Abstract
Background
Accurate and early identification of infection sites might help to drive crucial decisions regarding the treatment of sepsis. We aimed to determine the clinical and etiological features of infection according to sites among patients with severe sepsis in Japan.
Methods
This secondary analysis of a multicenter, prospective cohort study included 59 intensive care units (ICU) and proceeded between January 2016 and March 2017. The study cohort comprised 1184 adults (≥ 16 years) who were admitted to an ICU with severe sepsis and septic shock diagnosed according to the sepsis-2 criteria. Sites of infection diagnosed by physicians in charge at the time of arrival comprised the lung, abdomen, urinary tract, soft tissue, bloodstream, central nervous system (CNS), and undifferentiated infections. The primary outcome was in-hospital mortality.
Results
The most common sites of infection were the lungs (31.0%), followed by intra-abdominal sites (26.3%), the urinary tract (18.4%), and soft tissue (10.9%). The characteristics of the patients with severe sepsis across seven major suspected infection sites were heterogeneous. Septic shock was more frequent among patients with intra-abdominal (72.2%) and urinary tract (70.2%) infections than other sites. The in-hospital mortality rate due to severe sepsis and septic shock of a pooled sample was 23.4% (range, 11.9% [urinary tract infection] to 47.6% [CNS infection]). After adjusting for clinical background, sepsis severity, and stratification according to the presence or absence of shock, variations in hospital mortality across seven major sites of infection remained essentially unchanged from those for crude in-hospital mortality; adjusted in-hospital mortality rates ranged from 7.7% (95%CI, − 0.3 to 15.8) for urinary tract infection without shock to 58.3% (95%CI, 21.0–95.7) for CNS infection with shock in a generalized estimating equation model. Intra-abdominal and urinary tract infections were statistically associated with less in-hospital mortality than pneumonia. Infections of the CNS were statistically associated with higher in-hospital mortality rates than pneumonia in a logistic regression model, but not in the generalized estimating equation model.
Conclusions
In-hospital mortality and clinical features of patients with severe sepsis and septic shock were heterogeneous according to sites of infection.
Similar content being viewed by others
Introduction
Sepsis remains a major lethal healthcare problem, with a reported mortality of > 25% [1]. Sepsis differs from straightforward infection in that it is associated with life-threatening organ dysfunction, multiple organ failure, and death due to a dysregulated host response to infection [2]. The pathology of sepsis involves both inflammatory and anti-inflammatory responses [3, 4] and includes a broad spectrum of conditions with various clinical manifestations and patterns of acute organ dysfunction. From a clinical viewpoint, the history and characteristics of patients, infection sites, and comorbidities are remarkably heterogeneous. This, together with confusing nomenclature describing this syndrome and the scarcity of appropriate epidemiological data, has contributed to suboptimal findings from observational studies of sepsis [5,6,7].
Some authors have suggested that understanding clinical differences based on infection sites could aid clinicians to appropriately stratify risk, help guide clinical decisions regarding treatment, and facilitate understanding of variations in host responses [8, 9]. However, few studies have described differences in clinical characteristics and in-hospital mortality based on infection sites that might be important [6, 7]. Although our previous Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, Sepsis, and Trauma (FORECAST) study [10] determined management and clinical outcomes among patients with severe sepsis and septic shock in Japan, clinical features according to infection sites have not yet been evaluated. Therefore, the present study aimed to identify the clinical and etiological features and outcomes of severe sepsis based on infection sites to help guide clinical decisions.
Methods
Design, setting, and participants
This is a secondary analysis of a sub-study of patients with severe sepsis in the multicenter prospective cohort FORECAST study of acutely ill patients at 59 ICU in Japan that proceeded between January 2016 and March 2017 [10]. We investigated data from adult patients (≥ 16 years) diagnosed with severe sepsis based on the 2003 sepsis-2 criteria [11]. Inclusion criteria comprised the new onset of suspected infection based on clinical history, at least two systemic inflammatory response syndrome (SIRS) criteria, and evidence of dysfunction in at least one organ [11]. Exclusion criteria comprised requiring sustained life support or being in post-cardiopulmonary arrest resuscitation status when sepsis was diagnosed.
Data collection
Data were obtained from a database compiled by the FORECAST investigators. The variable of primary interest was the site of infection. Other variables included patient information such as demographics, admission source, comorbidities, activities of daily life (ADL) status, organ dysfunction, infection characteristics, laboratory data, blood culture findings, and antibiotics (no information was available about the amount and duration). Data were inputted by FORECAST investigators throughout the periods that patients remained in hospital. The primary outcome was in-hospital mortality.
Data definitions
Septic shock and organ dysfunction were defined according to the sepsis-2 criteria [11]. Sites of infection were assessed as suspected at initial examination by physicians in charge because the initial diagnosis contributes to predicting outcome [6, 7]. Sites of infection in this database initially included the lungs, intra-abdominal sites (the peritoneum, the pancreas, the gall bladder, the bowel, and other sites), urinary tract, soft tissue, wounds, osteo-articular sites, endocardium, and catheter-related, implant device-related, central nervous system (CNS), and undifferentiated infection (Additional file 1: Table S1). Because several categories contained few patients, we consolidated them into seven major categories comprising the lungs, intra-abdomen, urinary tract, soft tissue (including wounds), and bloodstream-related, CNS, and undifferentiated infection.
Analysis
Descriptive statistics included counts (proportions) for categorical variables, and continuous variables are expressed as medians with interquartile ranges (IQR) because many variables were not normally distributed. Data were not adjusted unless specifically stated otherwise. Since few values were missing, assumptions were not made for missing data; these are noted as footnotes to tables.
We compared the baseline characteristics of the patients, demographic data, infection characteristics including results of blood cultures, sepsis severity, organ dysfunction, and mortality outcomes categorized by infection sites. We also described the frequency and choice of initial antibiotics administered. We then stratified crude in-hospital mortality rates by sites of infection and the presence of shock because clinical approaches such as resuscitation differed between patients with and without shock. We adjusted the backgrounds of the patients and sepsis severity considering clustering by ICU using generalized estimating equation (GEE) models with an independent working correlation matrix. Models were adjusted for age, sex, Charlson comorbidity index (CCI), and organ-specific sepsis-related organ failure assessment (SOFA) scores and were selected a priori based on reported findings [6, 7] and clinical importance. We then used marginal standardization [12] based on probability determined from the GEE model to estimate adjusted in-hospital mortality by the seven major infection sites. To determine the clinical relationship between infection sites and shock, these models were further stratified by the presence or absence of shock. Results are reported as adjusted in-hospital mortality rates with 95% confidence intervals (CI). We assessed each variable for multicollinearity and also compared in-hospital mortality rates among the seven major infection sites using logistic regression as a sensitivity analysis. Models were adjusted for age, sex, BMI, ADL, admission sources, Charlson comorbidity index (CCI), presence of shock, and organ-specific SOFA scores, then further stratified by the presence of shock. The lungs served as the reference for all statistical models as they comprised the most frequent sites of infection.
All p values were two-sided, with p < 0.05 being considered statistically significant. Data were statistically analyzed using SPSS software, version 23.0 (IBM, Armonk, NY, USA) and Stata software, version 14.2 (StataCorp, College Station, TX, USA).
Results
Patients’ characteristics
We analyzed data from 1184 patients with severe sepsis who met the inclusion and exclusion criteria at 59 participating ICU in Japan between January 2016 and March 2017. The median age was 73 (IQR, 64–81) years and 60.7% were male. Most (57.1%) patients with severe sepsis arrived directly from emergency departments (ED), fewer were transferred from other departments or were diagnosed with severe sepsis while in an ICU, and 62.9% of patients were diagnosed with septic shock on arrival. The rate of blood culture positivity upon admission was 54.0%.
Patients were categorized by infection site upon arrival (Table 1). The most common site of infection was the lungs (31.0%), followed by the intra-abdomen (26.3%), the urinary tract (18.4%), and soft tissue (10.9%). The characteristics of the patients with severe sepsis were heterogeneous across all seven infection sites. Septic shock was more frequent among patients with intra-abdominal (72.2%) and urinary tract (70.2%) infections. Patients with lung, CNS, and undifferentiated infections had high APACHE II scores. Organ-specific SOFA scores were heterogeneous, but total scores were relatively similar. Rates of blood culture positivity were > 80% in bloodstream-related (osteo-articular 81.0%, endocardial 81.3%, catheter-related 90.9%, and implant device-related 87.5%) infections (Additional file 1: Table S1). Gram-negative rods were most frequently identified in cultured blood from intra-abdominal and urinary tract sites, whereas Gram-positive cocci were prevalent at other sites of infection (Additional file 2: Table S2). Fungal infection was rarely found in blood cultures. With respect to organ dysfunction, hypotension and hyperlactatemia were equally prevalent among patients with shock (Additional file 2: Table S2). Hyperlactatemia was prevalent (69.6%) among patients with CNS infection, although the rate of shock was relatively low (30.4%). The prevalence of thrombocytopenia was 43.5% and 55.1% in CNS and undifferentiated infections, respectively.
Carbapenem was the most frequent initial antibiotic (55.0%), followed by tazobactam/piperacillin (PIPC/TAZ) (20.9%) (Table 1). Carbapenem was usually administered to patients with intra-abdominal, urinary tract, and soft tissue infections (61.3%, 59.6%, and 63.7%, respectively), whereas bloodstream and CNS infections were usually treated with vancomycin (VCM; 47.8% and 63.6%, respectively) (Additional file 3: Table S3). Most (63.2%) patients received antibiotic monotherapy, particularly those with intra-abdominal (79.8%) or urinary tract (77.5%) infections, whereas those with soft tissue, bloodstream, and CNS infections more frequently received combined antibiotics (66.9%, 62.7%, and 72.7%, respectively).
Outcomes
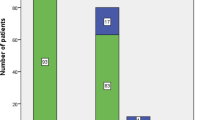
Mortality follow-up data were available for 97.0% of patients during their hospitalization. The overall in-hospital mortality rate was 23.4%. Regardless of shock status, crude in-hospital mortality ranged from 11.9 to 47.6% (urinary tract infection and CNS infection, respectively) across all sites of infection (Fig. 1; Additional file 1: Table S1). After stratification by the presence or absence of shock, crude in-mortality values ranged from 9.2% (urinary tract infection without shock) to 57.1% (CNS infection with shock) (Fig. 1). After adjusting for clinical background and sepsis severity, variations in hospital mortality across the seven major sites of infection remained essentially unchanged from those for crude in-hospital mortality; adjusted in-hospital mortality ranged from 7.7% (95%CI, − 0.3 to 15.8) for urinary tract infection without shock to 58.3% (95%CI, 21.0–95.7) for CNS infection with shock in a generalized estimating equation model (Fig. 1). Adjusted mortalities and its 95% confidence interval calculated by the generalized estimating equation model are demonstrated; those for pneumonia were 28.8% (95%CI, 21.4–36.2) in all, 33.9% (95%CI, 25.1–42.6) with shock, and 19.6% (95%CI, 11.4–27.8) without shock; those for CNS infection were 48.7% (95%CI, 25.7–71.7) in all, 58.3% (95%CI, 21.0–95.7) with shock, and 32.7% (95%CI, 5.8–59.7) without shock; and those for undifferentiated infection were 32.4% (95%CI, 23.5–41.4) in all, 43.1% (95%CI, 29.6–56.6) with shock, and 14.1% (95%CI, 1.2–27.1) without shock. Intra-abdominal infections were statistically associated with lower in-hospital mortality rates than pneumonia in the logistic regression model (P = 0.03) but not in the GEE model (P = 0.10). Urinary tract infections were statistically associated with lower in-hospital mortality rates than pneumonia in the logistic model and in the GEE model (P < 0.01). Infections of the CNS were statistically associated with higher in-hospital mortality rates than pneumonia in the logistic regression model (P = 0.04), but not in the GEE model (P = 0.07). Multicollinearity was not observed in the GEE model and the logistic regression model; all variance inflation factors were less than 3. After stratification by shock, variations in in-hospital mortality according to sites of infection persisted in patients with septic shock but not in those with non-septic shock (Figs. 1 and 2).
Crude and adjusted in-hospital mortality rates for patients with sepsis according to seven major infection sites stratified by shock. The crude mortalities are summarized in the table and adjusted mortalities and its 95% confidence interval calculated by the generalized estimating equation model are demonstrated with a bar graph with error bars.
Data were adjusted for age, sex, Charlson comorbidity index, and organ-specific sepsis-related organ failure assessment (SOFA) scores using marginal standardization. CNS central nervous system
Relationship between seven major sites of infection and in-hospital mortality among all patients with severe sepsis (n = 952), and those with (n = 613) and without (n = 339) septic shock using logistic regression models. Data were adjusted for age, sex, BMI, ADL, admission sources, CCI, presence of shock, and organ-specific SOFA scores. ADL, activities of daily living; BMI, body mass index; CCI, Charlson comorbidity index; CI, confidence interval; CNS, central nervous system; SOFA, sepsis-related organ failure assessment
Discussion
Brief summary
The multicenter, prospective FORECAST cohort study of patients with severe sepsis at 59 ICU in Japan confirmed that severe sepsis is a heterogeneous clinical syndrome that is associated with a poor prognosis. More importantly, highly variable in-hospital mortality was associated with infection sites, especially among patients with shock.
Heterogeneity of severe sepsis
The variation in severe sepsis was associated with infection sites and shock. After adjustment for initial characteristics and severity, intra-abdominal or urinary tract infections were associated with lower mortality rates than other sites of infection, but such patients were more likely to have shock. Our results were relatively consistent with these findings [6, 7], which was reasonable because patients with intra-abdominal or urinary tract infections often had stone pyelonephritis, cholangitis, or cholecystitis, namely, source-controllable infections [9]. On the other hand, mortality associated with intra-abdominal infection varied, which might be due to the wide heterogeneity of intra-abdominal infections such as peritonitis, pancreatitis, cholangitis, and ischemic bowel infections [6]. Mortality rates were the highest among patients with CNS infection and septic shock, but the statistical significance was not clear. Only 23 of 1184 patients with CNS infection were admitted to an ICU, and they were less likely to have shock (n = 7 of 23). This might have been underpowered due to the small patient cohort. Otherwise, selection bias might have been involved. Bloodstream and undifferentiated infections might have been also involved in the same discussion as CNS infection. Moreover, the GEE model was used as the main analysis instead of the logistic regression model because the prevalence of site of infection in sepsis and patient’s severity was considered to be clustered by facility, that is, the ordinal logistic regression model could be too efficient, whereas the point estimate from the logistic regression was consistent with that from the GEE model with an independent working correlation matrix. The present results which showed intra-abdominal and CNS infections showed contradictory results about a significance between the GEE model and the logistic regression model. It implied that the severity of intra-abdominal and CNS infections in each ICU was various. Otherwise, it could have needed more samples to show a significance. Previous studies have not found an association between positive blood cultures and mortality outcomes but have associated prognosis more closely with the severity of sepsis rather than the severity of underlying infection [13, 14]. More patients in the present study with infections of the urinary tract (major infection site) also had bacteremia, but the mortality rate was lower than that for other infections. Prognosis might have been associated with the distribution of infection sites in patients with bacteremia in each study.
Septic shock in 63% of the patients in the present study was associated with 28% in-hospital mortality, which was lower than previously published rates [6]. A systematic review identified a crude mortality rate associated with septic shock of 47% [15]. The present study found at least 40% mortality when septic shock was associated with the lungs, CNS, and undifferentiated infections. Although mortality rates are high among patients with septic shock, our findings emphasized the potential contribution of infection sites to mortality. The particular distribution of infection sites and shock might have contributed to the mortality rates in the present study. The contributing influence of infection sites among patients without shock was not clear. Point estimates of odds ratios according to sites of infection were similar despite the presence of shock in most patients, suggesting that the effect of the infection site on mortality was independent of shock. The number of patients without shock may have been insufficient to discern a statistical difference in mortality rates between those with and without shock. Otherwise, mortality rates might not differ according to infection sites among patients without shock. Validation studies with a larger sample size are needed to confirm our findings.
Possible explanations and implications
We found wide clinical heterogeneity regarding infection sites in patients with severe sepsis and septic shock. This highlights the importance of early and accurate diagnosis of infection sites, with the ability to diagnose warranting equivalent consideration of the skill required to select optimal treatment strategies [8]. By analogy, our findings suggested that because sepsis is not a monolithic disease, the approach should perhaps follow strategies more commonly found in oncology [16]. For example, cancer is uniform in the sense of dysregulated cell growth with distant invasion potential [17], but cancer treatment is now increasingly based on targeted cell receptors and is rapidly moving away from a chemotherapy-for-all paradigm [8]. The present findings imply that increased focus on specific organ-system presentation, namely, “sepsis at the infection source,” with appropriate subsequent risk stratification and diagnosis could be a reasonable strategy. Our results might contribute to similar profiling of infection sites, which we believe will be an important frontier of future sepsis research [8]. Moreover, differences of outcome according to the source infection are important to be considered in future randomized control trials in sepsis and septic shock.
Limitations
Several limitations of this study warrant discussion. First, the observational design of the study caused difficulties distinguishing causative from correlational relationships. Causal influence requires statistical evaluation, but clinical implications should also be considered. Second, we based our profile classification on suspected sites of infection at the time of admission, rather than on definitive sites of infection. Some patients might have been misdiagnosed, and our results suggest that the consequences of this impact patient outcomes. However, most infection sites were clarified from culture findings (Additional file 2: Table S2). Third, we consolidated 11 initial sites of infection into seven. This might have generated some bias but avoided overcomplexity. Fourth, we enrolled fewer patients than expected during consecutive sampling. The reasons for this were as follows. The prevalence of sepsis is relatively lower in tertiary care centers in Japan (< 5% in the 2010–2011 sepsis registry [18]). Some institutions became involved in the FORECAST study well after it began. Other institutions may have obtained convenience samples even though we had planned consecutive enrolment in the FORECAST study. Fifth, although our data were derived from a large database compiled from 59 ICU, all patients were diagnosed and treated in Japan. Therefore, epidemiological patterns of infection sites and microorganisms might be localized. We studied only patients who were admitted to ICU. Sixth, patients with CNS or soft tissue infections were more likely to be admitted to wards instead of ICU because they were less likely to have shock. Seventh, SSCG 2016 has provided updated definitions and clinical criteria for sepsis (sepsis-3) [2, 15, 19]. Nonetheless, application to the clinical settings would not be difficult due to the large overlap in sepsis-3 and sepsis-2 definitions. Finally, we do not have information about the amount of time that elapsed between the initiation of sepsis and ICU admission, and this might have been associated with outcomes. We also do not have information about source control. However, we believe that all the patients received appropriate source control because all participating institutions are nationally certified emergency centers.
Conclusions
We found that in-hospital mortality and the clinical features of patients with severe sepsis and septic shock were heterogeneous according to sites of infection.
Abbreviations
- ADL:
-
Activities of daily living
- APACHE II:
-
Acute physiology and chronic health evaluation II
- BMI:
-
Body mass index
- CCI:
-
Charlson comorbidity index
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- ED:
-
Emergency department
- FORECAST:
-
Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, Sepsis and Trauma
- GEE:
-
Generalized estimating equation
- ICU:
-
Intensive care unit(s)
- IQR:
-
Interquartile range
- PIPC/TAZ:
-
Piperacillin/tazobactam
- SIRS:
-
Systemic inflammatory response syndrome
- SOFA:
-
Sepsis-related organ failure assessment
- SSCG:
-
Surviving Sepsis Campaign Guidelines
- VCM:
-
Vancomycin
References
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Jacob JA. New sepsis diagnostic guidelines shift focus to organ dysfunction. JAMA. 2016;315(8):739–40.
Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340(3):207–14.
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):2063.
Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A. Co-operative Antimicrobial Therapy of Septic Shock Database Research G: association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189(10):1204–13.
Jeganathan N, Yau S, Ahuja N, Otu D, Stein B, Fogg L, Balk R. The characteristics and impact of source of infection on sepsis-related ICU outcomes. J Crit Care. 2017;41:170–6.
Iwashyna TJ, Govindan S. Did they just prove that a diagnosis of “septic shock” is meaningless? Am J Respir Crit Care Med. 2014;189(10):1156–7.
Martinez ML, Ferrer R, Torrents E, Guillamat-Prats R, Goma G, Suarez D, Alvarez-Rocha L, Pozo Laderas JC, Martin-Loeches I, Levy MM, et al. Impact of source control in patients with severe sepsis and septic shock. Crit Care Med. 2017;45(1):11–9.
Abe T, Ogura H, Shiraishi A, Kushimoto S, Saitoh D, Fujishima S, Mayumi T, Shiino Y, Nakada TA, Tarui T, et al. Characteristics, management, and in-hospital mortality among patients with severe sepsis in intensive care units in Japan: the FORECAST study. Crit Care. 2018;22(1):322.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–6.
Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962–70.
Brun-Buisson C, Doyon F, Carlet J. Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia-Sepsis Study Group. Am J Respir Crit Care Med. 1996;154(3 Pt 1):617–24.
Zahar JR, Timsit JF, Garrouste-Orgeas M, Francais A, Vesin A, Descorps-Declere A, Dubois Y, Souweine B, Haouache H, Goldgran-Toledano D, et al. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med. 2011;39(8):1886–95.
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M. Sepsis definitions task F: developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):775–87.
Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1989;17(5):389–93.
Defining Cancer. https://web.archive.org/web/20140625220940/http://www.cancer.gov/cancertopics/cancerlibrary/what-is-cancer. Accessed 28 Apr 2019.
Ogura H, Gando S, Saitoh D, Takeyama N, Kushimoto S, Fujishima S, Mayumi T, Araki T, Ikeda H, Kotani J, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. 2014;20(3):157–62.
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–74.
Acknowledgements
We are grateful to Yohei Hirano, MD, PhD; Kazutoshi Hirabayashi, MD, PhD; and Yuki Uehara, MD, PhD, for critical advice. We also thank Professor Toshiaki Iba, MD, PhD, for valuable advice and the JAAM FORECAST group for contributing to this study. We would like to thank FORTE (https://www.forte-science.co.jp) for the final English language editing.
JAAM FORECAST group
Osamu Tasaki1, Yasumitsu Mizobata2, Hiraku Funakoshi3, Toshiro Okuyama4, Iwao Yamashita5, Toshio Kanai6, Yasuo Yamada7, Mayuki Aibiki8, Keiji Sato9, Susumu Yamashita10,11, Kenichi Yoshida12, Shunji Kasaoka13, Akihide Kon14, Hiroshi Rinka15, Hiroshi Kato16, Hiroshi Okudera17, Eichi Narimatsu18, Toshifumi Fujiwara19, Manabu Sugita20, Yasuo Shichinohe21, Hajime Nakae22, Ryouji Iiduka23, Mitsunobu Nakamura24, Yuji Murata25, Yoshitake Sato26, Hiroyasu Ishikura27, Yasuhiro Myojo28, Yasuyuki Tsujita29, Kosaku Kinoshita30, Hiroyuki Yamaguchi31, Toshihiro Sakurai32, Satoru Miyatake33, Takao Saotome34, Susumu Yasuda35, Yasuaki Mizushima36
1. Nagasaki University Hospital
2. Osaka City University Hospital
3. Tokyo Bay Urayasu Ichikawa Medical Center
4. Aso Iizuka Hospital
5. Tomei Atsugi Hospital
6. Hiratsuka City Hospital
7. National Hospital Organization Sendai Medical Center
8. Ehime University Hospital
9. Okayama University Hospital
10. Tokuyama Central Hospital
11. Fukuyama City Hospital
12. JA Hiroshima General Hospital
13. Kumamoto University Hospital
14. Hachinohe City Hospital
15. Osaka City General Hospital
16. National Hospital Organization Disaster Medical Center
17. University of Toyama
18. Sapporo Medical University
19. Okayama Saiseikai General Hospital
20. Juntendo University Nerima Hospital
21. National Hospital Organization Hokkaido Medical Center
22. Akita University Hospital
23. Japanese Red Cross Society Kyoto Daini Hospital
24. Maebashi Red Cross Hospital
25. Sendai City Hospital
26. Subaru Health Insurance Society Ota Memorial Hospital
27. Fukuoka University Hospital
28. Ishikawa Prefectural Central Hospital
29. Shiga University of Medical Science
30. Nihon University School of Medicine
31. Seirei Yokohama General Hospital
32. National Hospital Organization Kumamoto Medical Center
33. Saiseikai Utsunomiya Hospital
34. National Hospital Organization Higashi-Ohmi General Medical Center
35. National Hospital Organization Mito Medical Center
36. Rinku General Medical Center
Funding
This study was supported by the Japanese Association for Acute Medicine (2014-01).
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Contributions
TA contributed to data acquisition and interpretation and study concept and design and drafted and revised the manuscript for important intellectual content. GD interpreted the data and revised the manuscript for important intellectual content. TS conceived and designed this study, interpreted the data, and revised the manuscript for important intellectual content. MU interpreted the data and revised the manuscript for important intellectual content. IN interpreted the data and revised the manuscript for important intellectual content. AS contributed to data acquisition, adjustment, and interpretation and revised the manuscript for important intellectual content. HO, SK, SG, DS, SF, and TM contributed to data acquisition and interpretation and study concept and design and revised the manuscript for important intellectual content. All authors contributed to the data acquisition and reviewed, discussed, and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committees at all institutions that participated in the Japanese Association for Acute Medicine (JAAM) study group reviewed and approved the study protocol. These committees waived the need for written informed consent from the study participants, given the retrospective and anonymized nature of the study. Institutional Review Board approval (No. 014–0306) was granted by Hokkaido University, the lead institution for FORECAST. All methods were implemented in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Characteristics and in-hospital mortality of patients with severe sepsis according to 11 sites of infection (n = 1184). Features and in-hospital mortality of patients with severe sepsis according to 11 infection sites. (DOCX 27 kb)
Additional file 2:
Table S2. Characteristics of patients with sepsis according to seven major infection sites (n = 1184). Additional features of patients with sepsis according to seven major sites of infection. (DOCX 29 kb)
Additional file 3:
Table S3. Initial antibiotic treatment administered to patients with severe sepsis (n = 1140). (DOCX 28 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Abe, T., Ogura, H., Kushimoto, S. et al. Variations in infection sites and mortality rates among patients in intensive care units with severe sepsis and septic shock in Japan. j intensive care 7, 28 (2019). https://doi.org/10.1186/s40560-019-0383-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-019-0383-3