Abstract
Background
Liver cirrhosis is characterized by fibrosis and nodule formation in the liver, due to a chronic injury, and subsequent alteration of the normal architecture of the liver. Even though there is a huge effort to elucidate the possible etiologic factors of liver cirrhosis, a significant number of cases are cryptogenic, especially in Sub Saharan Africa, where there is a high burden of aflatoxin exposure. Aflatoxins are known to cause hepatocellular carcinoma, which share similar etiologic factors with liver cirrhosis. This study aimed to assess the association between aflatoxin exposure and the risk of liver cirrhosis.
Methods
Relevant studies were identified through systematic searches conducted in Ovid MEDLINE, PubMed and Google Scholar. Also, by searching the references of retrieved articles. The abstracts and full text were screened for eligibility and the risk of bias was assessed for each study using Joanna Briggs Institute (JBI) critical appraisal checklist for observational studies. The extracted data from included studies using Microsoft Excel were exported to Stata software version 15.0 for analyses. The overall pooled estimation of outcomes was calculated using a random-effects model of DerSimonian–Laird method at a 95% confidence level. The heterogeneity of studies was determined using I2 statistics. The presence of publication bias between studies was evaluated using the Begg’s and Egger’s tests and funnel plot. The protocol of this systematic review and meta-analysis was registered in the Prospero database with reference number ID: CRD42019148481.
Results
A total of 5 studies published between the years 2005 and 2018 that met the pre-defined inclusion and exclusion criteria were included. The meta-analysis showed that a significant increase in the risk of liver cirrhosis is associated with aflatoxin exposure (unadjusted pooled odds ratio (OR) = 3.35, 95% CI: 2.74–4.10, p = 0.000; I2 = 88.3%, p = 0.000; adjusted OR = 2.5, 95% CI: 1.84–3.39, p = 0.000; I2 = 0%, p = 0.429).
Conclusions
The present meta-analysis suggests that aflatoxin exposure is associated with a higher risk of liver cirrhosis.
Similar content being viewed by others
Background
Cirrhosis is characterized by fibrosis and nodule formation in the liver, secondary to a chronic injury, which leads to alteration of the normal lobular organization of the liver [1, 2]. Cirrhosis is currently the 11th most common cause of death globally and liver cancer is the 16th leading cause of death; when combined, they account for 3.5% of all deaths worldwide [3]. Despite the tremendous amount of progress in our understanding the etiology of liver cirrhosis, many cases are cryptogenic, i.e. cirrhosis of the liver of undetermined etiology [4]. This is true especially in Sub Saharan Africa, where hepatitis B virus (HBV), hepatitis C virus (HCV) and alcohol consumption are involved in 34, 17, and 18% of cases as etiologic factors. However, in 31% of cases, the etiology is unknown, according to a recent global burden of disease report [5].
On the other hand, cirrhosis and hepatocellular carcinoma (HCC) are known to share numerous common etiologic factors, including chronic infection with HBV and/or HCV, heavy alcohol consumption, and non-alcoholic steatohepatitis/non-alcoholic fatty liver disease [5, 6]. An additional etiologic factor for HCC is exposure to aflatoxins (AFs) through the consumption of AF contaminated foods [7]. In this regard, Sub Saharan Africa is an area with a diet particularly high in AFs [8,9,10].
Emerging evidence has indicated that AF exposure may be involved in the pathogenesis of liver cirrhosis [11, 12]. Though there is no clear causation between AF and liver cirrhosis, the mutational activity of AF has been considered to be the main factor of AF-induced HCC [13]. As both AF exposure and liver cirrhosis are the main risk factors of HCC, it remains unclear whether AF also contributes to the earlier stage of HCC progression, i.e., liver cirrhosis. The objective of this systematic review was to analyze existing research to test the hypothesis that AFs cause liver cirrhosis by meta-analysis approach.
Methods
Study protocol
The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guideline was used to report the finding of this review [14]. This systematic review and meta-analysis was conducted by following the PRISMA Protocol [15]. The completed checklist has been provided as supplementary material (Additional file 1: Table S1). The study protocol is registered on PROSPERO with reference number ID: CRD42019148481.
Inclusion/exclusion criteria
During the screening and assessment of full texts for eligibility, there were predefined inclusion-exclusion criteria to arrive at the final included papers. Observational studies (Case-control or cohort studies) addressing AF exposure as a risk factor for liver cirrhosis were included. There were no restrictions on publication year, but only studies that were written in English were considered for inclusion. Studies having irretrievable full texts (after requesting full texts from the corresponding authors via email and/or Research Gate account) or studies with unrelated or insufficient outcome measures or studies with outcomes of interest that are missing or vague were excluded.
Data sources and search strategy
We performed an electronic literature search until December 31st, 2019, on Ovid MEDLINE and PubMed: using the following keywords and indexing terms: ‘aflatoxin’, ‘liver cirrhosis’, and ‘chronic liver disease’. Advanced Google Scholar search was also conducted to identify other relevant published and unpublished works including dissertations, institutional repositories, and organizational manuals, among others. Boolean operators (AND, OR) and truncation were used when appropriate to increase the number of relevant findings. Additionally, we searched (back-traced) reference lists from retrieved articles to identify further relevant studies.
Screening and eligibility of studies
The documents identified from different electronic sources were exported to ENDNOTE reference software version 7.8 (Thomson Reuters, Stamford, CT, USA) with compatible formats. Duplicate documents were removed with the help of ENDNOTE and manually. Each of the documents retrieved was assessed by the authors independently for eligibility by reading the title, abstract using the preset inclusion and exclusion criteria. This process was followed by retrieval and assessment of the full texts of the relevant citations. Any disagreement was solved by discussion.
Data extraction
Data extraction format prepared in Microsoft Excel was developed to extract data from each included study. The authors independently extracted the data related to study characteristics and outcome measures: including authors, publication year, study design and populations, study location, study period, diagnostic method, number of cases and controls, the age and sex of study subjects, method of AF exposure assessment (dietary intake of AF contaminated foods and biomarkers of AF exposure [249ser TP53 mutation, AF-albumin adduct, AF-N7-guanine adducts excreted in urine]), risk ratios (RRs)/odds ratios (ORs) and their 95% CI with or without adjustment for confounding factors, and variables adjusted for analysis, if any.
Critical appraisal of studies
To maintain methodological validity, before the inclusion of the eligible articles they were assessed by two independent reviewers using the Joanna Briggs Institute (JBI) critical appraisal checklist for case-control and cohort studies [16]. The assessment tool consisted of 10 questions about the quality of the study for which articles received values representing the extent to which they met the following criteria: Yes, No, Unclear and Not applicable. This critical appraisal was conducted to assess the internal (systematic error) and external (generalizability) validity of studies and to reduce the risk of biases. The mean score of the two authors was taken for final decision and studies with a score greater than or equal to five out of 10 were considered low risk and included in the study.
Outcome measurements
Our primary outcome of interest in this meta-analysis was the association between AF exposure and the risk of liver cirrhosis. Subgroup analyses based on information on the study design, geographic location and method of exposure assessment were performed.
Data processing and statistical analysis
The extracted data were exported from Excel to STATA 15.0 software for analyses of outcome measures and sub-grouping. Considering the variation in true effect sizes across the population, Der-Simonian-Laird’s random-effects model was applied for the analysis at 95% confidence level. The significance of heterogeneity of the studies was assessed using I2statistics based on Cochran’s Q test, I2 returns and the percent variation across studies. The presence of publication bias was evaluated using the Begg’s and Mazumdar’s correlation and Egger’s regression tests and presented with funnel plots [17, 18]. A statistical test with a p-value of less than 0.05 was deemed to be significant.
Results
Search result
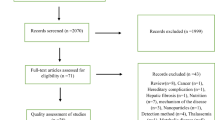
As shown in Fig. 1, the search identified 506 studies, of which 67 studies were found to be duplicates. From the 439 remaining records, 424 were excluded after reading their titles and abstracts. Full texts of 15 records were read to assess their eligibility. Of these, 10 records were further excluded because they did not satisfy the inclusion criteria. The remaining 5 studies [12, 19,20,21,22] were included in this systematic review and meta-analysis.
Study characteristics
Among the five studies that met the inclusion criteria, four of them were case-control studies and one study was a nested case-control study. They were conducted in Gambia [19, 20], Taiwan [12], India [21], and China [22] and involved 941 cases and 2, 281 controls. The included studies were published between 2005 and 2018. As shown in Table 1, the included studies employed AF-albumin adduct level [12, 21, 22], 249ser TP53 mutation [19,20,21] and groundnut consumption [19, 20] as methods of AF exposure assessment in liver cirrhosis patients. As depicted in Table 1, three of the included studies reported unadjusted and adjusted ORs and two studies [21, 22] did not report the adjusted odds ratio. Most studies were adjusted for factors such as age, gender, cigarette smoking, and alcohol drinking; two studies [19, 20] were also adjusted for recruitment site and date, socioeconomic status, HBV, and HCV status.
AF exposure and risk of liver cirrhosis
After pooling, the five studies that reported the unadjusted OR suggested a significantly higher risk of liver cirrhosis associated with AF exposure (OR = 3.35, 95% CI: 2.74–4.10, p = 0.000). However, high evidence of heterogeneity (I2 = 88.3%, p = 0.000) was observed in the pooled estimate (Fig. 2).
On the other hand, after pooling of the adjusted OR estimates of individual studies, AF exposure was still associated with a higher risk of liver cirrhosis (OR = 2.5, 95% CI: 1.84–3.39, p = 0.000) and no evidence of heterogeneity (I2 = 0%, p = 0.429) was found in the pooled estimate and subgroup analysis (Fig. 3).
Subgroup analyses
As shown in Table 2, subgroup analyses by study design, AF exposure assessment method and geographical region of study populations were performed to identify the sources of heterogeneity in the unadjusted OR estimates of individual studies. In the subgroup analysis by study design, the pooled estimate of case-control was 3.67 (95% CI: 2.93–4.59, p = 0.000; I2 = 89.4%, p = 0.000). In the subgroup analysis by AF exposure assessment method, the pooled estimate revealed that there was a significant association between AF-albumin adduct and liver cirrhosis [4.89 (95% CI: 3.77–6.35, p = 0.000; I2 = 88.8%, p = 0.000)], as well as between 249ser TP53 mutation and liver cirrhosis [4.30 (95% CI: 2.55–7.26, p = 0.000; I2 = 0.00%, p = 0.863)] though no statistically significant association was observed between groundnut consumption and liver cirrhosis [1.15 (95% CI: 0.76–1.72, p = 0.51; I2 = 82.4%, p = 0.017)].
In the subgroup analysis performed by geographical region, the corresponding pooled OR for Asia was 4.85 (95% CI: 3.75–6.26, p = 0.000; I2 = 83.3%, p = 0.000), and that of the African region was 1.84 (95% CI: 1.32–2.55, p = 0.000; I2 = 85.5%, p = 0.000) (Table 2).
Publication bias
The presence of publication bias was depicted using funnel plots of log OR and standard error of it and supplemented with statistical tests: Egger’s regression test (p = 0.683 for unadjusted ORs and p = 0.122 for adjusted ORs) and Begg’s and Mazumdar’s correlation test (continuity corrected) (p = 1.00 for unadjusted OR and p = 0.22 for adjusted OR) (Fig. 4). The finding indicated that there is no evidence of statistically significant publication bias among the included studies.
Discussion
This study is the first systematic review and meta-analysis to investigate the relationship between exposure to AF and the risk of liver cirrhosis. The results of the present study showed a significant association between AF exposure and the risk of liver cirrhosis. Despite the heterogeneity presented for most studies, those studies that performed the adjusted tests were able to demonstrate homogeneity in the comparisons. Subgroup analysis was conducted to reduce the degree of heterogeneity among studies. The random effect model has also been applied considering the variability of the effect size.
A likely explanation of this association is not yet identified, though consumption of AF-contaminated foods and feeds were reported to cause diverse degrees of liver injury comprising development of fatty cysts, fibrosis, and cirrhosis among humans and animals [23,24,25,26,27]. However, several lines of evidence support oxidative stress as a key factor in AF induced initiation and progression of liver cirrhosis [28,29,30,31].
The toxic effects of AFB1 against the liver and other organs are closely related to its metabolic activation into the free radical AFB1-exo-8,9-epoxide (AFBO) by cytochrome P450 (CYP450) enzymes [32] and associated formation of reactive oxygen species (ROS) including hydroxyl radical (HO.), per hydroxyl radical (HOO−) and superoxide anion [29, 33]. This can result in oxidative stress owing to an imbalance between limited antioxidant defenses and the excessive formation of ROS, resulting in the damage of biological molecules including lipids, proteins, and DNA in cellular systems [34, 35]. In support of this hypothesis, several studies have demonstrated the potential for antioxidants to lower the risk of hepatotoxicity caused by exposure to the AF [29, 36,37,38,39].
Moreover, many studies have reported the pivotal role of oxidative stress induced by AF in eliciting programmed cell death or apoptosis through mitochondrial signaling pathways [25, 40,41,42]. ROS induced mitochondrial damage is known to cause uncoupling of mitochondrial oxidative phosphorylation and the associated reduction in mitochondrial membrane potential following AFB1 administration in vivo and in vitro [25, 33, 35]. Consequently, mitochondrial alterations cause activation of cytochrome C that modulates Bcl2/Bax gene expression and activate caspase 9 and caspase 3, which results in cell death [41].
Conclusions
The current meta-analysis indicates that AF exposure is significantly associated with liver cirrhosis. However, large sample studies using standardized unbiased AF exposure assessment methods and well-matched controls are required to support this association further.
Availability of data and materials
All data generated or analyzed in this study are included in this article.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- AF:
-
Aflatoxin
- JBI:
-
Joanna Briggs Institute
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- ROS:
-
Reactive Oxygen Species
References
Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838–51.
Aizawa K, Liu C, Tang S, Veeramachaneni S, Hu KQ, Smith DE, et al. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int J Cancer. 2016;139(5):1171–81.
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71.
Mercado-Irizarry A, Torres EA. Cryptogenic cirrhosis: current knowledge and future directions. Clin Liver Dis. 2016;7(4):69–72.
Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145.
Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterol. 2002;123(1):134–40.
Hutanasu C, Sfarti C, Trifan A, Hutanasu M, Stanciu C. Aflatoxin contamination of food: additional risk factor for chronic liver diseases. Rev Med Chir Soc Med Nat Iasi. 2009;113(4):1061–5.
Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118(6):818–24.
Bankole S, Schollenberger M, Drochner W. Mycotoxins in food systems in sub Saharan Africa: a review. Mycotoxin Res. 2006;22(3):163–9.
Ladeira C, Frazzoli C, Orisakwe OE. Engaging one health for non-communicable diseases in Africa: perspective for Mycotoxins. Front Public Health. 2017;5:266.
Aydin M, Aydin S, Bacanli M, Basaran N. Aflatoxin levels in chronic hepatitis B patients with cirrhosis or hepatocellular carcinoma in Balikesir, Turkey. J Viral Hepatitis. 2015;22(11):926–35.
Chu YJ, Yang HI, Wu HC, Liu J, Wang LY, Lu SN, et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int J Cancer. 2017;141(4):711–20.
Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31(1):71–82.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–53.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Kirk GD, Lesi OA, Mendy M, Szymanska K, Whittle H, Goedert JJ, et al. 249(ser) TP53 mutation in plasma DNA, hepatitis B viral infection, and risk of hepatocellular carcinoma. Oncogene. 2005;24(38):5858–67.
Kuniholm MH, Lesi OA, Mendy M, Akano AO, Sam O, Hall AJ, et al. Aflatoxin exposure and viral hepatitis in the etiology of liver cirrhosis in the Gambia, West Africa. Environ Health Perspect. 2008;116(11):1553–7.
Anitha S, Raghunadharao D, Waliyar F, Sudini H, Parveen M, Rao R, et al. The association between exposure to aflatoxin, mutation in TP53, infection with hepatitis B virus, and occurrence of liver disease in a selected population in Hyderabad, India. Mutat Res Genet Toxicol Environ Mutagen. 2014;766:23–8.
Wang XZ, Huang XY, Yao JG, Wang C, Xia Q, Long XD. Genetic polymorphisms in ataxin-3 and liver cirrhosis risk related to aflatoxin B1. Oncotarget. 2018;9(44):27321–32.
Amla I, Kamala C, Gopalakrishina G, Jayaraj AP, Sreenivasamurthy V, Parpia H. Cirrhosis in children from peanut meal contaminated by aflatoxin. Am J Clin Nutr. 1971;24(6):609–14.
Wouters AT, Casagrande RA, Wouters F, Watanabe TT, Boabaid FM, Cruz CE, et al. An outbreak of aflatoxin poisoning in dogs associated with aflatoxin B1-contaminated maize products. J Vet Diagn Investig. 2013;25(2):282–7.
Wang X, Muhammad I, Sun X, Han M, Hamid S, Zhang X. Protective role of curcumin in ameliorating AFB1-induced apoptosis via mitochondrial pathway in liver cells. Mol Biol Rep. 2018;45(5):881–91.
Saad-Hussein A, Shahy EM, Shaheen W, Taha MM, Mahdy-Abdallah H, Ibrahim KS, et al. Comparative hepatotoxicity of Aflatoxin B1 among workers exposed to different organic dust with emphasis on polymorphism role of glutathione S-Transferase gene. Open Access Maced J Med Sci. 2016;4(2):312–8.
Zhou H, Wang J, Ma L, Chen L, Guo T, Zhang Y, et al. Oxidative DNA damage and multi-organ pathologies in male mice subchronically treated with aflatoxin B1. Ecotoxicol Environ Saf. 2019;186:109697.
Muhammad I, Wang H, Sun X, Wang X, Han M, Lu Z, et al. Dual Role of Dietary Curcumin Through Attenuating AFB (1)-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB (1) Bioactivation and Detoxification. Front Pharmacol. 2018;9:554.
Saad-Hussein A, Moubarz G, Mohgah SA, Wafaa GS, Aya HM. Role of antioxidant supplementation in oxidant/antioxidant status and hepatotoxic effects due to aflatoxin B1 in wheat miller workers. J Altern Complement Med. 2019;16(4):20180218.
Xu Q, Shi W, Lv P, Meng W, Mao G, Gong C, et al. Critical role of caveolin-1 in aflatoxin B1-induced hepatotoxicity via the regulation of oxidation and autophagy. Cell Death Dis. 2020;11(1):6.
Li S, Muhammad I, Yu H, Sun X, Zhang X. Detection of Aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol Environ Saf. 2019;176:137–45.
Deng J, Zhao L, Zhang NY, Karrow NA, Krumm CS, Qi DS, et al. Aflatoxin B1 metabolism: regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat Res. 2018;778:79–89.
Zhou Y, Jin Y, Yu H, Shan A, Shen J, Zhou C, et al. Resveratrol inhibits aflatoxin B1-induced oxidative stress and apoptosis in bovine mammary epithelial cells and is involved the Nrf2 signaling pathway. Toxicon. 2019;164:10–5.
Hamid AS, Tesfamariam IG, Zhang Y, Zhang ZG. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: geographical distribution, mechanism of action and prevention. Oncol Lett. 2013;5(4):1087–92.
Xu F, Wang P, Yao Q, Shao B, Yu H, Yu K, et al. Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct. 2019;10(7):3868–79.
Eftekhari A, Ahmadian E, Panahi-Azar V, Hosseini H, Tabibiazar M, Maleki DS. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: in vitro/in vivo studies. Artif Cells Nanomed Biotechnol. 2018;46(2):411–20.
de Freitas SC, Baldissera MD, Descovi S, Zeppenfeld C, Eslava-Mocha PR, Gloria EM, et al. Melaleuca alternifolia essential oil abrogates hepatic oxidative damage in silver catfish (Rhamdia quelen) fed with an aflatoxin-contaminated diet. Comp Biochem Physiol C Toxicol Pharmacol. 2019;221:10–20.
Singh C, Prakash C, Mishra P, Tiwari KN, Mishra SK, More RS, et al. Hepatoprotective efficacy of Premna integrifolia L. leaves against aflatoxin B1-induced toxicity in mice. Toxicon. 2019;166:88–100.
Wang XH, Li W, Wang XH, Han MY, Muhammad I, Zhang XY, et al. Water-soluble substances of wheat: a potential preventer of aflatoxin B1-induced liver damage in broilers. Poult Sci. 2019;98(1):136–49.
Mughal MJ, Xi P, Yi Z, Jing F. Aflatoxin B1 invokes apoptosis via death receptor pathway in hepatocytes. Oncotarget. 2017;8(5):8239–49.
Silva E, Bracarense AP, Oswald I. Mycotoxins and oxidative stress: where are we? World Mycotoxin J. 2018;11:1–22.
Huang L, Zhao Z, Duan C, Wang C, Zhao Y, Yang G, et al. Lactobacillus plantarum C88 protects against aflatoxin B1-induced liver injury in mice via inhibition of NF-kappaB-mediated inflammatory responses and excessive apoptosis. BMC Microbiol. 2019;19(1):170.
Acknowledgments
Not applicable.
Funding
No funding from any source was obtained for this study.
Author information
Authors and Affiliations
Contributions
ANM and MS were involved in the conception, design, analysis, interpretation, report writing, and manuscript writing. YYG and MNR were involved in the design, analysis, and critically reviewing the manuscript. All authors read and approved the final manuscript.
Authors’ information
ANM is a Lecturer of Pharmacology in School of Pharmacy, Haramaya University and PhD candidate at Addis Ababa University. MS is Assistance professor of Pharmacology in School of Pharmacy, Haramaya University. YYG is professor of Food Safety and Global Health in School of Food Science and Nutrition, University of Leeds. MNR is associate professor of environmental toxicology in School of Medicine, University of Leeds.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Table S1. Completed PRISMA checklist. The checklist highlights the important components addressed while conducting systematic review and meta-analysis from observational studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mekuria, A.N., Routledge, M.N., Gong, Y.Y. et al. Aflatoxins as a risk factor for liver cirrhosis: a systematic review and meta-analysis. BMC Pharmacol Toxicol 21, 39 (2020). https://doi.org/10.1186/s40360-020-00420-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-020-00420-7