunnel to be filled with graft, and shorter graft lengths can be used, as seen with tripling/quadrupling of the graft; (3) they are suitable for most tunnel sizes, eliminating the need for fixed-loop devices with different loop sizes [9, 14, 16, 18]. However, there are concerns about the elongation of cortical suspension adjustable-loop devices under cyclic loading post-fixation, which relate to the button-locking mechanism [26]. Studies show that cortical suspension fixed-loop devices elongate less than adjustable-loop devices, the latter has been shown to elongate by more than 3 mm, which introduces knee instability and is regarded as a clinical failure [13, 19].
There have been a variety of studies investigating the elongation and failure load of fixed-loop cortical suspension devices in vitro, however, to the authors’ knowledge there has been only one study testing the fixed-loop G-Lok device, and this was performed under low loads and a low number of cycles. The aim of this study was to compare the loop elongation after 5000 cycles, the loop-elongation at failure, and the load at failure of the fixed-loop G-Lok device and three adjustable-loop devices (UltraButton, RigidLoop Adjustable and ProCinch RT), during testing over an extended number of cycles under high loading. The authors hypothesised that the fixed-loop device would have a lower elongation after 5000 cycles and at failure, as well as a higher failure load, than the adjustable-loop devices.
Methods
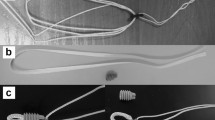
The G-Lok (Stryker Sports Medicine, Greenwood Village, Colorado, USA) fixed-loop device was compared against three adjustable-loop devices: UltraButton (Smith & Nephew, Andover, Massachusetts, USA), RigidLoop Adjustable (DePuy Mitek, Raynham, Massachusetts, USA), and ProCinch RT (Stryker Sports Medicine, Greenwood Village, Colorado, USA). All four devices consist of a loop and a locking-button, the loops on the adjustable devices have free ends to adjust the size of the loop (Fig. 1).
Fixed and adjustable femoral cortical suspension devices tested in the current study: a G-Lok (Stryker Sports Medicine, Greenwood Village, Colorado, USA), b UltraButton (Smith & Nephew, Andover, Massachusetts, USA), c RigidLoop Adjustable (DePuy Mitek, Raynham, Massachusetts, USA), and (d) ProCinch RT (Stryker Sports Medicine, Greenwood Village, Colorado, USA)
The three adjustable-loop devices were tightened to 20 mm to match the size of the fixed-loop device, using a custom-built 20 mm diameter cylinder, and confirmed using a Vernier calliper (Fig. 2). The devices were adjusted by pulling the free ends with a slow rocking motion, as recommended by the manufacturers [24, 32,33,34]. A trained technician was required to adjust the UltraButton loop according to the manufacturer’s strict protocol involving additional sideways movements; variations in this technique can affect the performance of this device, and lead to failure during testing.
Five devices of each type were tested on a simple, custom-built rig fixed to an Instron machine (Instron, Illinois Tool Works Inc., Norwood, Massachusetts, USA). Similarly to previous studies the rig comprised a bottom-mount attached to the baseplate, a top-mount attached to the crosshead and 5 kN load cell, and a 4.5 mm horizontal steel rod held between two holes in the top-mount (Fig. 3a) [3, 17]. In accordance to the manufacturer’s recommendations for the four devices, the loop of each device was fed upwards through a 5 mm deep and 4.5 mm diameter hole, representing the drilled femoral tunnel, until the button of the loop lay flat against the lower surface [24, 32,33,34]. The steel rod, representing the graft, was inserted through the loop, avoiding tension in the loop. When the loop was correctly positioned, the crosshead of the machine was moved upwards to remove any slack, until a 1 N load was measured by the load cell (Fig. 3b).
Testing was performed on a simple, custom-built rig, fixed to an Instron machine (Instron, Illinois Tool Works Inc., Norwood, Massachusetts, USA). The rig comprised a bottom-mount attached to the baseplate, a top-mount attached to the crosshead and 5 kN load cell, and a 4.5 mm horizontal steel rod held between two holes in the top-mount
The testing protocol had four stages: preloading, cyclic preconditioning, incremental cyclic loading and pull-to-failure (Fig. 4). A 20 N preload was first applied to simulate intraoperative tensioning, this was followed by 10 preconditioning cycles at 1 Hz with loads between 20 and 70 N, to simulate the surgeon bending the knee before fixation. After preconditioning the loops were re-tensioned using the same technique as during initial tensioning [17]. Upon completion, the elongation of the device was recorded and reset to 0. The incremental loading phase involved 5000 cycles at 1 Hz, with loads between 20 and 520 N, increasing in increments of 50 N, this simulated the forces that occur in the ACL graft during the initial phase of postoperative rehabilitation [28, 30, 35]. A test to failure was then performed at a rate of 20 mm/min.
The testing protocol had four stages: preloading, cyclic preconditioning, incremental cyclic loading and pull-to-failure. A 20 N preload was first applied, followed by 10 preconditioning cycles at 1 Hz with loads between 20 and 70 N, incremental loading involved 5000 cycles at 1 Hz with loads between 20 and 520 N, lastly a test to failure was performed at a rate of 20 mm/min
Load-displacement data was recorded using the Bluehill software (Instron, Illinois Tool Works Inc., Norwood, Massachusetts, USA). Outcome measures were loop elongation after 5000 cycles, loop-elongation at failure, and load at failure.
Statistical analysis
Using a sample-size calculator for a two-sample t-test (MiniTab Inc., State College PA, USA) at 80% power, it was estimated that testing five samples of each device would allow detection of a 0.3 mm difference in elongation, which represents 10% of the clinical laxity limit, or failure [13, 19].
Descriptive statistics were used to summarise the data. Comparisons between the devices were performed using Kruskal-Wallis tests, for loop elongation after 5000 cycles, elongation at failure and ultimate failure load, in addition, Wilcoxon rank sum tests were used to perform pairwise comparisons between devices, with corrections for multiple testing. Statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). A p-value of 0.05 was used to represent a statistically significant difference.
Results
The loop elongation after 5000 cycles for the G-Lok fixed-loop device was 1.46 ± 0.25 mm, which was comparable to that of the RigidLoop Adjustable (1.51 ± 0.16 mm, p = 1.000) and ProCinch RT (1.60 ± 0.09 mm, p = 1.000) (Tables 1 and 2). In comparison, the loop elongation for the UltraButton was 2.66 ± 0.28 mm, which was significantly larger than all other devices (p = 0.048).
The failure load for all devices ranged between 1455 and 2178 N (Table 1). The G-Lok fixed-loop device was significantly stronger than all adjustable-loop devices (p = 0.048), while ProCinch RT was significantly weaker than all other devices (p = 0.048) and there was no significant difference between UltraButton and RigidLoop Adjustable (p = 0.690). The most common method of device failure, which was seen in all devices, was breakage of the loop at the button level. In addition, the button itself also broke in some samples of G-Lok and UltraButton.
The elongation at failure was largest for the UltraButton adjustable-loop device (4.20 ± 0.33 mm), which was significantly greater than G-Lok (3.17 ± 0.33 mm, p = 0.048), RigidLoop Adjustable (2.88 ± 0.20 mm, p = 0.048) and ProCinch RT (2.78 ± 0.08 mm, p = 0.048) (Tables 1 and 2). There was no significant difference in elongation at failure for the rest of the devices.
The maximum loop elongation occurred for all specimens during the application of the 20 N preload, and the second largest elongation occurred during preconditioning between 20 and 70 N (Fig. 5). The elongation increased incrementally between cycle 1 and 5000 (Fig. 6).
Discussion
The most important finding of this study is that the fixed-loop G-Lok device and the three adjustable-loop devices all had mean elongations after 5000 cycles that were less than 3 mm. Thus, they all have the necessary biomechanical properties, in terms of reduced loop elongation and high failure load, for initial fixation of soft tissue grafts, when tested under extended cycles and high loads. Our study has been the first to compare the G-Lok cortical suspension fixed-loop device against the Ultrabutton, RigidLoop Adjustable and ProCinch RT adjustable-loop devices over an extended number of cycles (5000) at high loads (500 N), thus more closely replicating the early rehabilitation period in vitro.
Adjustable-loop femoral cortical suspension devices offer several advantages over fixed-loop devices [14, 16], however, there are concerns about their tendency to elongate when subjected to biomechanical loading, which may compromise the effective length of the graft-loop construct [3, 17, 25, 26]. The length of the graft and fixation device construct is critical during the first 8–12 postoperative weeks and early rehabilitation, while the graft heals. Elongation of the device by more than 3 mm could not only lead to clinical instability but may also impair tendon to bone healing [12, 13, 19]. In the current study, all cortical suspension devices elongated by less than 3 mm after 5000 cycles, although a significant difference was observed between the UltraButton and the three other devices. In terms of failure loads, the G-Lok was significantly stronger, while the ProCinch RT was significantly weaker than all other devices. However, failure loads of all devices exceeded the forces measured on ACL grafts during early rehabilitation which have peaks below 500 N [5, 23, 29]. The G-Lok showed the lowest extent of elongation and the highest failure load, thus supporting our hypothesis.
The low elongation of the G-Lok fixed-loop device was consistent with other published studies, most of which used the fixed-loop EndoButton CL (Smith & Nephew, Andover, Massachusetts, USA) as the reference fixed-loop device [2, 3, 17, 25] (Table 3). Rylander et al. have compared the G-Lok against the EndoButton CL and have found no significant differences in elongation between the two devices during cyclic loading to 250 N for 1000 cycles [27].
Chang et al. [7] are the only previous authors to use a similar testing protocol to the current study, with both an extended number of cycles (4500) and high loading (100–400 N). Chang et al. [7] compared the biomechanical properties of two different cortical suspension devices, the fixed-loop EndoButton CL and the adjustable-loop TightRope RT (Arthrex, Naples, FL) and their results were in good agreement with the current study, the fixed-loop device had a higher tensile strength and elongated less than the adjustable-loop device (p = 0.001), although both devices elongated less than 3 mm.
A number of other studies have used either an extended number of cycles [3, 25] or a high loading [2, 9], and similarly to the current study they have all reported significantly smaller elongations for fixed-loop cortical suspension devices in comparison to adjustable-loop devices, both when using a device-only model and a device-bone-soft tissue construct model [3, 9, 17, 25]. A few of the devices tested by these authors exceeded an elongation of 3 mm, thus resulting in clinical failure (Table 3). In agreement with our study, Chang et al. [7], Conner et al. [9] and Noonan et al. [25] also found that fixed-loop devices had significantly greater ultimate failure loads than adjustable-loop devices.
Similarly to the studies by Barrow et al. [3] and Noonan et al. [25], our study also found that the greatest amount of loop elongation occurred at low loads, during preloading (20 N) and preconditioning (20–70 N). Loop elongation at low loads has clinical implications, because the ACL is subject to low loads (0–20 N) in some ACL reconstruction rehabilitation exercises, such as dynamic squat to stand at 25 degrees, barbell squats and leg press [10, 29, 35]. Nonetheless, it is possible that in early rehabilitation the forces on the ACL are not sufficient to cause elongation, or that the cycling of graft and fixing at the tibial side with graft under tension mitigates the effects of elongation in the initial cycles [29, 37].
Although in vitro studies, including our own, have shown a greater elongation of adjustable-loop devices in comparison to fixed-loop devices, there is no clinical study showing significant differences in laxity between the two types of devices [4, 6, 8, 11, 21, 37]. The main limitations of this study are that testing was done in vitro, and we were unable to simulate in vivo conditions such as graft healing, the role of supporting structures, bone density and line-of-pull [3, 13, 17, 19, 31]. However, by testing the devices in vitro confounding variables such as bone quality were removed. In addition, only 5 samples of each device were tested, although this was enough to produce a statistical power above 80%. It is important to note that the protocol for implantation of the UltraButton was more complex compared to the other devices, which could affect clinical outcomes if the surgeon is not properly trained. The main strength of our study was the controlled testing of loops under high loads over an extended number of loading cycles.
Conclusions
The purpose of this study was to compare the biomechanical properties of the G-Lok fixed-loop device against three adjustable-loop devices during testing over 5000 cycles under high loading. Our study has shown that all devices have the necessary biomechanical properties for initial fixation of soft tissue grafts in the femoral tunnel for ACL reconstruction. Nonetheless, the fixed-loop device performed best in terms of least elongation and highest load at failure.
Abbreviations
- ACL:
-
Anterior cruciate ligament
References
Ahmad CS, Gardner TR, Groh M, Arnouk J, Levine WN (2004) Mechanical properties of soft tissue femoral fixation devices for anterior cruciate ligament reconstruction. Am J Sports Med 32(3):635–640
Ahmad SS, Hirschmann MT, Voumard B, Kohl S, Zysset P, Mukabeta T, Evangelopoulos DS, Ateschrang A (2018) Adjustable loop ACL suspension devices demonstrate less reliability in terms of reproducibility and irreversible displacement. Knee Surg Sports Traumatol Arthrosc 26(5):1392–1398
Barrow AE, Pilia M, Guda T, Kadrmas WR, Burns TC (2014) Femoral suspension devices for anterior cruciate ligament reconstruction: do adjustable loops lengthen? Am J Sports Med 42(2):343–349
Basson B, Philippot R, Neri T, Meucci JF, Boyer B, Farizon F (2016) The effect of femoral tunnel widening on one-year clinical outcome after anterior cruciate ligament reconstruction using ZipLoop(R) technology for fixation in the cortical bone of the femur. Knee 23(2):233–236
Bourne MN, Bruder AM, Mentiplay BF, Carey DL, Patterson BE, Crossley KM (2019) Eccentric knee flexor weakness in elite female footballers 1-10 years following anterior cruciate ligament reconstruction. Phys Ther Sport 37:144–149
Boyle MJ, Vovos TJ, Walker CG, Stabile KJ, Roth JM, Garrett WE Jr (2015) Does adjustable-loop femoral cortical suspension loosen after anterior cruciate ligament reconstruction? A retrospective comparative study. Knee 22(4):304–308
Chang MJ, Bae TS, Moon YW, Ahn JH, Wang JH (2018) A comparative biomechanical study of femoral cortical suspension devices for soft-tissue anterior cruciate ligament reconstruction: adjustable-length loop versus fixed-length loop. Arthroscopy 34(2):566–572
Choi NH, Yang BS, Victoroff BN (2017) Clinical and radiological outcomes after hamstring anterior cruciate ligament reconstructions: comparison between fixed-loop and adjustable-loop cortical suspension devices. Am J Sports Med 45(4):826–831
Conner CS, Perez BA, Morris RP, Buckner JW, Buford WL Jr, Ivey FM (2010) Three femoral fixation devices for anterior cruciate ligament reconstruction: comparison of fixation on the lateral cortex versus the anterior cortex. Arthroscopy 26(6):796–807
Escamilla RF, Macleod TD, Wilk KE, Paulos L, Andrews JR (2012) Anterior cruciate ligament strain and tensile forces for weight-bearing and non-weight-bearing exercises: a guide to exercise selection. J Orthop Sports Phys Ther 42(3):208–220
Firat A, Catma F, Tunc B, Hacihafizoglu C, Altay M, Bozkurt M, Kapicioglu MI (2014) The attic of the femoral tunnel in anterior cruciate ligament reconstruction: a comparison of outcomes of two suspensory femoral fixation systems. Knee Surg Sports Traumatol Arthrosc 22(5):1097–1105
Gamboa JT, Shin EC, Pathare NP, McGahan PJ, Chen JL (2018) Graft Retensioning technique using an adjustable-loop fixation device in arthroscopic anterior cruciate ligament reconstruction. Arthrosc Tech 7(2):e185–e191
Heijne A, Fleming BC, Renstrom PA, Peura GD, Beynnon BD, Werner S (2004) Strain on the anterior cruciate ligament during closed kinetic chain exercises. Med Sci Sports Exerc 36(6):935–941
Houck DA, Kraeutler MJ, McCarty EC, Bravman JT (2018) Fixed- versus adjustable-loop femoral cortical suspension devices for anterior cruciate ligament reconstruction: a systematic review and meta-analysis of biomechanical studies. Orthop J Sports Med 6(10):2325967118801762
Inderhaug E, Stephen JM, Williams A, Amis AA (2017) Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med 45(2):347–354
Jin C, Paluvadi SV, Lee S, Yoo S, Song EK, Seon JK (2018) Biomechanical comparisons of current suspensory fixation devices for anterior cruciate ligament reconstruction. Int Orthop 42(6):1291–1296
Johnson JS, Smith SD, LaPrade CM, Turnbull TL, LaPrade RF, Wijdicks CA (2015) A biomechanical comparison of femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction under high loads. Am J Sports Med 43(1):154–160
Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C (2009) Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy 25(7):767–776
Kawakami H, Shino K, Hamada M, Nakata K, Nakagawa S, Nakamura N, Toritsuka Y, Yoshikawa H, Ochi T (2004) Graft healing in a bone tunnel: bone-attached graft with screw fixation versus bone-free graft with extra-articular suture fixation. Knee Surg Sports Traumatol Arthrosc 12(5):384–390
Krause M, Freudenthaler F, Frosch KH, Achtnich A, Petersen W, Akoto R (2018) Operative versus conservative treatment of anterior cruciate ligament rupture. Dtsch Arztebl Int 115(51–52):855–862
Lanzetti RM, Monaco E, De Carli A, Grasso A, Ciompi A, Sigillo R, Argento G, Ferretti A (2016) Can an adjustable-loop length suspensory fixation device reduce femoral tunnel enlargement in anterior cruciate ligament reconstruction? A prospective computer tomography study. Knee 23(5):837–841
Levine WN, Vogel LA, Perfetti DC, Moen TC (2011) ACL injury and surgical treatment options. Phys Sportsmed 39(1):108–115
Nagura T, Matsumoto H, Kiriyama Y, Chaudhari A, Andriacchi TP (2006) Tibiofemoral joint contact force in deep knee flexion and its consideration in knee osteoarthritis and joint replacement. J Appl Biomech 22(4):305–313
Nephew S (2016) Technique guide: Ultrabutton adjustable fixation device
Noonan BC, Dines JS, Allen AA, Altchek DW, Bedi A (2016) Biomechanical evaluation of an adjustable loop suspensory anterior cruciate ligament reconstruction fixation device: the value of Retensioning and knot tying. Arthroscopy 32(10):2050–2059
Pasquali M, Plante MJ, Monchik KO, Spenciner DB (2017) A comparison of three adjustable cortical button ACL fixation devices. Knee Surg Sports Traumatol Arthrosc 25(5):1613–1616
Rylander L, Brunelli J, Taylor M, Baldini T, Ellis B, Hawkins M, McCarty E (2014) A biomechanical comparison of anterior cruciate ligament suspensory fixation devices in a porcine cadaver model. Clin Biomech (Bristol, Avon) 29(2):230–234
Shao Q, MacLeod TD, Manal K, Buchanan TS (2011) Estimation of ligament loading and anterior tibial translation in healthy and ACL-deficient knees during gait and the influence of increasing tibial slope using EMG-driven approach. Ann Biomed Eng 39(1):110–121
Shelburne KB, Pandy MG (2002) A dynamic model of the knee and lower limb for simulating rising movements. Comput Methods Biomech Biomed Engin 5(2):149–159
Shelburne KB, Pandy MG, Anderson FC, Torry MR (2004) Pattern of anterior cruciate ligament force in normal walking. J Biomech 37(6):797–805
Shin CS, Chaudhari AM, Andriacchi TP (2007) The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. J Biomech 40(5):1145–1152
Stryker (2014) Technique guide: Femoral fixation ACL/PCL reconstruction featuring GLok
Stryker (2015) Technqiue guide: ProCinch RT.
Synthes D (2015) Technique guide: the RigidLoop adjustable cortical system
Toutoungi DE, Lu TW, Leardini A, Catani F, O'Connor JJ (2000) Cruciate ligament forces in the human knee during rehabilitation exercises. Clin Biomech (Bristol, Avon) 15(3):176–187
van der List JP, DiFelice GS (2017) Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon 15(3):161–168
Wise BT, Patel NN, Wier G, Labib SA (2017) Outcomes of ACL reconstruction with fixed versus variable loop button fixation. Orthopedics 40(2):e275–e280
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analyses were performed by SRP and KC. The manuscript was written by SS and SRP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, S., Ramos-Pascual, S., Czerbak, K. et al. Biomechanical testing of fixed and adjustable femoral cortical suspension devices for ACL reconstruction under high loads and extended cyclic loading. J EXP ORTOP 7, 27 (2020). https://doi.org/10.1186/s40634-020-00235-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-020-00235-9