Abstract
Low moisture foods like spices are known to be contaminated with pathogens, sun-dried chili pepper powder not left out. A study was conducted to determine the effect of gamma irradiation on the decontamination of sun-dried Legon-18 pepper powder and its impact on some quality parameters. Samples were obtained from a local farmer. Sterility tests was performed. Samples were inoculated with known cfu/g of L. monocytogenes, E. coli and S. Typhimurium. The inoculated samples were irradiated at 1, 2, 4 and 5 kGy and unirradiated samples were used as control (0 kGy). The samples were stored for 60 days at 28 ± 2 °C and pathogens enumerated in the appropriate medium. For quality parameters, samples were irradiated at 5 kGy, unirradiated samples served as control (0 kGy) and stored at 28 ± 2 °C. HPLC was used for the quantification of carotenoids and capsaicinoids in the samples that were stored for 8 weeks at 28 ± 2 °C. A dose-dependent effect on the inactivation of the pathogens used in the study as well as the effect of storage days was observed (p < 0.05). All the pathogens used in the study were inactivated at 5 kGy on day 0. Gamma irradiation at 5 kGy caused an increase of 21.81% in capsaicin, 16.79% in dihydrocapsaicin and 20.32% in total capsaicinoids in the irradiated samples. The effect of gamma irradiation on the carotenoids led to losses of 8.84% in beta carotene, 26.07% in beta cryptoxanthin and 8.16% in capsanthin. Storage weeks caused losses in all carotenoids and capsaicinoids analyzed during the study.
Similar content being viewed by others
Introduction
Legon-18 chili pepper (Capsicum annuum) is one of the pepper varieties cultivated in Ghana (MOFA 2007). It is used as a spice in various meals and in Ghana a special sauce known as ‘shito’ is prepared from it (Doku 2015). Spices in general are known to be contaminated with pathogens which are of health importance because they (spices) are of agricultural origin, processing and handling methods mostly make them contaminated (Banerjee and Sarkar 2003; Buckenhuskes and Rendlen 2004; Cheon et al. 2015).
The FAO/WHO (2014) indicated that spices (including pepper powder) which are low moisture foods get contaminated with pathogens such as Listeria monocytogenes, Bacillus cereus, Salmonella species, Escherichia coli and other pathogens. Some of these have been implicated in disease outbreaks leading to mortality and morbidity in man (CDC 2010). There have been several conventional means of decontaminating spices, however some of them tend to be source of carcinogens (ethylene dioxide), others lead to reduced quality of produce (steam treatment) and others have been known to leave pesticide residues (Fowels et al. 2001; Tainter and Grenis 2001; Rico et al. 2010).
Gamma irradiation has been used for the decontamination of spices due to its high penetrability into commodities (Rico et al. 2010; Deng et al. 2015). In view of this, a study was conducted to determine the effect of gamma irradiation on the decontamination and its effect on some quality parameters of sun-dried Legon-18 pepper powder.
Materials and methods
Source of Legon-18 pepper powder
Sun dried Legon-18 powdered pepper samples were obtained from a local farmer for the experiment.
Microbiological analysis
Source of bacteria strains used
The strains used in the study were pure strains of Salmonella enterica serotype Typhimurium and Esherichia coli (25922) which were obtained from the Microbiological Collection Centre of the Noguchi Memorial Institute for Medical Research of the University of Ghana and Listeria monocytogenes (NCTC 7973/ATCC 35152) purchased from Becton Dickinson in the United States of America.
Sterilization of pepper and sterility tests
The powdered pepper samples in sealed pouches were irradiated at 20 kGy at a dose rate of 1.97kGyhr− 1 by a category IV wet storage gamma irradiator from a cobalt-sixty source (60Co) at the Gamma Irradiation Facility of the Radiation Technology Centre of the Biotechnology and Nuclear Agriculture Research Institute, Ghana Atomic Energy Commission, with the purpose of the removal of background microflora (Deng et al. 2015). Sterility tests were conducted to confirm the removal of the background microflora using appropriate media (XLD agar (BD), PLS agar (BD) and CT-SMAC (BD) for S. Typhimurium, L. monocytogenes and E. coli respectively) under sterile conditions. The procedures of Deng et al. (2015) and Jeong et al. (2010) were used with some modifications. Ten grams of the pepper samples from the pouches (dimensions of 0.118 m in width and 0.170 m in length) were weighed into sterile stomacher (Seward, UK) pouches, and 90 ml of sterile buffered saline water added. The irradiated samples were homogenized, serially diluted, spread-plated and incubated for 24 h at 37 0C for S. Typhimurium, E. coli and 48 h for Listeria monocytogenes.
Culture preparation and inoculation
The pathogens S. Typhimurium and E. coli were inoculated into the sundried Legon-18 powdered pepper samples according to the procedures of Cheon et al. (2015) with some modifications. The stock culture was stored at -80 °C in 0.7 ml of Tryptic Soy Broth (TSB; Difco) and 0.3 ml of 50% glycerol was streaked onto Tryptic Soy Agar (TSA; Difco) which was later incubated at 37 °C for 24 h and stored at 4 °C. A loop of each pathogen was taken and incubated in TSB in falcon tubes on a shaker at 37 °C for 24 h. The cells were harvested by centrifugation at 4000 g for 20 min at 4 °C and washed thrice with phosphate buffered saline (PBS). Final pellets were re-suspended in 10 ml of PBS which corresponded to approximately 107–108 CFU/ml.
L. monocytogenes strain was inoculated according to the method of Ducic et al. (2016). The inoculum was prepared by transferring 0.1 ml of the stock cultures into 10 ml of tryptic soy broth (TSB; Becton Dickinson Diagnostic Systems, Sparks, Maryland; BD) which was incubated at 37 °C for 24 h. A loop of the pathogen was taken and incubated in TSB in falcon tubes on a shaker at 37 °C for 24 h. The cells were harvested and centrifuged at 3000 g for 10 min at a temperature of 4 °C. The pellets that were formed during the centrifuge were washed and re-suspended in 10 ml of 0.1% sterile PBS which was then adjusted to make a final cell concentration of approximately 10 9 CFU/ml.
A cocktail (approximate equal proportions) of all the organisms was prepared and used for the inoculation of the sun-dried Legon-18 pepper powder samples. A millilitre of the cocktail (suspension) was added to 10 g of the samples in sterile pouches corresponding to 105–106. The inoculated pepper samples were thoroughly mixed and dried in a biosafety hood for a period of sixty minutes.
Irradiation of pepper powder sample
The inoculated pepper samples in the pouches (dimensions of 0.118 m in width and 0.170 m in length) were irradiated at 1, 2, 4 and 5 kGy at a dose rate of 2.01 kGy/h at the Gamma Irradiation Facility of the Radiation Technology Centre. Samples which were not irradiated served as control (0 kGy).
Microbial enumeration
The enumeration of all the organisms used in the study was carried out according to the procedures of Ducic et al. (2016), Cheon et al. (2015), Jeong et al. (2010) and Deng et al. (2015) with some modifications. Ninety millilitres of sterile PBS was poured into 10 g of the inoculated pepper in sterile stomacher bags and homogenized. Samples were serially diluted and spread plated under sterile conditions onto the various media (XLD agar (BD) PLS agar (BD) and CT-SMAC (BD) for S. Typhimurium, L. monocytogenes and E. coli respectively) . E. coli and S. Typhimurium were incubated at 37 °C for 24 h for and L. monocytogenes was incubated for 48 h.
Chemical composition analysis
Carotenoids
The carotenoids analysed in the sun-dried Legon-18 pepper powder samples were capsanthin, beta carotene and beta cryptoxanthin. The standards for the carotenoids were purchased from Extrasynthese (Lyon, France). HPLC (high-performance liquid chromatography) analysis of the carotenoids was done according to the procedure of Topuz and Odzemir (2003).
Extraction of carotenoids
Extraction of the carotenoids was done according to the procedure of Barbero et al. (2006). One gram of sun-dried Legon-18 pepper powder samples were weighed and the contents were extracted using an accelerated solvent extractor which was equipped with an 100-ml stainless steel extraction cells. The cells were loaded with the pepper samples which was mixed with inert sea sand (cellular components had been homogenised). The cells were filled with 90% ethanol to a pressure of `1500 psi. Heat was applied for the initial period of heat-up time, static extraction took place after all valves closed. The cells were rinsed with the extraction solvent and the cells purged with N2 gas for 2 min. The extracts from the cells were collected with 20 ml falcon tubes after the system of the accelerated solvent extractor was depressurised.
Quantification of carotenoids
The carotenoids were quantified according to the procedure of Topuz and Odzemir (2003) using an HPLC. The extracts were filtered through a 0.45 µm membrane filter (Millipore) into a 2 ml glass vial and used for HPLC injection. The Chromatographic separation of each carotenoid was performed on a reversed-phase column AQUA 5u C18 125A (150 × 4.60, 5um) The binary gradient (acetone:water at the beginning 75:25). Calibration curves of the various carotenoids (standards) were drawn using Microsoft Excel, 2010.
Capsaicinoids
Capsaicin and dihydrocapsaicin were the main capsaicinoids that were analysed since they are the main capsaicinoids responsible for the hotness of pepper (Giuffrida et al. 2013). Standards of capsaicin and dihydrocapsaicin were purchased from Extrasynthese (Lyon, France).
Extraction of capsaicinoids
The extraction of the capsaicinoids was done according to the procedure of Barbero et al. (2006). Pressurized liquid extractor (Fluid Management Systems, USA) was used for the extraction of the capsaicinoids. Powdered Legon-18 pepper samples were weighed at 1.0 g and contents extracted using accelerated solvent extractor equipped with an 100-ml stainless steel extraction cells. Cells were loaded with the pepper samples, mixed with inert sea sand which had been homogenised. Cells were filled with 90% ethanol to a pressure of `1500 psi. Heat was applied for the initial period of heat-up time, static extraction was effected after all valves were closed. Cellular contents were rinsed with the extraction solvent and purged with N2 gas for 120 s. The extracts were collected from the cells after depressurization of the system (Barbero et al. 2006).
Quantification of capsaicinoids
Extracts from the samples were filtered through a 0.45 µm Millipore membrane filter and injected into HPLC. Analysis (qualitative and quantitative) of the capsinoids profile was carried out by the HPLC PDA detector (PerkinElmer Flexar) equipped with a revered phase column SunFire TM C18 (5um, 4.6 × 150 mm, Waters) thermostated at 30 °C. The capsaicinoids were separated by an isocratic mixture of water: acetonitrile 55:44 v/v. The detection wavelength was set at 280 nm (Giuffrida et al. 2013). Calibration curves of the standards were drawn using Microsoft Excel, 2010.
Total Capsaicinoids and Scoville heat units
Total capsaicinoids and Scoville Heat units (SHU) of the sundried Legon-18 pepper powder were computed based on the procedure of Jung et al. (2015) and Orellana-Escobedo et al. (2013) respectively. Where total capsaicinoids (TC) and SHU were expressed as
\( {\displaystyle \begin{array}{c}\mathrm{TC}=\mathrm{capsaicin}+\mathrm{dihydrocapsaicinand}\\ {}\mathrm{SHU}=\left[\left(\%\mathrm{dihydrocapsaicin}\times 16.1\right)+\left(\%\mathrm{capsaicin}\times 16.1\right)\right]\times 10000.\end{array}} \)
Data analysis
The data obtained during this study was analysed with StatGraphics Centurion XV.I. and means were separated using Least Significant Difference (p < 0.05).
Results and discussion
Several authors have indicated the effect of gamma irradiation on the inactivation of pathogenic organisms associated with the food as well as spices (Jung et al. 2015; Deng et al. 2015; Rico et al. 2010; Song et al. 2014).
During this study, some pathogenic organisms associated with chili pepper were inoculated into Legon-18 pepper powder to determine the effect of gamma irradiation and storage days on the inactivation of these pathogenic organisms.
Inactivation of L. monocytogenes
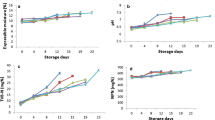
There was a general reduction in the count (log cfu/g) of the L. monocytogenes in the samples of pepper powder from day 0 to day 60 with reference to all the doses of gamma irradiation applied except 5 kGy at which there was no detection of organisms below the detection limit (which continued throughout the period of storage of the samples). Complete inactivation was achieved at day 60 for all the samples that were irradiated at 2 and 4 kGy (Table 1). Jo et al. (2004) and Mukhopadhyay et al. (2013) indicated a corresponding effect of gamma irradiation on the inactivation of pathogens after exposure to gamma irradiation. In this study, there was a corresponding effect of gamma irradiation on the inactivation of L. monocytogenes in the pepper samples. As doses of gamma irradiation increased, there was higher inactivation of L. monocytogenes. The gradual inactivation of L. monocytogenes due to gamma irradiation may be attributed to the inability of the pathogen to recover (repair) due to the injuries caused to the cellular components (Byun et al. 2001).
Inactivation model of L. monocytogenes was regressionally (stepwise regression (Table 2)) determined as
Where X1 = days of storage and X2 (kGy) as doses of gamma irradiation. The R-square for the model was 86.76% or 0.8676 which was statistically significant (p < 0.05).
Inactivation of S. enterica serotype typhimurium
There was a general reduction in the count (log cfu/g) for all doses of gamma irradiation applied during the study including the samples that were not irradiated. There was no detection of S. Typhimurium for all the samples that were irradiated at 5 kGy and stored for 60 days (Table 3). There was no detection of S. Typhimurium in the samples that were irradiated at 1, 2, 4 and 5 kGy at day 60. A dose-dependent effect on the inactivation of S. Typhimurium in the samples from day 0 to day 45 (p < 0.05). It has been indicated that the level of injuries caused by gamma irradiation to the cells of microorganisms led to the inactivation of the organisms. Thus, the observed pattern in the inactivation of S. Typhimurium can be attributed to this. The significant differences (p < 0.05) in the count of S. Typhimurium with reference to the doses may be due to the effect of the extent to which injuries or lethality of the doses had on the cells. Song et al. (2014) and Deng et al. (2015) had indicated that there is a dose-dependent effect on the inactivation of microorganisms when they are exposed to gamma irradiation. In this study, there was a dose-dependent effect on the inactivation of S. Typhimurium. This might be due to the level of injuries caused to the cells by gamma irradiation. The general reduction in the irradiated samples during the storage period may be attributed to the inability of the S. Typhimurium to recover from the injuries caused to cells as well as cellular content with time.
A stepwise regression analysis (Table 4) was done to determine the effects of the gamma irradiation and storage days on the inactivation of S. enterica serotype Typhimurium.
The inactivation model was determined as log cfu/g (S. enterica serotype Typhimurium)
Where X1 = days of storage and X2 (kGy) as doses of gamma irradiation. The R-square for the model was 88.23% or 0.8823 which was statistically significant (p < 0.05).
Table 4.0 indicates the stepwise regression analysis for the model development for the indication of (S. enterica serotype Typhimurium).
Inactivation of E. coli
Count (log cfu/g) of E. coli reduced with storage in the samples that were irradiated at 1, 2 and 4 until day 30 as well as the unirradiated samples (Table 5). There was no detection of E. coli in the samples that were irradiated at 5 kGy for all the days. There was a dose-dependent effect on the inactivation of E. coli in the samples during the period of the study (p < 0.05). A significant impact of gamma irradiation on the cells of E. coli was observed as the pathogen was inactivated in relation to the doses applied (p < 0.05). Ban and Kang (2014), in a previous study indicated a dose-dependent effect (the higher the dose of gamma irradiation that the organisms were exposed to, the higher the inactivation), in this study, it was observed that there was a dose-dependent effect on the inactivation of E. coli. The reduction in the count of E. coli during the storage period up to day 30 for the irradiated samples might be due to the inability of the injured cells to recover from the effect of gamma irradiation exposure and the inability of the organisms to adapt to the environment (Byun et al. 2001).
A model was developped using the stepwise regression analysis (Table 6) to determine the effects of the gamma irradiation and storage days on the inactivation of E. coli in the samples. The inactivation is expressed as
Where X1 = days of storage and X2 (kGy) as doses of gamma irradiation. The R-square for the model was 94.40% or 0.9440 which was statistically significant (p < 0.05).
Effect of gamma irradiation and storage on some physicochemical components of Legon-18 pepper powder
Capsaicinoids
The main capsaicinoids analyzed during the study were dihydrocapsaicin and capsaicin.
There was a dose-dependent effect on the capsaicinoids of the samples used in the study (p < 0.05). Capsaicinoid content in the samples that were irradiated at 5 kGy were higher than the ones in the unirradiated samples (p < 0.05). Reduction of the contents of the capsaicinoids in the samples (both irradiated and unirradiated) was observed during the storage period (p < 0.05). The observed pattern in the individual capsaicinoids was similar to the pattern in the total capsaicinoids and the Scoville Heat units (since these two are computations based on the individual capsaicinoids analyzed (Table 7)). Byun et al. (1996) indicated that capsaicinoids (dihydrocapsaicin and capsaicin) are stable after irradiation whiles Giuffrida et al. (2014) indicated that gamma irradiation caused an increase in the capsaicinoid content of some pepper varieties. In this study, there was an increase in the capsaicinoid content (21.81% in capsaicin, 16.79% in dihydrocapsaicin and 20.32% in total capsaicinoids in the irradiated samples) of the pepper samples irradiated at 5 kGy in comparison to the unirradiated samples. This observation can be attributed to the change in the conformation of molecules of capsaicinoids and the other compounds from such conformations, as well as the extraction process (Subbulakshmi et al. 1991). The observed reduction (a loss of 34.54% capsaicin in the unirradiated samples and a 22.71% in the irradiated samples; a 48.65% loss in dihydrocapsacin content in the unirradiated samples and a 10.67% in the irradiated samples; 39.54% loss in total capsaicinoid content in the unirradiated samples and a 19.23% in the total capsaicinoid content of the irradiated samples) during the storage of the capsaicinoids (dihydrocapsaicin, capsaicin, total capsaicinoids as well as the SHU) may be attributed to oxidation. The oxidation of the capsaicinoids is due to the effect of some residual enzymatic action as well as the effect of milling (Wang et al. 2009).
Carotenoids
Beta carotene, beta cryptoxanthin and capsanthin were the main carotenoids analyzed in this study (Table 8). The carotenoids in the samples that were irradiated at 5 kGy were lower (8.84% in beta carotene, 26.07% in beta cryptoxanthin and 8.16% in capsanthin) than the ones in the unirradiated (p < 0.05). There was a general reduction in all the carotenoid contents (30.32% loss in beta carotene content of the unirradiated samples and 42.86% in the irradiated samples; a 38.57% loss in beta cryptoxanthin content of the unirradiated samples and 32.90% in the irradiated samples) irrespective of the doses of gamma irradiation applied during storage (p < 0.05). The reduction of the content of the carotenoids analyzed may be due to the effect of gamma irradiation and storage, the absorbed energy from gamma irradiation, oxidation of the carotenoids, free radicals formed during the gamma irradiation process and post gamma irradiation (Topuz and Odzemir 2003; Perez-Galvez and Minguez-Mosquera 2001; Giuffrida et al. 2014).
Conclusion
From the study, gamma irradiation at 5 kGy could inactivate all the pathogens used even on day zero. However, 2 kGy and 4 kGy could also be used for the inactivation of all the pathogens which is subject to storage days. E. coli could be inactivated completely after 30 days of storage with even 1 kGy. L. monocytogenes and S. Typhimurium could be inactivated after irradiation at 2 and 4 kGy if stored for 60 days. The hotness of the pepper samples were higher in the samples that were irradiated at 5 kGy as compared with the unirradiated samples during the storage period despite the effect of storage on the hotness of the samples. The carotenoids analyzed in this study were lower in the irradiated samples as compared with the unirradiated. Thus, gamma irradiation and storage weeks caused a reduction in the carotenoid content of sun-dried Legon-18 pepper powder (p < 0.05).
Availability of data and materials
Data cannot be shared because components are part of an academic work.
Abbreviations
- BD:
-
Becton Dickenson
- BNARI:
-
Biotechnology and Nuclear Agriculture Research Institute
- CDC:
-
Centre for disease control and prevention
- CT-SMAC:
-
Cefixime tellurite sorbitol MacConkey
- GAEC:
-
Ghana Atomic Energy Commission
- HPLC High:
-
Performance liquid chromatography
- kGy:
-
kilogray
- MOFA:
-
Ministry of Food and Agriculture
- PBS:
-
Phosphate buffered saline
- PLS:
-
Palcam listeria selective
- SHU:
-
Scoville heat units
- TC:
-
Total capsaicinoids
- TSB:
-
Tryptic soy broth
- XLD:
-
Xylose lysine deoxycholate agar (XLD agar)
References
Ban G-H, Kang D-H. Effects of gamma irradiation for inactivating Salmonella Typhimurium in peanut butter product during storage. Int J Food Microbiol. 2014;171:48–53.
Banerjee M, Sarkar PK. Microbiological quality of some retail spices in India. Food Res Int. 2003;36:469–74.
Barbero GF, Palma M, Barraso CG. Pressurized liquid extraction of capsaicinoids from peppers. J Agric Food Chem. 2006;54:3231–6.
Buckenhuskes HJ, Rendlen M. Hygienic problems of phytogenic raw material for food production with special emphasis on herbs and spices. Food Sci Biotechnol. 2004;13:262–8.
Byun MW, Kim DH, Yook HS, Cha BS, Kim JO. Changes in microbiological and general qualities in gamma irradiated Doenjang (fermented soyabean paste). Food Science Biotechnol. 2001;10:7–11.
Byun MW, Yook HS, Kwon JH, Kim JO. Improvement of hygiene quality and long-term storage of dried red pepper by gamma-irradiation. Korean Soci Food Sci Technol. 1996;28:482–9.
CDC (2010) Investigation update: multistate outbreak of human Salmonella Montevideo infections. Available at www.cdc.gov/salmonella/montevideo/index.html. Accessed Sept 2017
Cheon H-L, Shin J-S, Park K-H, Chung M-S, Kang D-H. Inactivation of pathogens in red pepper powder (Capsicum annuum L.) using combined UV-C irradiation and mild heat. Food Control. 2015;50:441–5.
Deng W, Wu G, Guo L, Long M, Li B, Liu S, Cheng L, Pan X, Zou L. Effect of gamma irradiation on Escherichia coli, Salmonella Enterica typhimurium and Aspergillus niger in peppers. Food Sci Technol Res. 2015;21(2):241–5.
Doku SK (2015) Genetic diversity studies. In: Twenty accessions of hot pepper (Capsicum Spp L.) in Ghana. A thesis presented to the Department of Nuclear Agriculture and Radiation Processing School of nuclear and allied Sciences University of Ghana.
Ducic M, Klisara N, Markov S, Blagojevic B, Vidakovic A. The fate and pasteurization of-based inactivation of Escherichia coli O157, Salmonella typhimurium and Listeria monocytogenes in dry, fermented sausages. Food Control. 2016;59:400–6.
FAO/WHO Food and Agriculture Organization of the United Nations; World Health Organization. Summary report: joint FAO/WHO expert meeting on microbiological hazards in spice and dried aromatic herbs, 7-10 October 2014. Preliminary report. Rome: Food and Agriculture Organization of the United Nations; 2014.
Fowels J, Mitchell J, McGrath H. Assessment of cancer risk from ethylene oxide residues in spices imported into New Zealand. Food Chem Toxicol. 2001;39:1055e1062.
Giuffrida D, Dugo P, Torre G, Bignardi C, Cavazza A, Corrandi C, Dugo G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid and pungency determination. Food Chem. 2013;140:794–802.
Giuffrida D, Dugo P, Torre G, Bignardi C, Cavazza A, Corrandi C, Dugo G. Evaluation of carotenoid and capsaicinoid and capsaicinoid contents in powder of red chili peppers during one year of storage. Food Res Int. 2014;65:163–70.
Jeong A-R, Jo MJ, Koo M, Ku K-H, Park J-B, Kim HJ. Microbial contamination of fresh-red pepper and packaged-red pepper powder in South Korea. J Food SC Nutr. 2010;15:233–8.
Jo C, Lee NY, Kang HJ, Shin DH, Byun MN. Inactivation of foodborne pathogens in marinated beef rib by ionizing radiation. Food Microbiol. 2004;21:543–8.
Jung K, Song B-S, Kim MJ, Moon B-G, Go S-M, Kim J-K, Lee Y-J, Park J-H. Effect of X-ray, gamma ray, and electron beam irradiation on the hygienic and physicochemical qualities of red pepper powder. LWT-Food Sci Technol. 2015;63:846–51.
MOFA. Chilli Pepper Production Guide. Horticulture Export Industry Initiative. Accra; 2007. p. 9.
Mukhopadhyay S, Ukuku D, Fan X, Juneja VK. Efficacy of integrated treatment of UV light and low dose gamma irradiation on inactivation of Escherichia coli O157:H7 and Salmonella enterica on grape tomatoes. J Food Sci. 2013;78(7):M1049–56.
Orellana-Escobedo L, Garcia-Amezquita LE, Olivas GI, Ornelas-Paz JJ, Sepulveda DR. Capsaicinoids content and proximate composition of Mexican chili peppers (Capsicum spp.) cultivated in the state of Chihuahua. CytA-J Food. 2013;11(2):179–84.
Perez-Galvez A, Minguez-Mosquera MI. Structure-reactivity relationship in the oxidation of carotenoid pigments of the pepper (Capsicum annuum L.). J Agric Food Chem. 2001;49(10):4864–9.
Rico CW, Kim G-R, Ahn J-J, Kim H-K, Furuta M, Kwon J-H. The comparative effect of steaming and irradiation on the physicochemical and microbiological properties of dried red pepper (Capsicum annum L.). Food Chem. 2010;119:1012–6.
Song WJ, Sung HJ, Kim SY, Kim KP, Ryu S, Kang DH. Inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium in black pepper and red pepper by gamma irradiation. Int J Food Microbiol. 2014;172:125–9.
Subbulakshmi G, Udipi S, Raheja R, Sharma A, Padwal-Desai SR, Nair PM. Evaluation of sensory attributes and some quality indices of irradiated spices. J Agric Food Chem. 1991;28(6):396–7.
Tainter DR, Grenis AT. Spices and seasonings—a food technology handbook. 2nd ed. New York: Wiley-VCH; 2001.
Topuz A, Odzemir F. Influences of gamma irradiation and storage on the carotenoids of sun-dried and dehydrated paprika. J Agric Food Chem. 2003;51:4972–7.
Wang Y, Xia Y, Wang J, Luo F, Huang Y. Carotenoids in chili pepper (Capsicum annuum, L.) powder as affected by heating and storage methods. Am Soc Agric Biol Eng. 2009;52(6):2007–10.
Acknowledgments
The authors wish to express their profound gratitude to the technologists at the Department of Bacteriology, Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, Accra-Ghana for the assistance rendered during the microbiology aspect, Radiation Technology Centre (BNARI-GAEC) for the dosimetry and Central Laboratory of KNUST for the analysis of the carotenoids and the capsaicinoids.
Funding
The authors self-funded the study from the data collection, analysis and interpretation to the final write-up of the manuscript.
Author information
Authors and Affiliations
Contributions
BTO, KTD, KKA, FKS and DA were involved in the design of the experiment. BTO, MLA, SWNOM were involved in data collection. BTO was involved in data analysis. All authors were involved in the proof reading of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The data generated for this article did not involve both animal and human subjects which would have warranted the need for ethical clearance. All data obtained and used in the study were on plant material (pepper powder).
Consent for publication
The data herein presented in this article does not contain any individual’s personal data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Odai, B.T., Tano-Debrah, K., Addo, K.K. et al. The role of gamma irradiation and storage at 28 ± 2 °C on the inactivation of Listeria monocytogenes, Escherichia coli and Salmonella enterica serotype Typhimurium in sun-dried Legon-18 pepper (Capsicum annuum) powder. FoodContamination 6, 7 (2019). https://doi.org/10.1186/s40550-019-0077-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40550-019-0077-6