Abstract
Background
There is evidence that atypical antipsychotics (APs) increase risk of osteoporosis in schizophrenia patients, however the mechanism is unclear. The aim of the study was to explore the molecular mechanisms about Wnt/β-catenin signal pathway underlying the osteal side effects of APs.
Methods
We cultured human osteoblast cell line hFob1. 19 (OB) treatments with olanzapine, risperidone, amisulpride, aripiprazole or resveratrol in vitro. OB cells viability was detected by cell viability assay. OB cells apoptosis was analyzed by flow cytometry (FCM). Further apoptosis-related marker and β-catenin expression was analyzed by Western blot and Immunofluorescence analysis.
Results
Compared with the control group, proliferation of OB cells decreased and apoptosis rates of OB cells increased significantly in APs group (p < 0.05). There were a reduced level of Bcl-2, Mcl-1 (antiapoptotic marker) and an elevated level of Bax, Cleaved-Caspase3 (proapoptotic marker) in APs group (p < 0.05). Simultaneously, β-catenin expression decreased in cytoplasm and nucleus (p < 0.05). Compared with the just APs group, the apoptosis rates decreased and β-catenin expression increased significantly in resevratrol combined with APs group (p < 0.05). Correlation analysis showed positive correlation between β-catenin expression and the apoptotic rate in OB cells (r = − 0.515, p < 0.05).
Conclusions
APs cause OB cells apoptosis relating to Wnt/β-catenin signaling while resevratrol could reverse this phenomenon. Our study could lay the foundation for overcoming the APs-induced osteal side effects to improve the life quality of schizophrenia patients.
Similar content being viewed by others
Background
Schizophrenia has a prevalence of ~ 1% worldwide and brings these individuals suffering from chronic symptoms and disabilities, while antipsychotic drugs maintain the standard for schizophrenia treatment [1, 2]. With the wide use of antipsychotic drugs, especially atypical antipsychotics (APs), the focus of treatment on schizophrenia gradually shifted from controlling symptoms to improving the quality of patients life [3, 4]. APs could be more effective in treating positive symptoms (e.g., hallucinations and delusions), negative symptoms (e.g., impaired motivation and reduction in spontaneous speech), cognitive symptoms but fewer movement disorders. Unfortunately, there is evidence that long-term treatment with APs would carry a higher risk of clinically significant metabolic adverse events, including weight gain, metabolic syndrome, lipid and glycemic values fluctuation, diabetes mellitus and insulin resistance [5,6,7,8,9]. Furthermore, this clinical observation supported schizophrenia patients with long-term exposure to APs had decreased bone mineral density (BMD) [10]. Mortality in schizophrenic patients with major fractures increased by 54% compared with the schizophrenic patients without fractures [11], and the hip fractures in schizophrenia patients would cause mental state and ambulatory worsening [12]. It is a pity that the basic molecular mechanism by which antipsychotic drug treatment leads to a negative effect on bone is unclear.
Low BMD and microarchitectural alterations of bone tissue be caused by imbalance between osteoblasts rebuilt and resorbed bone [13]. Osteoblastic bone formation is one of the important factors in bone mass maintenance [14]. From the level of molecular biology, a number of signal pathways include BMP-Smads, Wnt/β-catenin, Notch, Hedgehog, FGF et al. are involved in the osteoblast regulation [15]. Wnt/β-catenin signaling regulated osteoblast apoptosis is a new insight. These study reported that β-catenin accumulated in the cytoplasm and subsequently translocates into the nucleus [16], where it associated with the T cell factor/ lymphocyte growth factor (TCF/LEF) into the complex, thus regulated the apoptotic gene transcription [17]. Wnt/β-catenin signaling pathway may be a target for avoiding the APs-induced osteal side effects. However, the classic activator of β-catenin, Lithium chloride (LiCl), is a toxic compound which is harmful to humans [18]. Fortunately, resveratrol, a natural polyphenolic compound and abundantly found in plant foods, is also thought to be involved in Wnt/β-catenin signaling pathway activation [19, 20]. If resveratrol is effective, it will be more suitable for preventing osteoporosis associated with Aps.
Hence, the aim of this study was to measure the viability, apoptosis rate, apoptosis-related protein and β-catenin in OB cells to determine whether APs-induced apoptosis in OB cells as well as that involved in Wnt/β-catenin signal pathway and whether resevratrol reduce the apoptosis through Wnt/β-catenin signal pathway. Our study could provide evidence about antipsychotics have adverse effects on osteoblasts. Additionally, it could lay the foundation for overcoming the APs-induced osteal side effects.
Methods
Chemicals and antibodies
The following four antipsychotics drugs Olanzapine, Risperidone, Amisulpride and Aripiprazole were obtained from Sigma-aldrich (Sigma, USA). Fetal bovine serμM (Gibco BRL); RPMi-1640 medium (Gibco BRL); Dimethyl Sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, SUA); Cell counting kit-8 (CCK8) (Tongren, Tokyo, Japan) Annexin V-Fuorescein Isothiocyanate (FITC)/Propidium Iodide (PI) apoptosis detection kit (BD Biosciences, San Jose, CA, USA); Primary antibodies such as β-actin, BAX, BCL-2, MCL-1,Caspase-3, Cleaved-Caspase3 and β-catenin were obtained from Cell Signaling Technology (Beverly, MA, USA); Secondary antibodies for western blot analysis were obtained from Santa Cruz Biotechnology (Inc, CA, USA) and Cell Signaling Technology (Beverly, MA, USA).
Cell lines and cell culture conditions
The human osteoblast cell line hFob1. 19 (OB) was purchased from ATCC. The cell line was cultured at 37 °C in a 5%CO2 saturated humidity in RPMi-1640 medium supplemented with 20% fetal bovine serum (Gibco BRL; Life Technologies, Carlsbad, CA, USA), penicillin (100 U/ml), and streptomycin (100 μg/ml).
Cell viability assay
Different groups of OB cells were seeded at the density which 5000 cells per well in 96-well plates. There were 3 duplicated wells, and negative control wells and blank wells were set. After overnight incubation, the cells were treated with Olanzapine (20, 40, 60, 80, 100 and 120 μM), Risperidone (20, 40, 60, 80, 100 and 120 μM), Amisulpride (20, 40, 60, 80, 100 and 120 μM), Aripiprazole (2.5, 5, 15, 10, 20 and 40 μM) or 0.1%DMSO. After 12, 24 or 48 h, added CCK-8 10ul per well. Then incubation for 3 h, the absorbance of each well was measured at the wavelength of 490 nm by ELIASA.
Apoptosis analysis
OB cells treated with Olanzapine, Risperidone, Amisulpride, Aripiprazole, resveratrol or 0.1%DMSO for 24 h. The cells were harvested and washed with phosphate-buffered saline (PBS), and then cell pellets were harvested and stained with an Annexin V-FITC/PI apoptosis kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instruction. After being stained at room temperature for 15 min in the dark, the cells were measured with FCM and the Cell Quest software (BD Biosciences). The cells were measured with FCM and the Cell Quest software (BD Biosciences).
Protein extraction
Total protein extraction: OB cells were digested with trypsin and centrifuged, and then the supernatant was removed and washed by PBS. Joined protein cracking liquid RAPI and centrifuged at 4 °C. The supernatant is the total protein. Extraction of cytoplasm protein and nuclear protein: cytoplasm protein extraction reagent and nuclear protein extraction reagent respectively were added to PMSF, made final concentration of PMSF was 1 mM. OB cells were digested with trypsin and centrifuged, and then the supernatant was removed and washed by PBS. Added 200 μL mixture of cytoplasm protein extraction reagents and PMSF per 20 μL precipitate, high-speed vortex made precipitation fully spread out into suspense and centrifugation at 4 °C, the supernatant is cytoplasmic protein. Added 50–100 μL mixture of nuclear protein extraction reagent and PMSF in the precipitate, high-speed vortex made precipitation fully spread out into suspense and centrifugation at 4 °C, the supernatant is nuclear protein.
Western blot analysis
Western blot analysis (WB) was performed to analyze protein expression and activation after cells were treated with Olanzapine (40 μM), Risperidone (40 μM), Amisulpride (30 μM) and Aripiprazole (10 μM). Briefly, cells were washed in PBS, collected and then lysed in RIPA buffer (radio immunoprecipitation assay buffer, 50 mM Tris-HCl; 150 mM NaCl; 0.1% SDS; 0.5% Na-deoxycholate; 1% NP40) containing proteinase inhibitor cocktail and phosphatase inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). The lysate was centrifuged at 12000 rpm at 4 °C for 15 min, and equal protein lysate was used for Western blot analyses. For WB, 50 μg of total cell lysate was subjected to SDS-PAGE. The proteins were then transferred to a polyvinylidene difluoride membrane (Pall Corp, Ann Arbor, MI), and the membranes were blocked in 1 × PBS, 0.1% Tween-20 and 5% skim milk. After blocking, the membranes were incubated with primary antibodies diluted 1:1000 in 1 × PBS, 5% skim milk and 0.1% Tween-20 overnight at 4 °C. Immunodetection was performed by the Western blotting Luminol Reagent (Santa Cruz Biotechnology Inc. CA, USA). Actin immunoblotting was performed to verify that equal protein had been loaded in each lane. Their optical density was analyzed with Quantity One software. The expressions of target proteins were normalized to β-actin.
Immunofluorescence analysis
OB cells were plated confocal microscopy special cell culture dish, and incubated with Olanzapine (40 μM), Risperidone (40 μM), Amisulpride (30 μM) and Aripiprazole (10 μM) or 0.1%DMSO for 24 h. Then the cells were rinsed in PBS and fixed by incubation with 4% formaldehyde for 20 min at room temperature. After washing with PBS, cells were permeabilized with PBS containing 0.25% Triton X-100. The cells were blocked in PBS containing 5% BSA for 30 min. Next, with further washing cells were incubated with a rabbit anti-β-catenin monoclonal antibody, overnight at 4 °C temperature. After washing with PBS, cells were incubated with FITC-conjugated goat anti-rabbit antibody (1:200) for 30 min. Then, the nucleus was stained with DAPI (4, 6-diamidino-2-phenylindole) and cells were observed under a 20× field of view by confocal microscopy (LSM710, ZEISS).
Statistical analysis
Each experiment or assay was performed at least three times, and representative examples were shown. Data are reported as means ± SEM. Statistically significant differences between the treated groups were calculated using Student’s t-test. Differences were considered statistically significant at P < 0.05. Pearson correlation was used to assess relationship.
Results
Inhibition of OB cells viability after treatment with APs
To determine the effect of APs on proliferation of OB cells, we examined cell viability using CCK8 assay after the four APs (olanzapine, risperidone, amisulpride and aripiprazole) treatment respectively. Our results showed that the proliferation of OB cells decreased significantly compared with the control group (Fig. 1 a, c, e, g). In Bar graph 1 B, the IC50 values of olanzapine were 62.29 ± 12.23 μM, 43.73 ± 3.27 μM, and 27.24 ± 2.35 μM at 12 h, 24 h and 48 h. In Bar graph 1 D, the IC50 values of risperidone were 47.82 ± 3.78 μM, 37.06 ± 7.01 μM, and 26.13 ± 4.82 μM at 12 h, 24 h and 48 h. In Bar graph 1 F, the IC50 values of amisulpride were 45.51 ± 9.96 μM, 31.10 ± 2.53 μM, and 23.04 ± 1.90 μM at 12 h, 24 h and 48 h. In Bar graph 1 H, the IC50 values of aripiprazole were 16.18 ± 2.19 μM, 12.86 ± 1.30 μM, and 9.73 ± 0.82 μM at 12 h, 24 h and 48 h. Our data indicated that APs had an inhibitory effect on OB cells in a dose-dependent and time-dependent manner.
Inhibition of OB cells viability after APs treatment. a, OB cell viability was examined by CCK-8 assay after exposured to olanzapine (0.1%DMSO, 10, 20, 40, 80 or 120 μM), risperidone (0.1%DMSO, 10, 20, 40, 80 or 120μM), amisulpride (0.1%DMSO, 10, 20, 40, 80 or 120μM) or aripiprazole (0.1%DMSO, 2.5, 5, 10, 20 or 40 μM) at 12h, 24h or 48h (a, c, e, g). Bar graph indicated the IC-50 values of olanzapine, risperidone, amisulpride, aripiprazole in 1640 mediuM at 12, 24 and 48 hours (b, d, f, h). The data were calculated with GraphPad Prism. *P < 0.05; **P < 0.01
APs-induced apoptosis in OB cells
To further determine whether the four APs induce apoptosis in OB cells, we analyzed OB cells apoptosis by flow cytometry. The apoptosis rates of OB cells treated with olanzapine were 3.83 ± 2.34% (0.1% DMSO), 16.05 ± 2.12% (5 μM), 25.63 ± 3.90% (20 μM), 71.43 ± 5.23% (80 μM) (Fig. 2 a). The apoptosis rates of OB cells treated with risperidone were 4.73 ± 0.90% (0.1% DMSO), 17.67 ± 4.15% (5 μM), 29.37 ± 1.25% (20 μM), 66.70 ± 4.26% (80 μM) (Fig. 2 b). The apoptosis rates of OB cells treated with amisulpride were 5.80 ± 2.40% (0.1% DMSO), 21.83 ± 3.68% (5 μM), 32.93 ± 6.65% (20 μM), 71.26 ± 4.47% (80 μM) (Fig. 2 c). The apoptosis rates of OB cells treated with aripiprazole were 4.93 ± 2.31% (0.1% DMSO), 7.87 ± 2.44% (2.5 μM), 37.37 ± 3.78% (10 μM), 82.07 ± 7.10% (40 μM) (Fig. 2 d). Compared with the control group, apoptosis rates of OB cells treated by APs were significantly increased in a dose-dependent manner.
Effect of APs on OB cells apoptosis. OB cells treated with olanzapine (0.1%DMSO or 5, 20, 80, μM), risperidone (0.1%DMSO or 5, 20, 80, μM), amisulpride (0.1%DMSO or 5, 20, 80, μM) or aripiprazole (0.1%DMSO or 2.5, 10, 40, μM) incubated in 1640 medium at 24 h and the apoptosis of OB cells were analyzed by flow cytometry (a-d). Bar graph indicates the percent of Annexin V-positive cells (apoptotic cells) of experiments three times. The data were calculated with GraphPad Prism. *P < 0.05; **P < 0.01
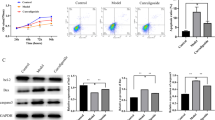
The broken balance between proapoptotic and antiapoptotic markers causing apoptosis
We had previously shown that treatment with the APs induced apoptosis rates upregulation in OB cells. To gain insight into the mechanism of APs-induced apoptosis in OB cells, We measured apoptotic protein Bcl-2, Mcl-1, Bax which belong to B cell lymphoma 2 (BCL2) family by WB. We found a reduced level of Bcl-2, Mcl-1 (antiapoptotic protein) and an elevated level of Bax (proapoptotic protein) after olanzapine (40 μM), risperidone, amisulpride and aripiprazole treatment compared with the control group. Additionally, Cleaved Caspase-3 increased while Caspase-3 decreased compared with the control group (Fig. 3 a). In the four treatment groups, olanzapine and risperidone had the stronger inhibitory effect on β-catenin than amisulpride and aripiprazole at the IC50 concentration (Fig. 3 a).
APs-induced apoptosis related to inhibition of Wnt/β-catenin signaling in OB cells. a, apoptosis-related protein and β-catenin protein expression was measured by western blot after exposured to olanzapine (Ola, 40 μM), risperidone (Ris, 40 μM), amisulpride (Ami, 30 μM) or aripiprazole (Ari, 12 μM) treatment at 24 h in OB cells. b, Nuclear and cytoplasmic protein of β-catenin were analyzed by western blot after exposured to olanzapine (40 μM), risperidone (40 μM), amisulpride (30 μM) or aripiprazole (12 μM) at 24 h in OB cells. c, The different expression of β-catenin protein between nuclear and cytoplasm was determined by Immunoflourescence analysis after exposured to olanzapine (40 μM), risperidone (40 μM), amisulpride (30 μM) or aripiprazole (12 μM) at 24 h in OB cells. The data were calculated with GraphPad Prism. *P < 0.05; **P < 0.01
The correlation between β-catenin and apoptotic markers
The importance of inhibition Wnt/β-catenin signaling had been confirmed in osteopenia related to osteoblast [16]. Our study previously indicated that APs increased apoptosis rate of OB cells. Together, we hypothesized that APs drugs might cause osteoblast apoptosis through Wnt/β-catenin signaling. To test this possibility, we measured protein expression of β-catenin after APs treatment respectively by WB. We found that β-catenin protein expression decreased compared with control group (Fig. 3 a). Since the functions of β-catenin depended on its expression in nucleus [17], subcellular fractionation immunoflourescence analysis and WB were performed. β-catenin was showed respectively decreased in cytoplasm and nuclear of OB cells after the four APs treatment (Fig. 3 c and b). These results suggested that inhibition of Wnt/β-catenin signaling was connected with increased apoptosis.
Heightened Wnt/β-catenin signaling prevented APs-induced apoptosis
Based on the ability of resveratrol to activate the β-catenin/TCF-mediated transcriptional activity [21], we selected it as activator of β-catenin. To study the effects of Wnt/β-catenin signaling on apoptosis rate of OB cells, we examined the apoptosis rate of OB cells again after resevratrol combined with APs treatment or just APs treatment. The apoptosis rate of olanzapine group was 51.2 ± 2.3%. The apoptosis rate of olanzapine combined with resevratrol group was 22.1 ± 0.3%. The apoptosis rate of risperidone group was 45.6 ± 2.5%. The apoptosis rate of risperidone combined with resevratrol group was 22.8 ± 0.5%. The apoptosis rate of amisulpride group was 47.3 ± 2.7%. The apoptosis rate of amisulpride combined with resevratrol group was 21.7 ± 0.6%. The apoptosis rate of aripiprazole group was 52.7 ± 2.5%. The apoptosis rate of aripiprazole combined with resevratrol group was 22.8 ± 0.8%. Compared with the just APs group, the apoptotic rates of APs combined with resevratrol group decreased significantly (Fig. 4 a). In the WB, β-catenin expression in APs combined with resevratrol group was significantly higher than just APs group (Fig. 4b). Correlation analysis showed negative correlation between β-catenin expression and the apoptotic rate in OB cells (Fig. 4c) (r = − 0.515, p < 0.05).
Activation of Wnt/β-catenin signaling to protect osteoblasts. a, the apoptosis of OB cells were analyzed by flow cytometry after olanzapine (40 μM), risperidone (40 μM), amisulpride (30 μM), aripiprazole (12 μM) with or without resveratrol (Res, 50 μM) treatment at 24 h. Bar graph indicates the percent of Annexin V-positive cells (apoptotic cells). b, β-catenin protein expression was measured by WB after olanzapine (40 μM), risperidone (40 μM), amisulpride (30 μM), aripiprazole (12 μM) with or without resveratrol (50 μM) treatment at 24 h in OB cells. The data were calculated with GraphPad Prism. c, Correlation between OB cells apoptosis rate and β-catenin protein expression in OB cells. *P < 0.05; **P < 0.01
Discussion
This study demonstrated that APs induced OB cell apoptosis. Further, the study provided evidence that expression of apoptosis-related proteins was adjusted after APs treatment. However, the current study suggest that APs may induce low BMD by sustaining elevate the secretion of prolactin and subsequently inhibit estrogen secretion as their dopamine D2 receptor-blocking effect [22]. High prolactin level being negatively correlation with estrogen concentration strongly relates to BMD loss [23]. A clinical trial showed that the prolactin-raising medication group (n = 26) had higher rate of bone pathology compared with the control group (n = 12). Unfortunately, the detailed mechanisms have not been investigated, particularly direct impact of APs on osteoblast. Just a few experiments superficially described one of APs impaired viability and function of osteoblasts [24,25,26]. Our study is the first to suggest a novel mechanism of APs induced apoptosis in OB cells.
The study identified the reduced level of Bcl-2, Mcl-1 and elevated level of Bax by WB. It is well known that BCL2 gene family is fundamental to the balance between cell survival and death, which encodes multiple proapoptotic and antiapoptotic proteins to regulate the intrinsic apoptosis pathway [27, 28]. Increased proapoptotic Bax bound to the mitochondrial outer results in membrane outer membrane permeabilization (MOMP) and the release of cytochrome c [29]. Trend in the apoptosis inducing signal, leaved caspase-3, a key executive in the process of apoptosis [24], hydrolyzes the target substance within the cell to degrade intracellular protein, resulting in irreversible death [30]. While the decreased expression of antiapoptotic protein Bcl-2, Mcl-1 reduced the effect which blocked the proapoptotic protein-mediated MOMP lead to increased apoptosis [31]. In Our work, cleaved caspase-3 expression increased also together with the current study.
In addition, our work highlighted the connection between Wnt/β-catenin signaling and atypical antipsychotic-induced apoptosis. The data indicated that Wnt/β-catenin signaling suppression is related to proapoptotic Bax, Caspase-3 up-regulation and antiapoptotic Bcl-2, Mcl-1 down-regulation. The Wnt/β-catenin signaling is an important modulator in bone homeostasis because it robustly promoted mesenchymal stem cells differentiated into osteoblasts and osteogenesis [32]. Previous studies also showed that wnt/β-catenin signaling involved in protecting osteoblasts and osteocytes from apoptosis [33,34,35]. In parallel with our study, a cell culture experiments showed that activating β-catenin by LiCl or Wnt1 inhibited the H2O2-mediated cell viability decreased and restored the mitochondrial Bcl-2/Bax ratio in human osteoblasts [36].
Interestingly, our study indicated that resevratrol could reverse the drop of β-catenin even reduced APs induced-apoptosis. Resevratrol had protective effect on inflammatory, oxida, aging, carcinoma had been reported [37,38,39]. Indeed, resevratrol was shown promote osteoblast proliferation and differentiation from multipotent mesenchymal cells [40]. Recent studies also demonstrated that resveratrol promoted osteoblast proliferation via activation of Wnt/β-catenin signaling [41]. More recently, the published data indicated that resevratrol upregulated sirt1 in osteoblast and volved in the control of osteoblast proliferation, differentiation and apoptosis [42]. Of note, sirt1 as a NAD-dependent histone deacetylase be provided controlling anxiety in mice [43]. There was also a link between the expression of sirt1 gene and depression [44]. Herein, we venturesome hypothesized whether resevratrol could be combined with APs to resist APs-induced low BMD and to relieve emotional disorders with schizophrenia patients. We would test this interesting hypothesis in the future experiments.
Taken together, our study suggested that APs may induce osteoblast apoptosis related to Wnt/β-catenin signal pathway inhibition while resevratrol could reverse this phenomenon. In addition to APs, common risk factors for osteoporosis including smoking, physical inactivity, polydipsia, alcohol misuse, hypogonadism, a family or personal history of fractures, and vitamin D deficiency are all increased in schizophrenia patients [45]. Studying the side effects of atypical antipsychotics, it is not to deny APs but to use it rationally in clinical such as schizophrenia patients with high osteoporotic risk need special attention with APs treatment. What is more, rational use of APs and active management of BMD loss in schizophrenia patients who have antipsychotic-associated bone disease could delay or even reverse this process [46].
Conclusions
Our study suggested that APs may induce osteoblast apoptosis through decrease of antiapoptotic protein expression and increase of proapoptotic protein expression related to decreased β-catenin expression and restrained β-catenin translocated into the nucleus which a committed step in the wnt/β-catenin signal pathway, and resevratrol could prevent APs-induced osteoblast apoptosis through increased β-catenin expression (Fig. 5).
Schematic representation mechanism of APs inducing OB cells apoptosis. The Aps inhibited Wnt/β-catenin signal pathway, as a result, Bcl-2, Mcl-1 (antiapoptotic protein) transcript reduced and Bax, (proapoptotic protein) transcripted elevated. Then, Caspase-3 was activated. Eventually, this led to OB cells apoptosis. On the contrary, resevratrol could reverse the above effects
Abbreviations
- APs:
-
Atypical antipsychotics
- BCL-2:
-
B cell lymphoma 2
- BMD:
-
Bone mineral density
- CCK8:
-
Cell counting kit-8
- FCM:
-
Flow cytometry
- FITC:
-
Annexin V-fluorescein isothiocyanate
- LiCl:
-
Lithium chloride
- MOMP:
-
Membrane permeabilization
- OB:
-
Osteoblast cell line hFob1. 19
- PI:
-
propidiμm iodide
- TCF/LEF:
-
T cell factor/ lymphocyte growth factor
- WB:
-
Western blot analysis
References
Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, Gallinat J, Giedd J, Grayson DR, Heinrichs M, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485–515.
Ibi D, de la Fuente Revenga M, Kezunovic N, Muguruza C, Saunders JM, Gaitonde SA, Moreno JL, Ijaz MK, Santosh V, Kozlenkov A, et al. Antipsychotic-induced Hdac2 transcription via NF-kappaB leads to synaptic and cognitive side effects. Nat Neurosci. 2017;20(9):1247–1259.
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388(10039):86–97.
Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406.
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62.
Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14(1):73–96.
Ferno J, Ersland KM, Duus IH, Gonzalez-Garcia I, Fossan KO, Berge RK, Steen VM, Skrede S. Olanzapine depot exposure in male rats: dose-dependent lipogenic effects without concomitant weight gain. Eur Neuropsychopharmacol. 2015;25(6):923–32.
Orsolini L, Tomasetti C, Valchera A, Vecchiotti R, Matarazzo I, Vellante F, Iasevoli F, Buonaguro EF, Fornaro M, Fiengo AL, et al. An update of safety of clinically used atypical antipsychotics. Expert Opin Drug Saf. 2016;15(10):1329–47.
Rojo LE, Gaspar PA, Silva H, Risco L, Arena P, Cubillos-Robles K, Jara B. Metabolic syndrome and obesity among users of second generation antipsychotics: a global challenge for modern psychopharmacology. Pharmacol Res. 2015;101:74–85.
Zhang B, Deng L, Wu H, Lu X, Peng L, Wu R, Guo W, Chen J, Li L, Zhao J. Relationship between long-term use of a typical antipsychotic medication by Chinese schizophrenia patients and the bone turnover markers serum osteocalcin and beta-CrossLaps. Schizophr Res. 2016;176(2–3):259–63.
Stubbs B, Gaughran F, Mitchell AJ, De Hert M, Farmer R, Soundy A, Rosenbaum S, Vancampfort D. Schizophrenia and the risk of fractures: a systematic review and comparative meta-analysis. Gen Hosp Psychiatry. 2015;37(2):126–33.
Holt RI. Osteoporosis in people with severe mental illness: a forgotten condition. Maturitas. 2010;67(1):1–2.
Bernabei R, Martone AM, Ortolani E, Landi F, Marzetti E. Screening, diagnosis and treatment of osteoporosis: a brief review. Clin Cases Miner Bone Metab. 2014;11(3):201–7.
Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349–55.
Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13(1):27–38.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513–32.
Shan T, Zhou C, Yang R, Yan F, Zhang P, Fu Y, Jiang H. Lithium chloride promotes the odontoblast differentiation of hair follicle neural crest cells by activating Wnt/beta-catenin signaling. Cell Biol Int. 2015;39(1):35–43.
Wang HD, Shi YM, Li L, Guo JD, Zhang YP, Hou SX. Treatment with resveratrol attenuates sublesional bone loss in spinal cord-injured rats. Br J Pharmacol. 2013;170(4):796–806.
Feng G, Zheng K, Song D, Xu K, Huang D, Zhang Y, Cao P, Shen S, Zhang J, Feng X, et al. SIRT1 was involved in TNF-alpha-promoted osteogenic differentiation of human DPSCs through Wnt/beta-catenin signal. In Vitro Cell Dev Biol Anim. 2016;52(10):1001–11.
Abed E, Couchourel D, Delalandre A, Duval N, Pelletier JP, Martel-Pelletier J, Lajeunesse D. Low sirtuin 1 levels in human osteoarthritis subchondral osteoblasts lead to abnormal sclerostin expression which decreases Wnt/beta-catenin activity. Bone. 2014;59:28–36.
De Hert M, Detraux J, Stubbs B. Relationship between antipsychotic medication, serum prolactin levels and osteoporosis/osteoporotic fractures in patients with schizophrenia: a critical literature review. Expert Opin Drug Saf. 2016;15(6):809–23.
Howes OD, Wheeler MJ, Meaney AM, O'Keane V, Fogelman I, Blake G, Murray RM, Smith S. Bone mineral density and its relationship to prolactin levels in patients taking antipsychotic treatment. J Clin Psychopharmacol. 2005;25(3):259–61.
Costa JL, Smith G, Watson M, Lin JM, Callon K, Gamble G, Cheng A, Vickers MH, Shepherd PR, Cornish J, et al. The atypical anti-psychotic clozapine decreases bone mass in rats in vivo. Schizophr Res. 2011;126(1–3):291–7.
Motyl KJ, Beauchemin M, Barlow D, Le PT, Nagano K, Treyball A, Contractor A, Baron R, Rosen CJ, Houseknecht KL. A novel role for dopamine signaling in the pathogenesis of bone loss from the atypical antipsychotic drug risperidone in female mice. Bone. 2017;103:168–76.
Motyl KJ, DeMambro VE, Barlow D, Olshan D, Nagano K, Baron R, Rosen CJ, Houseknecht KL. Propranolol attenuates risperidone-induced trabecular bone loss in female mice. Endocrinology. 2015;156(7):2374–83.
Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: orchestrators of apoptosis. Biochim Biophys Acta. 2011;1813(4):508–20.
Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63.
Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273–84.
White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159(7):1549–62.
Bender CE, Fitzgerald P, Tait SW, Llambi F, McStay GP, Tupper DO, Pellettieri J, Sanchez Alvarado A, Salvesen GS, Green DR. Mitochondrial pathway of apoptosis is ancestral in metazoans. Proc Natl Acad Sci U S A. 2012;109(13):4904–9.
Kim HY, Yoon JY, Yun JH, Cho KW, Lee SH, Rhee YM, Jung HS, Lim HJ, Lee H, Choi J, et al. CXXC5 is a negative-feedback regulator of the Wnt/beta-catenin pathway involved in osteoblast differentiation. Cell Death Differ. 2015;22(6):912–20.
Gortazar AR, Martin-Millan M, Bravo B, Plotkin LI, Bellido T. Crosstalk between caveolin-1/extracellular signal-regulated kinase (ERK) and beta-catenin survival pathways in osteocyte mechanotransduction. J Biol Chem. 2013;288(12):8168–75.
Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML, Bonewald LF. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25(12):2657–68.
Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280(50):41342–51.
Kook SH, Lee D, Cho ES, Heo JS, Poudel SB, Ahn YH, Hwang JW, Ji H, Kim JG, Lee JC. Activation of canonical Wnt/beta-catenin signaling inhibits H2O2-induced decreases in proliferation and differentiation of human periodontal ligament fibroblasts. Mol Cell Biochem. 2016;411(1–2):83–94.
Lancon A, Frazzi R, Latruffe N. Anti-oxidant, anti-inflammatory and anti-Angiogenic properties of resveratrol in ocular diseases. Molecules. 2016;21(3):304.
Liu FC, Tsai YF, Tsai HI, Yu HP. Anti-inflammatory and organ-protective effects of resveratrol in trauma-hemorrhagic injury. Mediat Inflamm. 2015;2015:643763.
Venigalla M, Sonego S, Gyengesi E, Sharman MJ, Munch G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease. Neurochem Int. 2016;95:63–74.
Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, Xiao Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14(12):806–14.
Zhou H, Shang L, Li X, Zhang X, Gao G, Guo C, Chen B, Liu Q, Gong Y, Shao C. Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells. Exp Cell Res. 2009;315(17):2953–62.
Abed E, Delalandre A, Lajeunesse D. Beneficial effect of resveratrol on phenotypic features and activity of osteoarthritic osteoblasts. Arthritis Res Ther. 2017;19(1):151.
Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S, Preisig M, Otowa T, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147(7):1459–72.
Consortium C. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523(7562):588–91.
Stubbs B, De Hert M, Sepehry AA, Correll CU, Mitchell AJ, Soundy A, Detraux J, Vancampfort D. A meta-analysis of prevalence estimates and moderators of low bone mass in people with schizophrenia. Acta Psychiatr Scand. 2014;130(6):470–86.
O'Keane V. Antipsychotic-induced hyperprolactinaemia, hypogonadism and osteoporosis in the treatment of schizophrenia. J Psychopharmacol. 2008;22(2 Suppl):70–5.
Acknowledgements
Not applicable.
Funding
This study had support from the National Natural Science Foundation of China (NO. 81761128036, 81560235 and 31760294), High-level Innovative Talents Cultivation Program of Guizhou Province (QianKeHe SY [2016] 5679), Platform for Talent of Guizhou QianKeHe ([2018]5802, [2016]1120), Fund of Guiyang Science and Technology department ([2017]5–14) and Joint Fund Project of Guizhou Porvincial Technology Department (QianKeHe LH [2015]7430). Neither the funding source nor the sponsor had any role in the design of this study or its execution, analyses, interpretation of the data, or decision to submit results.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
YW, XL and ZZ conceived and designed the study. PL, ZZ, JW, HZ, LZ and LY performed research and analysed the results. PL and ZZ made substantial contributions to interpretation of data. PL, YW and XL drafted the original manuscript. All authors meet the criteria for authorship in accordance with ICJME having made: a) substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; b) drafting the work or revising it critically for important intellectual content; c) final approval of the version to be published; and d) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors were involved in critically revising the manuscript for important intellectual content and gave final approval of the published version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, P., Wang, Y., Liu, X. et al. Atypical antipsychotics induce human osteoblasts apoptosis via Wnt/β-catenin signaling. BMC Pharmacol Toxicol 20, 10 (2019). https://doi.org/10.1186/s40360-019-0287-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-019-0287-9