Abstract
Given their dormancy capability (long-term resistant stages) and their ability to colonise and reproduce, microscopic aquatic animals have been suggested having cosmopolitan distribution. Their dormant stages may be continuously moved by mobile elements through the entire planet to any suitable habitat, preventing the formation of biogeographical patterns. In this review, I will go through the evidence we have on the most common microscopic aquatic animals, namely nematodes, rotifers, and tardigrades, for each of the assumptions allowing long-distance dispersal (dormancy, viability, and reproduction) and all the evidence we have for transportation, directly from surveys of dispersing stages, and indirectly from the outcome of successful dispersal in biogeographical and phylogeographical studies. The current knowledge reveals biogeographical patterns also for microscopic organisms, with species-specific differences in ecological features that make some taxa indeed cosmopolitan with the potential for long-distance dispersal, but others with restricted geographic distributions.
Similar content being viewed by others
Introduction
Microscopic animals are generally assumed to be extremely widespread, up to the level of not showing any biogeographical pattern: the first European expeditions to remote areas brought back home surprising new groups of plants and large animals, whereas all the microscopic organisms collected during the same expeditions resembled the species that were already known in Europe [1]. Such empirical scenario of widespread distribution was found across different taxa and at different spatial scales. Among the different rationales that tried to explain the pattern of cosmopolitism in microscopic animals the most known remains nowadays the ‘everything is everywhere’ or the ubiquity hypothesis [2,3,4,5]. The discussion on the lack of biogeography for microscopic organisms focused originally on prokaryotes and unicellular eukaryotes, to then extend also to all microscopic organisms below one or two millimetres in body length, including microscopic multicellular animals [1, 6]. The ubiquity hypothesis for microscopic aquatic animals holds true for continental [7] as well as for marine species [8], for which it is known as the meiofauna paradox: microscopic benthic organisms with little mobility seem to be cosmopolitan, contrary to their lack of dispersal ability and their lack of planktonic larval stages [9].
The inclusion of microscopic animals in the discussion on the ubiquity hypothesis makes comparisons with larger organisms more obvious: microscopic and macroscopic animals have common physiology, similar ecology, and shared evolutionary trajectories [10]. Yet, biogeographical patterns seem to be different between microscopic and large animals, because of the differences in size and the ecological consequences of being microscopic [1, 6].
Three assumptions should be met in order to allow long-distance dispersal in microscopic aquatic animals: dormancy capability, long-term resistance of dormant stages, and ability to colonise and reproduce quickly. Here I review these assumptions and the direct and indirect evidence we have for long-distance dispersal. The review is not meant to be exhaustive on each of the topics, but is designed to introduce the subject and its relevance for our general understanding of movement ecology and biogeography of microscopic aquatic animals, focusing on nematodes, rotifers, and tardigrades, the most notorious microscopic animals with high potentials for long-distance dispersal.
Review
Microscopic aquatic animals, smaller than one or two millimetres [5], are small by definition: they cannot be easily seen with a naked eye and they are considered ecologically different from the large, macroscopic organisms that we see around us [1, 9]. One of the main consequences of being microscopic for aquatic animals is that the ratio between volume and surface of the organism is so small that when the animal is taken out of water it may be unable to retain its internal liquids, which would evaporate very quickly unless some sort of protection is present [11]. The volume to surface ratio may not usually be a problem for an unprotected microscopic animal that lives in osmotically and chemically stable, permanently hydrated habitats, such as large lakes and open oceans. Such animal will have very small or almost no probability of encountering desiccation problems. Moreover, some animals, especially arthropods, have protective integuments to contrast such rapid desiccation. Yet, several microscopic animals with no thick protective integuments live in temporary water bodies that desiccate frequently, for example shallow ponds, bogs, small puddles, intermittent rivers, cryoconites, rock pools, salt marshes, etc. Some microscopic aquatic animals even adapted to live in the thin and ephemeral water layers surrounding mosses, lichens, and soils, in what is called a limno-terrestrial habitat: an aquatic microscopic environment (the ‘limno’ part) in an otherwise terrestrial ecosystem. The most common and abundant animals living in these habitats, especially in continental freshwater settings, are nematodes, rotifers, and tardigrades, a community of microscopic animals collectively known as meiofauna [8, 9]. Other microscopic aquatic animals exist and are part of the meiofauna, e.g. gastrotrichs, loriciferans, kinorhynchs, etc., but they do not have the dormancy capabilities of nematodes, rotifers, and tardigrades that make them suitable candidates for long-distance transportation, dispersal and cosmopolitan distribution [12].
The three animal groups included in this review belong to different evolutionary lineages in the metazoan tree of life [10], but they all share small body size, usually much less than 1 mm, together with the ability to survive lack of water through dormancy [13, 14] and other peculiar features that will be introduced in this review. Overall, more than size in itself, the consequences of small size are relevant for dispersal abilities [11, 15], and these peculiar ecological features will be the subject of the first part of the review.
Dormancy
Nematodes, rotifers, and tardigrades independently evolved adaptations to survive adverse periods without water [16,17,18,19]: as soon as conditions are not favourable with liquid water becoming unavailable because of evaporation or of freezing, some animals are able to produce dormant stages to allow the following generation to recover a viable population when liquid water becomes available again [16]. Such dormant stages are known as resting eggs in rotifers and in tardigrades [20, 21], and eggs in nematodes [22]. They are dormant embryos and not actual eggs [23], and they are produced both by parthenogenesis and by sexual reproduction. There is an ample literature on the triggers that drive the production of resting stages, and also on the mechanisms that are put in place to maximise the efficacy of such stages to maintain viable populations through bet-hedging strategies, especially for microscopic zooplankton animals [24]. The production of dormant stages that accumulate at the bottom of an ephemeral water body is also considered the main mechanism that structures community and population dynamics for microscopic animals with high genetic differentiation at the local and landscape level [25], in what was named monopolisation hypothesis [26]. These communities, structured by the interplay between the buffering effect of dormant stages acting against new colonisers and the dispersal of dormant propagules from other populations [27, 28], are one of the most studied examples within the metacommunity framework [29, 30]. The research output of community ecology connected to dispersal in microscopic aquatic animals is highly productive, but will be only marginally considered in this review, given that it involves local or landscape-level settings, and not proper long-distance dispersal across continents.
Microscopic animals can withstand lack of liquid water through other mechanisms, not only through the production of dormant embryos: bdelloid rotifers and tardigrades can simply contract into a ‘tun’ shape and nematodes can coil, losing most if not all of their internal water, halting any metabolic activity, and remaining in this dormant condition until water becomes available again [31, 32]. The physiological mechanisms allowing these animals to remain viable while desiccated during dormancy are still under study, and are thought to involve protecting molecules such as sugars, late embryogenesis abundant proteins, antioxidants, etc. [33,34,35], in addition to the ability to recover their broken DNA when rehydrated [36]. Two main scenarios have been identified on the effect of dormancy on life span: either metabolism is completely halted and animals do not age during dormancy (the ‘sleeping beauty’ model) or some stress acts on the animals, which do age slowly while dormant (the ‘picture of Dorian Gray’ model) [37]. Such differences have obvious consequences for long-term viability, which can be much longer for groups that follow the ‘sleeping beauty’ model [38]. Rotifers and tardigrades seem to follow the ‘sleeping beauty’ model, whereas nematodes seem to follow the ‘picture of Dorian Gray’ model [37,38,39,40].
Dormancy may be attained several times during the life span of an organism: for example, meiofauna living on a lichen patch on a tree trunk at temperate latitudes may desiccate every day under the sun in the morning and resume activity every evening when moisture levels increase again with enough water in the thin layers surrounding the lichen to allow the animals to move and feed. Given that the life span of these animals is usually more than a month [20, 22, 41, 42], they may experience several tens of cycles of desiccation and resurrection during their life. Desiccated dormant animals can be easily moved by wind through the landscape, and this is considered the main mechanism that strictly asexual bdelloid rotifers use to survive their arm-race against fungal parasites in a hide-and-seek continuous movement to new lichen patches temporarily without parasites [43].
One caveat about generalisations on dormancy is that interspecific variation in survival during dormancy is known in nematodes [44, 45], rotifers [46,47,48], and tardigrades [49,50,51]. Thus, even if these three groups of microscopic aquatic animals indeed have the capacity to survive adverse conditions through dormancy, there is a degree of species-specific survival capabilities while dormant. Some species are indeed known not to survive dormancy at all [21, 22, 52].
Long-term viability
The simple fact of being small forces microscopic aquatic animals to develop mechanisms to survive lack of water, either through the production of dormant propagules such as protected resting embryos that will resume development after rehydration, or through the dormancy capability of the animals themselves, able to enter a desiccated stage at any time of their life [16]. For dormant stages of any type to be successful in long-distance transportation and successful dispersal, they have to remain under dormancy for long time, enough at least to be moved by mobile vectors across continents, and then to be able to recover and ‘resurrect’ [16]. Dormant stages have also to survive adverse conditions other than desiccation, such as high and low temperatures, oxidative stress, UV radiation, digestive enzymes, etc. [53,54,55]; at the same time, they still need to react to external stimuli, in order to hatch when environmental conditions are favourable [19, 56, 57].
The potential maximum length of long-term viability of dormant stages is unknown and not easy to test; yet, there is evidence that such survival can be extremely long. Dormant animals of the limno-terrestrial meiofauna could be recovered after more than 10 years of artificial dry storage of lichens in museum collections [58]. The longest unambiguous survival times for adult dormant animals are those of tardigrades from dry mosses, which are up to 20 years [50, 59,60,61], and those of nematodes from sediments, even more than 30 years [22, 62, 63].
Regarding resting embryos, the oldest viable resting eggs of rotifers that have been successfully hatched from sediments were older than 100 years and belonged to two species of the genus Brachionus, B. calyciflorus and B. plicatilis [64, 65]. Resting stages of other small aquatic animals like copepods are known to remain viable even longer, for a few centuries [66, 67]. Thus, the potential for long-term viability is an actual feature of meiofauna, both for resting stages and for dormant animals.
As a consequence of their dormancy capabilities, the three main groups of the meiofauna, nematodes, rotifers, and tardigrades, are able to cope with the extreme conditions of the most hostile places for life on Earth, being for example the only metazoans thriving in polar deserts [68,69,70,71,72]. As a by-product of their resistance, they have been shown to remain viable also when exposed to environmental conditions that they likely never experienced during their evolution, such as those of the outer space [73,74,75,76].
Another ecological consequence of long-term viability during dormancy for microscopic aquatic animals is that they can be found even outside of their ecological niche [77]. For large animals, because of dispersal limitations, the realised niche (where the organism is actually found) is geographically nested within the fundamental niche (the areas where the organism could survive) [78]: for example, a mammal or a beetle living in Eurasiatic temperate forests may be absent from the North-American equivalent forests not because of habitat unsuitability, but simply because it never arrived there. For microscopic aquatic animals, one can speculate that if the assumption of reduced dispersal limitation because of dormancy resistance is true at least for some species, the realised niche (where such species are found) can in principle expand well beyond the fundamental niche (where the species survives): sink populations, where population growth rate is negative, can be maintained by propagules coming from large source populations placed in suitable areas [78]. This pattern is known for other organisms with dormant dispersing propagules, such as thermophilic bacteria in cold soils [79], or in the continuous recruitment of marine algae in unstable environments from more stable source populations [80]. The discrepancy between a large fundamental niche and a smaller realised niche in large organisms makes them considered as “alien species” when displaced elsewhere by human activities [81]. If microscopic aquatic animals are already cosmopolitan, in principle, no alien species should exist, because they are already present wherever human activities may bring them: the issue of alien species in nematodes, rotifers, and tardigrades will be discussed later, when dealing with biogeographical evidence of long-distance dispersal.
Parthenogenesis
Reproductive capabilities do not matter for long-distance transportation and dispersal per se, but will affect the possibility of the propagule to effectively colonise the new place where it lands. Nematodes, rotifers, and tardigrades share dormancy capabilities and the possibility of parthenogenetic reproduction: a single dormant female or a dormant embryo can start a new population after recovering from dormancy. Different groups have different parthenogenetic strategies. Bdelloid rotifers are strictly asexual, monogonont rotifers reproduce by cyclical parthenogenesis [82], tardigrades and nematodes have different degrees of parthenogenesis and hermaphroditism between species [22, 83] and sometimes even between different populations within species depending on the habitat [84]. Thus, almost all species of nematodes, rotifers, and tardigrades have the potential to rapidly develop a new population, even if only one single propagule arrives through long-distance transportation.
Evidence of long-distance dispersal
Microscopic aquatic animals like nematodes, rotifers, and tardigrades fulfil the assumptions of being small, surviving in dormant state, and reproducing rapidly when conditions return suitable. Because of these features, they have been assumed to be able to disperse passively across the globe [4, 5, 85, 86]. Long-distance successful dispersal, defined as low frequency dispersal events in the tail of the dispersal kernel [87], does not need to be a common phenomenon, and even a relatively small number of successful propagules can be enough to allow these animals to be geographically widespread [88]. Yet, do we have evidence that indeed they do disperse? Direct studies of transportation and dispersal through capture, marking, and recapturing techniques [89] are impossible for meiofauna. So, how can people discuss about their long-distance dispersal? We have two main lines of evidence: on one hand, there is direct evidence that microscopic animals can passively move across the landscape; on the other hand, there is indirect evidence of actual cosmopolitism from biogeographical studies that use a morphological approach in species identification, and phylogeographical studies that analyse the geographic distribution of DNA sequences within species. Here, I provide a brief overview of such direct and indirect evidence.
Direct evidence
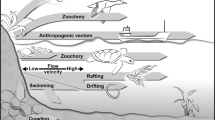
Microscopic animals can be passively dispersed from one place to another by mobile vectors such as larger animals that are able to actively move across the landscape or by wind and water [90]. Dispersal of dormant stages allows meiofaunal organisms to escape unsuitable environmental conditions, to temporarily avoid competition and parasitism, and thus to move to distant areas and new habitats [91].
Transportation and dispersal by a mobile element such as an animal vector is a known mechanism in movement ecology and is commonly called phoresy [92]. Phoresy is a temporary symbiotic interaction between one organism and a host for the purpose of travel. Known examples of phoresy involve ticks, mites, and pseudoscorpions attaching to larger arthropods and to vertebrates and has been described even in fossils from several million of year ago [92]. Microscopic aquatic animals are also known to hitchhike with phoretic relationships [93], but the phenomenon is not so common.
Microscopic aquatic animals have been found dispersing by epizoochory, externally attached to a variety of larger animals [94]: for example, on earthworms [95], beetles [96], water bugs [97], fish [98], treefrogs [99], flying foxes [100], wild boars [101], nutrias [102], and even humans [103, 104]. Endozoochory, the transportation through the digestive gut of large animals, is also possible for microscopic aquatic animals, and viable invertebrates have been found in faeces of vertebrates [105, 106]. These examples are relevant to show that meiofauna can be passively dispersed by mobile elements across the landscape [107]. Yet, such mobile elements (except for humans) cannot be the cause of the widespread distribution of several species of nematodes, rotifers, and tardigrades, because the hosts themselves are not so widely distributed across continents and their distributions are indeed limited by barriers to active dispersal [108]. Thus, notwithstanding the relevant effect of phoresy and endozoochory by macroscopic animals for community ecology at the landscape level, only limited effects can be expected for long-distance dispersal and biogeography of meiofauna.
The only mobile animals that could allow meiofauna to disperse across large distances are migratory birds [109]: indeed microscopic animals have been found associated to birds. Since Darwin’s time researchers placed feet of ducks in water and performed other experiments to find viable microscopic animals attached to or in the gut of migratory birds [7, 110,111,112]. Yet, dispersal of meiofauna through birds may not be as effective as for seeds of plants [105], and can be considered plausible only for those microscopic animals that live in shallow water or in other habitats that are frequently visited by birds. This can be true for marshes and ponds [110], rock pools [113], littoral sediments [114], and even vegetation eaten by birds [109], but birds cannot be considered a suitable mobile element for meiofauna living for example in vertical lichen patches on tree trunks or in other habitats not visited by birds.
The most plausible mobile elements that can disperse dormant propagules of all microscopic aquatic animals regardless of the habitat are wind for terrestrial meiofauna and water for marine meiofauna. Colonisation experiments of mesocosms designed to selectively exclude different mobile elements indeed found meiofauna when allowing only wind as a carrier, and also demonstrated that the effect of wind was much stronger than that of phoretic animals in dispersing propagules across the landscape [115,116,117,118,119]. Meiofauna can be found dispersing through wind and water [120,121,122,123,124,125,126], and dormant propagules of meiofauna can be found in windsocks [127], on sticky traps [128], or in desert dust storms [129]. It is true that the number of dormant propagules found in direct sampling of wind and air is usually low, but even a small number of successful dormant propagules is anyway predicted to allow for effective long-distance dispersal, especially if those propagules can rapidly develop large populations via parthenogenesis [87, 125, 130, 131].
A mechanism for trans-oceanic dispersal between continents, demonstrated at least for nematodes, is rafting: some nematodes are able to move across long distances inside rhizome fragments that are dispersed by seawater [132]. The same holds true also within the marine environment, where benthic meiofauna can be found in the water column [133, 134] and in suspended sediment traps physically disconnected from the bottom [135].
Overall, our current understanding is that dormant propagules of nematodes, rotifers, and tardigrades have the potential for long-distance dispersal and propagules for some species have been found to be transported and dispersed globally. Nevertheless, a quantification of the role of such potential long-distance dispersal on the biogeography of microscopic aquatic animals is unavailable, and the relative role of animal-mediated, wind-mediated, and water-mediated effect on dispersal is still debated. Unfortunately, no quantification of long-distance transportation and dispersal is possible and no quantitative modeling has been developed yet to address such issue, contrary to what has been successfully done for seed dispersal in plants [136, 137].
Biogeography
The widespread distribution of microscopic organisms was the first evidence to indirectly suggest the possibility of long-distance dispersal, within the rationale of the ‘everything is everywhere’ or ubiquity hypothesis [5]. Indeed, several species of nematodes, rotifers, and tardigrades are known to occur in different continents. On the other hand, there is also a long list of species from these phyla with restricted distribution, making any generalisation on their biogeography ambiguous.
Nematodes with limited dispersal abilities have biogeographical patterns even at the genus level, with several genera having different species in different continents [138]. Different continents have a different composition in genera and families with consistent differences [12]. Differences in species composition between nematode communities correlate with geographic distances across continental scales [139]. Thus, for nematodes, biogeography seems to exist and cosmopolitism is an exception.
In rotifers, most species are considered cosmopolitan [140] and surveys usually find a large proportion of cosmopolitan species [141, 142], but endemic species exist too [143]. In addition to a large number of cosmopolitan species, a large representation of the global diversity is usually found in local samples: any single temperate or tropical water body is expected to host between 150 and 250 species, that is up to 12% of global worldwide diversity found locally in one single water body [144]. Rotifer biogeography is a complicated issue: biogeographical patterns exist, but are hard to see and occur mostly at a continental scale [145, 146].
In tardigrades, the distribution of genera and families is different between landmasses originating from Laurasia and Gondwana [147,148,149]. Of the 64 limno-terrestrial genera of tardigrades, 11 are considered endemic at continental level [12], and continental endemism at the species level can be up to 60% [148]. In addition, species of the genus Mopsechiniscus follows the geological division of Gondwanan landmasses [150]. For tardigrades, biogeography indeed makes sense.
Among the main problems in assessing biogeographical patterns for all organisms, large and small, are lack of data and sampling bias [151, 152]. Our knowledge on the distribution of microscopic aquatic animals suffers from such issues even more than for larger organisms [145, 153]: for example, after compiling all published records on monogonont rotifers from over 1800 published papers across more than 30 years, the most significant predictor of species richness remained sampling effort, obscuring a negligible effect of spatial and environmental drivers [146]. The same lack of data and sampling bias hold true also for tardigrades [154].
The probability of finding endemic species in meiofauna negatively correlates with species-specific dispersal abilities [155]. In general, rotifers seem to have low endemism, whereas nematodes and tardigrades seem to have clear biogeographical patterns.
An interesting support against the claim of cosmopolitism in microscopic aquatic animals is the presence of alien species: they exist also for microscopic aquatic animals, even in rotifers [156,157,158,159,160], suggesting that at least some species were not able to colonise some areas before they were introduced recently by human activities.
Phylogeography
Phylogeographic structures in small organisms can be very complex, with interacting effects of local adaptation buffering against newcomers and the action of geographically structured gene flow between diverse lineages from different geographic areas [161, 162]. Priority effects, founder events, and genetic bottlenecks are common scenarios found in spatially explicit surveys of genetic diversity for nematodes [163,164,165], rotifers [166], and tardigrades [167], both in freshwater and in marine water bodies.
When using DNA sequence data to analyse spatial structure in the distribution of microscopic aquatic animals, researchers realised that our understanding of diversity was highly biased, with several species clearly indicated as independently evolving entities that could not be identified as separated with a morphological approach [168]. A large number of cryptic species is continuously discovered in almost all analysed meiofaunal taxa [169,170,171,172]. Thus, if species that were considered cosmopolitan are in reality complexes of geographically restricted species, the biogeographical inference based on morphology could be misled by inappropriate identification of taxonomic units. Yet, again, also in the case of cryptic species complexes, there are examples where several of the actual species of the complex came out as widespread [173] and examples where each of the species in the complex had geographically restricted distribution.
In nematodes, both long-distance dispersal and restricted gene flow was found using DNA sequence information [164, 174].
In rotifers, the most studied species complex, Brachionus plicatilis, for which hundreds of populations have been sequenced, is composed in reality by at least 15 different species, but most of them have cosmopolitan distributions [175, 176], even if often with local phylogeographic structures [177,178,179,180]. The same global distribution but with local genetic structure is found in bdelloid rotifers too [181, 182].
In tardigrades, geographically restricted species within a species complex, previously considered cosmopolitan but now known to be composed of several taxa with limited distribution were found [170], for example in the marine tardigrade Echiniscoides sigismundi, where populations display extreme subdivision and contain deeply divergent lineages that are fully or nearly restricted to single sampling sites [167, 183, 184]. Echiniscus testudo seems to be a single species and not a complex, but indeed very widespread, if not cosmopolitan [61, 185]. Antarctic tardigrades seem to have several narrowly distributed species [186].
Most of the studies using DNA sequence data, similarly to the studies on biogeography, reveal little or no connection between geographic and genetic distances between populations, but provide evidence of limited distributions, potentially linked to dispersal limitation, together with some examples of truly cosmopolitan distributions. Thus, until now, not even quantitative methods from population genetics in spatially explicit contexts allowed generalisations and quantifications of the role of long-distance dispersal in shaping the distribution of nematodes, rotifers, and tardigrades.
Conclusions
The whole range of biogeographic patterns seems to be present in nematodes, rotifers and tardigrades, from actual cosmopolitan species through long-distance dispersal, according to the ‘everything is everywhere’ hypothesis [5], to endemic ones, more in line with the moderate endemicity hypothesis [187], similar to what is now known also in other microscopic organisms [188, 189], dismissing the general view that microscopic aquatic organisms are uninteresting for biogeography because they are all cosmopolitan [1].
Such broad diversity in biogeographical, and consequently phylogeographical, patterns seem to be related to species-specific dormancy capability: similar to what happens in many terrestrial species of plants [190], species-specific limited dormancy and dispersal capacity can restrict the distribution of some species of microscopic aquatic animals. Species with higher dispersal potential exhibit greater viability of dormant stages [191, 192]. The importance of dispersal differs greatly among and within species, depending on their life histories [7, 193]. Some species of microscopic aquatic animals are indeed cosmopolitan, but not all [1].
It is true that microscopic aquatic animals have incredible dormancy capabilities [40] in principle allowing them to be passively dispersed, but their actual dispersal depends on several factors, both internal (e.g. species-specific dormancy strategy, long-term viability, etc.) and external (e.g. habitat type, mobile element, etc.). Because of species-specific differences in dormancy, potential for long-distance dispersal, biogeographical and phylogeographical patterns, and in spatial structure, the study of dispersal and biogeography of microscopic aquatic animals, contrary to being uninteresting, may become highly relevant to support or refute the generality of the patterns and processes assumed for larger organisms [193, 194]. Moreover, because of their small size, the use of such animals as biogeographical models may open new frontiers in biodiversity and biogeography, even with experimental biogeography, which will be impossible to achieve with larger organisms [193,194,195].
References
Fontaneto D. Biogeography of microscopic organisms, is everything small everywhere? Cambridge: Cambridge University Press; 2011.
Beijerinck MW. De infusies en de ontdekking der backteriën. Jaarboek van de Koninklijke Akademie v. Wetenschappen. Amsterdam: Müller; 1913.
Baas-Becking LGM. Geobiologie of inleiding tot de milieukunde. The Hague: W.P. Van Stockum & Zoon; 1934.
Finlay BJ. Global dispersal of free-living microbial eukaryote species. Science. 2002;296:1061–3.
Fenchel T, Finlay BJ. The ubiquity of small species: patterns of local and global diversity. BioScience. 2004;54:777–84.
Incagnone G, Marrone F, Barone R, Robba L, Naselli-Flores L. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia. 2015;750:103–23.
Bohonak AJ, Jenkins DG. Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecol Lett. 2003;6:783–96.
Warwick RM. The contrasting histories of marine and freshwater meiobenthic research – a result of differing life histories and adaptive strategies? J Exp Mar Biol Ecol. 2018;502:4–11.
Giere O. Meiobenthology, the microscopic fauna in aquatic sediments. Berlin: Springer-Verlag; 1993.
Edgecombe GD, Giribet G, Dunn CW, Hejnol A, Kristensen RM, Neves RC, Rouse GW, Worsaae K, Sørensen MV. Higher-level metazoan relationships: recent progress and remaining questions. Org Divers Evol. 2011;11:151–72.
Hildrew AG, Raffaelli DG, Edmonds-Brown R. Body size: the structure and function of aquatic ecosystems. Cambridge: Cambridge University Press; 2007.
Artois T, Fontaneto D, Hummon WD, McInnes SJ, Todaro MA, Sorensen MV, Zullini A. Ubiquity of microscopic animals? Evidence from the morphological approach in species identification. In: Fontaneto D, editor. Biogeography of microscopic organisms. Cambridge: Cambridge University Press; 2011. p. 244–83.
Wharton DA. Anhydrobiosis. Curr Biol. 2015;25:R1114–6.
Møbjerg N, Halberg KA, Jørgensen A, Persson D, Bjørn M, Ramløv H, Kristensen RM. Survival in extreme environments–on the current knowledge of adaptations in tardigrades. Acta Physiol. 2011;202:409–20.
Jenkins DG, et al. Does size matter for dispersal distance? Glob Ecol Biogeogr. 2007;16:415–25.
Càceres CE. Dormancy in invertebrates. Invert Biol. 1997;116:371–83.
Alpert P. The limits and frontiers of desiccation-tolerant life. Int Comp Biol. 2005;45:685–95.
Watanabe M. Anhydrobiosis in invertebrates. Appl Entomol Zool. 2006;41:15–31.
Schröder T. Diapause in monogonont rotifers. Hydrobiologia. 2005;546:291–306.
Wallace RL, Snell TW, Ricci C, Nogrady T. Rotifera. Vol. 1. Biology, Ecology and Systematics (2nd ed.). In: HJF D, editor. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World (vol. 23). Ghent: Kenobi Productions; 2006. p. 1–299.
Guidetti R, Altiero T, Rebecchi L. On dormancy strategies in tardigrades. J Ins Physiol. 2011;57:567–76.
McSorley R. Adaptations of nematodes to environmental extremes. Florida Ent. 2003;86:138–42.
Boschetti C, Leasi F, Ricci C. Developmental stages in diapausing eggs: an investigation across monogonont rotifer species. Hydrobiologia. 2011;662:149–55.
Garcia-Roger EM, Carmona MJ, Serra M. Modes, mechanisms and evidence of bet hedging in rotifer diapause traits. Hydrobiologia. 2017; https://doi.org/10.1007/s10750-016- 2869-5.
Brendonck L, Pinceel T, Ortells R. Dormancy and dispersal as mediators of zooplankton population and community dynamics along a hydrological disturbance gradient in inland temporary pools. Hydrobiologia. 2017;796:201–22.
De Meester L, Gómez A, Okamura B, Schwenk K. The monopolization hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol. 2002;23:121–35.
Shurin JB. Dispersal limitation, invasion resistance, and the structure of pond zooplankton communities. Ecology. 2000;81:3074–86.
Shurin JB, Cottenie K, Hillebrand H. Spatial autocorrelation and dispersal limitation in freshwater organisms. Oecologia. 2009;159:151–9.
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett. 2004;7:601–13.
De Bie T, De Meester L, Brendonck L, Martens K, Goddeeris B, et al. Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecol Lett. 2012;15:740–7.
Ricci C, Melone G, Santo N, Caprioli M. Morphological response of a bdelloid rotifer to desiccation. J Morphol. 2003;257:246–53.
Rebecchi L, Altiero T, Guidetti R. Anhydrobiosis: the extreme limit of desiccation tolerance. Invert Surv J. 2007;4:65–81.
Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812.
Boschetti C, Pouchkina-Stantcheva N, Hoffmann P, Tunnacliffe A. Foreign genes and novel hydrophilic protein genes participate in the desiccation response of the bdelloid rotifer Adineta ricciae. J Exp Biol. 2011;214:59–68.
Rebecchi L. Dry up and survive: the role of antioxidant defences in anhydrobiotic organisms. J Limnol. 2013;72:62–72.
Hespeels B, Knapen M, Hanot-Mambres D, Heuskin A-C, Pineux F, Lucas S, Koszul R, Van Doninck K. Gateway to genetic exchange? DNA double-strand breaks in the bdelloid rotifer Adineta vaga submitted to desiccation. J Evol Biol. 2014. https://doi.org/10.1111/jeb.12326.
Ricci C, Pagani M. Desiccation of Panagrolaimus rigidus (Nematoda): survival, reproduction and the influence on the internal clock. Hydrobiologia. 1997;347:1–13.
Ricci C, Covino C. Anhydrobiosis of Adineta ricciae: costs and benefits. Hydrobiologia. 2005;546:307–14.
Hengherr S, Brummer F, Schill RO. Anhydrobiosis in tardigrades and its effects on longevity traits. J Zool. 2008;275:216–20.
Ricci C. Bdelloid rotifers: ‘sleeping beauties’ and ‘evolutionary scandals’, but not only. Hydrobiologia. 2017;796:277–85.
Traunspurger W. The biology and ecology of lotic nematodes. Freshwat Biol. 2000;44:29–45.
Nelson DR. Current status of the Tardigrada: evolution and ecology. Int Comp Biol. 2002;42:652–9.
Wilson C, Sherman P. Anciently asexual bdelloid rotifers escape lethal fungal parasites by drying up and blowing away. Science. 2010;327:574–6.
Grewal PS. Anhydrobiotic potential and long-term storage of entomopathogenic nematodes (Rhabditida: Steinernematidae). Int J Parasitol. 2000;30:995–1000.
Shannon AJ, Browne JA, Boyd J, Fitzpatrick DA, Burnell AM. The anhydrobiotic potential and molecular phylogenetics of species and strains of Panagrolaimus (Nematoda, Panagrolaimidae). J Exp Biol. 2005;208:2433–45.
Ricci C. Anhydrobiotic capabilities of bdelloid rotifers. Hydrobiologia. 1998;387/388:321–6.
Schröder T. Colonising strategies and diapause of planktonic rotifers (Monogononta, Rotifera) during aquatic and terrestrial phases in a floodplain (lower Oder Valley, Germany). Int Rev Hydrobiol. 2001;86:635–60.
Ricci C, Caprioli M. Anhydrobiosis in bdelloid species, populations and individuals. Int Comp Biol. 2005;45:759–63.
Wright JC. Desiccation tolerance and water-retentive mechanisms in tardigrades. J Exp Biol. 1989;142:267–92.
Rebecchi L, Guidetti R, Borsari S, Altiero T, Bertolani R. Dynamics of long-term anhydrobiotic survival of lichen-dwelling tardigrades. Hydrobiologia. 2006;558:23–30.
Faurby S, Jönsson KI, Rebecchi L, Funch P. Variation in anhydrobiotic survival of two eutardigrade morphospecies: a story of cryptic species and their dispersal. J Zool. 2008;275:139–45.
Eyres I, Boschetti C, Crisp A, Smith TP, Fontaneto D, Tunnacliffe A, Barraclough TG. Horizontal gene transfer in bdelloid rotifers is ancient, ongoing and more frequent in species from desiccating habitats. BMC Biol. 2015;13:90.
Rebecchi L, Cesari M, Altiero T, Frigieri A, Guidetti R. Survival and DNA degradation in anhydrobiotic tardigrades. J Exp Biol. 2009;212:4033–9.
Rizzo AM, Negroni M, Altiero T, Montorfano G, Corsetto P, Berselli P, Berra B, Guidetti R, Rebecchi L. Antioxidant defences in hydrated and desiccated states of the tardigrade Paramacrobiotus richtersi. Comp Biochem Physiol B: Biochem Mol Biol. 2010;156:115–21.
Wełnicz W, Grohme MA, Kaczmarek Ł, Schill RO, Frohme M. Anhydrobiosis in tardigrades—the last decade. J Insect Physiol. 2011;57:577–83.
Hagiwara A, Hoshi N, Kawahara F, Tominaga K, Hirayama K. Resting eggs of the marine rotifer Brachionus plicatilis Müller: development, and effect of irradiation on hatching. Hydrobiologia. 1995;313:223–9.
Altiero T, Bertolani R, Rebecchi L. Hatching phenology and resting eggs in tardigrades. J Zool. 2010;280:290–96. https://doi.org/10.1111/j.1469-7998.2009.00664.x.
Fontaneto D, Bunnefeld N, Westberg M. Long-term survival of microscopic animals under desiccation is not so long. Astrobiology. 2012;12:863–9.
Guidetti R, Jönsson KI. Long-term anhydrobiotic survival in semi-terrestrial micrometazoans. J Zool. 2002;257:181–7.
Bertolani R, Guidetti R, Jönsson KI, Altiero T, Boschini D, Rebecchi L. Experiences with dormancy in tardigrades. J Limnol. 2004;63:16–25.
Jørgensen A, Møbjerg N, Kristensen RM. A molecular study of the tardigrade Echiniscus testudo (Echiniscidae) reveals low DNA sequence diversity over a large geographical area. J Limnol. 2007;66(Suppl):77–83.
Steiner G, Albin FM. Resuscitation of the nematode Tylenchus polyhypnus, n. Sp., after almost 39 years dormancy. J Wash Acad Sci. 1946;36:97–9.
Fielding MJ. Observations on the length of dormancy in certain plant infecting nematodes. Proc Helminth Soc Wash. 1951;18:110–2.
Fu Y. Studies on genetic variations of the rotifer Brachionus plicatilis O.F. Muller. PhD Thesis, Nagasaki University. 1991.
Piscia R, Guilizzoni P, Fontaneto D, Vignati DAL, Appleby PG, Manca M. Dynamics of rotifer and cla- doceran resting stages during copper pollution and recovery in a subalpine lake. Ann Limnol – Int J Limnol. 2012;48:151–60.
Hairston NG Jr, Van Brunt RA, Kearns CM, Engstrom DR. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology. 1995;76:1706–11.
Hairston NG Jr. Zooplankton egg banks as biotic reservoirs in changing environments. Limnol Oceanogr. 1996;41:1087–92.
Treonis AM, Wall DH, Virginia RA. Invertebrate biodiversity in Antarctic dry valley soils and sediments. Ecosystems. 1999;2:482–92.
Courtright EM, Wall DH, Virginia RA. Determining habitat suitability for soil invertebrates in an extreme environment: the McMurdo dry valleys. Antarctica Antarct Sci. 2001;13:9–17.
Convey P, McInnes SJ. Exceptional tardigrade-dominated ecosystems in Ellsworth Land. Antarctica Ecology. 2005;86:519–27.
Sohlenius B, Boström S. The geographic distribution of metazoan microfauna on East Antarctic nunataks. Polar Biol. 2005;28:439–48.
Fontaneto D, Iakovenko N, De Smet WH. Diversity gradients of rotifer species richness in Antarctica. Hydrobiologia. 2015;761:235–48.
Ricci C, Caprioli M, Boschetti C, Santo N. Macrotrachela quadricornifera featured in a space experiment. Hydrobiologia. 2005;534:239–44.
Jönsson KI, Rabbow E, Schill RO, Harms-Ringdahl M, Rettberg P. Tardigrades survive exposure to space in low earth orbit. Curr Biol. 2008;18:R729–31.
Rebecchi L, Altiero T, Guidetti R, Cesari M, Bertolani R, Negroni M, Rizzo AM. Tardigrade resistance to space effects: first results of experiments on the LIFE-TARSE mission on FOTON-M3 (September 2007). Astrobiology. 2009;9:581–91.
Persson D, Halberg KA, Jorgensen A, Ricci C, Mobjerg N, Kristensen RM. Extreme stress tolerance in tardigrades: surviving space conditions in low earth orbit. J Zool Syst Evol Res. 2011;49(S1):90–7.
Fontaneto D, Hortal J. At least some protist species are not ubiquitous. Mol Ecol. 2013;22:5053–5.
Soberón J, Nakamura M. Niches and distributional areas: concepts, methods, and assumptions. Proc Natl Acad Sci U S A. 2009;106:19644–50.
Marchant R, Franzetti A, Pavlostathis SG, Tas DO, Erdbrűgger I, Űnyayar A, Mazmanci MA, Banat IM. Thermophilic bacteria in cool temperate soils: are they metabolically active or continually added by global atmospheric transport? Appl Microbiol Biotechnol. 2008;78:841–52.
Reed DC, Laur DR, Ebeling AW. Variation in algal dispersal and recruitment: the importance of episodic events. Ecol Monogr. 1988;58:321–35.
Catford JA, Jansson R, Nilsson C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib. 2009;15:22–40.
Fontaneto D, De Smet WH. Rotifera, Chapter 4. In: Schmidt-Rhaesa A, editor. Handbook of Zoology, Gastrotricha, Cycloneuralia and Gnathifera. Volume 3, Gastrotricha and Gnathifera. Berlin: De Gruyter GmbH; 2015. p. 217–300.
Gilabert A, Wasmuth JD. Unravelling parasitic nematode natural history using population genetics. Trends Parasitol. 2013;29:438–48.
Bertolani R, Rebecchi L, Beccaccioli G. Dispersal of Ramazzottius and other tardigrades in relation to type of reproduction. Invert Reprod Dev. 1990;18:153–87.
Bilton DT, Freeland JR, Okamura B. Dispersal in freshwater invertebrates. Annu Rev Ecol Syst. 2001;32:159–81.
Rundle SD, Foggo A, Choiseul V, Bilton DT. Are distribution patterns linked to dispersal mechanisms? An investigation using pond invertebrate assemblages. Freshwat Biol. 2002;47:1571–81.
Nathan R. Long-distance dispersal of plants. Science. 2006;313:786–8.
Ronce O. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol Syst. 2007;38:231–53.
Southwood TRE, Henderson PA. Ecological methods. Oxford, UK: Blackwell; 2000.
Maguire B. The passive dispersal of small aquatic organisms and their colonization of isolated bodies of water. Ecol Monogr. 1963;33:161–85.
Bonte D, Dahirel M. Dispersal: a central and independent trait in life history. Oikos. 2017;126:472–9.
Signe White P, Morran L, de Roode J. Phoresy. Curr Biol. 2017;27:R578–80.
Krishnan A, et al. A hithchiker’s guide to a crowded synconium: how do fig nematodes find the right ride? Funct Ecol. 2010;24:741–9.
Vanschoenwinkel B, Waterkeyn A, Nhiwatiwa T, Pinceel T, Spooren E, Geerts A, Clegg B, Brendonck L. Passive external transport of freshwater invertebrates by elephant and other mud-wallowing mammals in an African savannah habitat. Freshwat Biol 2011;56:1606–19.
MacMillan K, Haukeland S, Rae R, Young I, Crawford J, Hapca S, Wilson M. Dispersal patterns and behaviour of the nematode Phasmarhabditis hermaphrodita in mineral soils and organic media. Soil Biol Biochem. 2009;41:1483–90.
Milliger LE, Stewart KW, Silvey JKG. The passive dispersal of viable algae, protozoans, and fungi by aquatic and terrestrial Coleoptera. Ann Ent Soc Am. 1971;64:36–45.
Schlichting HE, Milliger LE. The dispersal of microorganisms by a hemipteran, Lethocerus uhleri (Montandon). Trans Am Micr Soci. 1969;88:452–4.
Coughlan NE, Stevens AL, Kelly TC, Dick JTA, Jansen MAK. Zoochorous dispersal of freshwater bivalves: an overlooked vector in biological invasions? Knowl Manag Aquat Ecosyst. 2017;418:42.
Sabagh LT, Dias RJP, Branco CWC, Rocha CFD. News records of phoresy and hyperphoresy among treefrogs, ostracods, and ciliates in bromeliad of Atlantic forest. Biodivers Conserv. 2011;20:1837–41.
Parsons JG, Cairns A, Johnson CN, Robson SKA, Shilton LA, Westcott DA. Bryophyte dispersal by flying foxes: a novel discovery. Oecologia. 2007;152:112–4.
Vanschoenwinkel B, Waterkeyn A, Vandecaetsbeek T, Pineau O, Grillas P, Brendonck L. Dispersal of freshwater invertebrates by large terrestrial mammals: a case study with wild boar (Sus scrofa) in Mediterranean wetlands. Freshwat Biol. 2008;53:2264–73.
Waterkeyn A, Pineau O, Grillas P, Brendonck L. Invertebrate dispersal by aquatic mammals: a case study with nutria Myocastor coypus (Rodentia, Mammalia) in southern France. Hydrobiologia. 2010;654:267–71.
Robinet C, Roques A, Pan H, Fang G, Ye J, et al. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS One. 2009;4(2):e4646.
Waterkeyn A, Vanschoenwinkel B, Elsen S, Anton-Pardo M, Grillas P, Brendonck L. Unintentional dispersal of aquatic invertebrates via footwear and motor vehicles in a Mediterranean wetland area. Aquatic Conserv. 2010;20:580–7.
Green AJ, Figuerola J, Sánchez MI. Implications of waterbird ecology for the dispersal of aquatic organisms. Acta Oecol. 2002;23:177–89.
Brochet A-L, Gauthier-Clerc M, Guillemain M, Fritz H, Waterkeyn A, Baltanás Á, Green AJ. Field evidence of dispersal of branchiopods, ostracods and bryozoans by teal (Anas crecca) in the Camargue (southern France). Hydrobiologia. 2010;637:255–61.
Tesson SVM, Okamura B, Dudaniec RY, Vyverman W, Löndahl J, Rushing C, Valentini A, Green AJ. Integrating microorganism and macroorganism dispersal: modes, techniques and challenges with particular focus on codispersal. Ecoscience. 2016;22:109–24.
Caplat P, Edelaar P, Dudaniec RY, Green AJ, Okamura B, Cote J, Ekroos J, Jonsson PR, Löndahl J, Tesson SVM, Petit EJ. Looking beyond the mountain: dispersal barriers in a changing world. Front Ecol Env. 2016;14:261–8.
Figuerola J, Green AJ. Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshwat Biol. 2002;47:483–94.
Frisch D, Green AJ, Figuerola J. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquat Sci. 2007;69:568–74.
Coughlan NE, Kelly TC, Davenport J, Jansen MAK. Up, up and away: bird-mediated ectozoochorous dispersal between aquatic environments. Freshwat Biol. 2017a;62:631–48.
Mogle MJ, Kimball SA, Miller WR, McKown RD. Evidence of avian-mediated long distance dispersal in American tardigrades. PeerJ. 2018;6:e5035.
Simonis JL, Ellis JC. Bathing birds bias b-diversity: frequent dispersal by gulls homogenizes fauna in a rock-pool metacommunity. Ecology. 2014;95:1545–55.
Battauz YS, José de Paggi SB, Paggi JC. Passive zooplankton community in dry littoral sediment: reservoir of diversity and potential source of dispersal in a subtropical floodplain lake of the middle Paraná River (Santa Fe, Argentina). Int Rev Hydrobiol. 2014;99:277–86.
Langley JM, Shiel RJ, Nielsen DL, Green JD. Hatching from the sediment egg-bank, or aerial dispersing? – the use of mesocosms in assessing rotifer biodiversity. Hydrobiologia. 2001;446/447:203–11.
Càceres CE, Soluk DA. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia. 2002;131:402–8.
Cohen GM, Shurin JB. Scale-dependence and mechanisms of dispersal in freshwater zooplankton. Oikos. 2003;103:603–17.
Allen MR. Measuring and modeling dispersal of adult zooplankton. Oecologia. 2007;153:135–43.
Lopes PM, Bozelli R, Bini LM, Santangelo JM, Declerck SAJ. Contributions of airborne dispersal and dormant propagule recruitment to the assembly of rotifer and crustacean zooplankton communities in temporary ponds. Freshwat Biol. 2016;61:658–69.
White JH. Wind-borne dispersal of potato-root eelworm. Nature. 1953;172:686–7.
de RooiJ-van der Goes PCEM, van Dijk C, van der Putten WH, Jungerius PD. Effects of sand movement by wind on nematodes and soil-borne fungi in coastal foredunes. J Coast Conserv. 1997;3:133–42.
Villenave C, et al. Transport of free-living nematodes by runoff water in a Sudano-Sahelian area. Appl Soil Ecol. 2003;23:85–91.
Nkem JN, et al. Wind dispersal of soil invertebrates in the McMurdo dry valleys. Antarctica Pol Biol. 2006;29:346–52.
Moreno E, Pérez-Martínez C, Conde-Porcuna JM. Dispersal of zooplankton dormant propagules by wind and rain in two aquatic systems. Limnetica. 2016;35:323–36.
Ptatscheck C, Gansfort B, Traunspurger W. The extent of wind-mediated dispersal of small metazoans, focusing nematodes. Sci Rep. 2018;8:6814.
Sudzuki M. An analysis of colonization in freshwater micro-organisms. II. Two simple experiments on the dispersal by wind. Japan J Ecol. 1972;22:222–5.
Jenkins DG, Underwood MO. Zooplankton may not disperse readily in wind, rain, or waterfowl. Hydrobiologia. 1998;387/388:15–21.
Brendonck L, Riddoch BJ. Wind-borne short-range egg dispersal in anostracans (Crustacea: Branchiopoda). Biol J Linn Soc. 1999;67:87–95.
Rivas JA, Mohl JE, VanPelt RS, Leung M-Y, Wallace RL, Gill TE, Walsh EJ. Evidence for regional aeolian transport of freshwater micrometazoans in arid regions. Limnol Oceanogr Lett. 2018;3:320–30.
Kokko H. López-Sepulcre AFrom individual dispersal to species ranges: perspectives for a changing world. Science. 2006;313:789–91.
Vanschoenwinkel B, Gielen S, Seaman M, Brendonck L. Any way the wind blows - frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos. 2008;117:125–34.
de la Peña E, Vandegehuchte ML, Bonte D, Moens M. Nematodes surfing the waves: long-distance dispersal of soil-borne microfauna via sea swept rhizomes. Oikos. 2011;120:1649–56.
Hagerman GM, Rieger RM. Dispersal of benthic meiofauna by wave and current action in Bogue sound, North Carolina. USA Mar Ecol. 1981;2:245–70.
Palmer MA. Dispersal of marine meiofauna: a review and conceptual model explaining passive transport and active emergence with implications for recruitment. Mar Ecol Prog Ser. 1988;48:81–91.
Commito JA, Tita G. Differential dispersal rates in an intertidal meiofauna assemblage. J Exp Mar Biol Ecol. 2002;268:237–56.
Levin SA, Muller-Landau HC, Nathan R, Chave J. The ecology and evolution of seed dispersal: a theoretical perspective. Annu Rev Ecol Evol Syst. 2003;34:575–604.
Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A. Mechanisms of long-distance seed dispersal. Trends Ecol Evol. 2008;23:638–47.
Ferris VR, Goseco CG, Ferris JM. Biogeography of free-living soil nematodes from the perspective of plate tectonics. Science. 1976;193:508–9.
Zullini A. Cosmopolitism and endemism in free-living nematodes. Biogeographia. 2017;33:33–9.
Segers H. A global checklist of the rotifers (phylum Rotifera). Zootaxa. 2007;1564:1–104.
Ricci C, Shiel RJ, Fontaneto D, Melone G. Bdelloid rotifers recorded from Australia with description of Philodinavus aussiensis n.sp. Zool Anz. 2003;242:241–8.
Wallace RL, Walsh EJ, Schrõder T, Rico-Martínez R, Rios-Arana JV. Species composition and distribution of rotifers in Chihuahuan Desert waters of México: is everything everywhere? Verh lnternat Verein Limnol. 2008;30:73–6.
Segers H, De Smet W. Diversity and endemism in Rotifera: a review and Keratella Bory de St Vincent. Biodivers Conserv. 2008;17:303–16.
Dumont H, Segers H. Estimating lacustrine zooplankton species richness and complementarity. Hydrobiologia. 1996;341:125–32.
Dumont HJ. Biogeography of rotifers. Hydrobiologia. 1983;104:19–30.
Fontaneto D, Barbosa AM, Segers H, Pautasso M. The ‘rotiferologist’ effect and other global correlates of species richness in monogonont rotifers. Ecography. 2012;35:174–82.
McInnes SJ, Pugh PJA. Biogeography of limno-terrestrial Tardigrada, with particular reference to the Antarctic fauna. J Biogeogr. 1998;25:31–6.
McInnes SJ, Pugh PJA. An attempt to revisit the global biogeography of limno-terrestrial Tardigrada. J Limnol. 2007;66(Suppl. 1):90–6.
Pilato G, Binda MG. Biogeography and limno-terrestrial tardigrades: are they truly incompatible binomials? Zool Anz. 2001;240:511–6.
Guidetti R, McInnes SJ, Cesari M, Rebecchi L, Rota-Stabelli O. Evolutionary scenarios for the origin of an Antarctic tardigrade species based on molecular clock analyses and biogeographic data. Contrib Zool. 2017;86:97–110.
Sánchez-Fernández D, Lobo JM, Abellán P, Ribera I, Millán A. Bias in freshwater biodiversity sampling: the case of Iberian water beetles. Divers Distrib. 2008;14:754–62.
Boakes EH, McGowan PJK, Fuller RA, Chang-qing D, Clark NE, O'Connor K, Mace GM. Distorted views of biodiversity: spatial and temporal bias in species occurrence data. PLoS Biol. 2010;8:e1000385.
Rundle SD, Bilton DT, Shiozawa DK. Global and regional patterns in lotic meiofauna. Freshwat Biol. 2000;44:123–34.
McInnes SJ. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. J Nat Hist. 1994;28:257–352.
Curini-Galletti M, Artois T, Delogu V, De Smet WH, Fontaneto D, et al. Patterns of diversity in soft-bodied meiofauna: dispersal ability and body size matter. PLoS One. 2012;7:e33801.
Arnemo R, Berzins B, Grönberg B, Mellgren I. The dispersal in Swedish waters of Kellicottia bostoniensis (Rousselet) (Rotatoria). Oikos. 1968;19:351–8.
Boggero A, Basset A, Austoni M, Barbone E, et al. Weak effects of habitat type on susceptibility to invasive freshwater species: an Italian case study. Aquat Conserv. 2014;24:841–52.
Ejsmont-Karabin J. Rotifer invasion? On appearance and abundance of tropical species in lakes of North-Eastern Poland. Pol J Ecol. 2014;62:821–7.
Pociecha A, Solarz W, Najberek K, Wilk-Wozniak E. Native, alien, cosmopolitan, or cryptogenic? A framework for clarifying the origin status of rotifers. Aquat Biol. 2016;24:141–9.
Zhdanova SM, Lazareva VI, Bayanov NG, Lobunicheva EV, Rodionova NV, Shurganova GV, Kulakov DV, Il’in MY. Distribution and ways of dispersion of american rotifer Kellicottia bostoniensis (Rousselet, 1908) (Rotifera: Brachionidae) in waterbodies of European Russia. Russ J Biol Inv. 2016;7:308–20.
Derycke S, Remerie T, Vierstraete A, Backeljau T, Vanfleteren J, Vincx M, Moens T. Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Mar Ecol Progr Ser. 2005;300:91–103.
Campillo S, Serra M, Carmona MJ, Gómez A. Widespread secondary contact and new glacial refugia in the halophilic rotifer Brachionus plicatilis in the Iberian Peninsula. PLoS One. 2011;6:e20986.
Plantard O, Porte C. Population genetic structure of the sugar beet cyst nematode Heterodera schachtii: a gonoristhic and aphimictic species with highly inbred but weakly differentiated populations. Mol Ecol. 2004;13:34–41.
Derycke S, Remerie T, Backeljau T, Vierstraete A, Vanfleteren J, Vincx M, Moens T. Phylogeography of the Rhabditis (Pellioditis) marina species complex: evidence for long-distance dispersal, and for range expansions and restricted gene flow in the Northeast Atlantic. Mol Ecol. 2008;17:3306–22.
Derycke S, Backeljau T, Moens T. Dispersal and gene flow in free-living marine nematodes. Front Zool. 2013;10:1.
Mills S, Gómez A, Lunt DH. Global isolation by distance despite strong regional phylogeography in a small metazoan. BMC Evol Biol. 2007;7:22.
Faurby S, Barber PH. Extreme population subdivision despite high colonization ability: contrasting regional patterns in intertidal tardigrades from the west coast of North America. J Biogeogr. 2015;42:1006–17.
Pfenninger M, Schwenk K. Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evol Biol. 2007;7:121.
Fontaneto D, Kaya M, Herniou EA, Barraclough TG. Extreme levels of hidden diversity in microscopic animals (Rotifera) revealed by DNA taxonomy. Mol Phylogenet Evol. 2009;53:182–9.
Guil N. Molecular approach to micrometazoans. Are they here, there and everywhere? In: Fontaneto D, editor. Biogeography of microscopic organisms. Cambridge: Cambridge University Press; 2011. p. 284–306.
Tang CQ, Leasi F, Obertegger U, Kieneke A, Barraclough TG, Fontaneto D. The widely used small subunit 18S rDNA molecule greatly underestimates true diversity in biodiversity surveys of the meiofauna. Proc Natl Acad Sci U S A. 2012;109:16208–12.
Ristau K, Steinfartz S, Traunspurger W. First evidence of cryptic species diversity and significant population structure in a widespread freshwater nematode morphospecies (Tobrilus gracilis). Mol Ecol. 2013;22:4562–75.
Finlay BJ, Esteban GF, Brown S, Fenchel T, Hoef-Emden K. Multiple cosmopolitan ecotypes within a microbial eukaryote morphospecies. Protist. 2006;157:377–90.
Nieberding C, Libois R, Douady CJ, Morand S, Michaux R. Phylogeography of a nematode (Heligmosomoides polygyrus) in the western Palearctic region: persistence of northern cryptic populations during ice ages? Mol Ecol. 2005;14:765–79.
Suatoni E, Vicario S, Rice S, Snell T, Caccone A. An analysis of species boundaries and biogeographic patterns in a cryptic species complex: the rotifer – Brachionus plicatilis. Mol Phylogen Evol. 2006;41:86–98.
Mills S, Alcantara-Rodriguez JA, Ciros-Pérez J, Gómez A, Hagiwara A, et al. Fifteen species in one: deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia. 2017;796:39–58.
Gómez A, Carvalho GR, Lunt DH. Phylogeography and regional endemism of a passively dispersing zooplankter: mitochondrial DNA variation in rotifer resting egg banks. Proc R Soc Lond B. 2000;267:2189–97.
Gómez A, Adcock GJ, Lunt DH, Carvalho GR. The interplay between colonization history and gene-flow in passively dispersing zooplankton: microsatellite analysis of rotifer resting egg banks. J Evol Biol. 2002a;15:158–71.
Gómez A, Serra M, Carvalho GR, Lunt DH. Speciation in ancient cryptic species complexes: evidence from the molecular phylogeny of Brachionus plicatilis (Rotifera). Evolution. 2002;56:1431–44.
Gómez A, Montero-Pau J, Lunt DH, Serra M, Campillo S. Persistent genetic signatures of colonization in Brachionus manjavacas rotifers in the Iberian Peninsula. Mol Ecol. 2007;16:3228–40.
Fontaneto D, Barraclough TG, Chen K, Ricci C, Herniou EA. Molecular evidence for broad-scale distributions in bdelloid rotifers: everything is not everywhere but most things are very widespread. Mol Ecol. 2008;17:3136–46.
Robeson MS, King AJ, Freeman KR, Birky CW, Martin AP, Schmidt SK. Soil rotifer communities are extremely diverse globally but spatially autocorrelated locally. Proc Natl Acad Sci U S A. 2011;108:4406–10.
Faurby S, Jørgensen A, Kristensen RM, Funch P. Phylogeography of North Atlantic intertidal tardigrades: refugia, cryptic speciation and the history of the mid-Atlantic Islands. J Biogeogr. 2011;38:1613–24.
Faurby S, Jørgensen A, Kristensen RM, Funch P. Distribution and speciation in marine intertidal tardigrades: testing the roles of climatic and geographical isolation. J Biogeogr. 2012;39:1596–607.
Gąsiorek P, Vončina K, Michalczyk Ł. Echiniscus testudo (Doyère, 1840) in New Zealand: anthropogenic dispersal or evidence for the ‘Everything is Everywhere’ hypothesis? New Zeal J Zool. DOI: https://doi.org/10.1080/03014223.2018.1503607.
Velasco-Castrillón A, McInnes SJ, Schultz MB, Arróniz-Crespo M, D’Haese CA, et al. Mitochondrial DNA analyses reveal widespread tardigrade diversity in Antarctica. Invert Syst. 2015;29:578–90.
Foissner W. Biogeography and dispersal of microorganisms: a review emphasizing protists. Acta Protozool. 2006;45:111–36.
Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JB. Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol. 2012;10:497–506.
Naselli-Flores L, Padisák J. Blowing in the wind: how many roads can a phytoplanktont walk down? A synthesis on phytoplankton biogeography and spatial processes. Hydrobiologia. 2016;764:303–13.
Primack RB, Miao SL. Dispersal can limit local plant distribution. Cons Biol. 1992:6513–9.
Jenkins DG. Dispersal-limited zooplankton distribution and community composition in new ponds. Hydrobiologia. 1995;313/314:15–20 191 Howe HF, Miriti MN. When seed dispersal matters. BioScience. 2004;54:651–660.
Howe HF, Mirit MN, When seed dispersal matters. BioScience. 2004;54:651–60
Hortal J. Geographic variation in the diversity of microbial communities: research directions and prospects for experimental biogeography. In: Fontaneto D, editor. Biogeography of microscopic organisms. Cambridge: Cambridge University Press; 2011. p. 335–57.
Jenkins DG, Medley KA, Franklin RB. Microbes as a test of biogeographic principles. In: Fontaneto D, editor. Biogeography of microscopic organisms. Cambridge: Cambridge University Press; 2011. p. 309–23.
Jeltsch F, Bonte D, Pe'er G, Reineking B, Leimgruber P, et al. Integrating movement ecology with biodiversity research - exploring new avenues to address spatiotemporal biodiversity dynamics. Mov Ecol. 2013;1:6.
Acknowledgements
I thank Ran Nathan for inviting me to write this overview, and Dave Jenkins and an anonymous reviewer for providing comments to improve an earlier version of the manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fontaneto, D. Long-distance passive dispersal in microscopic aquatic animals. Mov Ecol 7, 10 (2019). https://doi.org/10.1186/s40462-019-0155-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40462-019-0155-7