Abstract
Background
The novel coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is leading to a worldwide pandemic. Except representative manifestation of pneumonia and acute respiratory symptoms, COVID-19 patients have also shown different levels of liver injury or liver dysfunction. The aim of our study was to explore the probable clinical severity and mortality of COVID-19 patients and their liver dysfunction.
Method
A combination of computer and manual retrieval was used to search in Medline through PubMed, EMBASE and Web of Science. Review Manager 5.3 software was used to examine the heterogeneity among the studies and to calculate the combined effect value (OR, 95CI). Subgroup analysis, sensitivity analysis, and publication bias test were also performed.
Results
We found a significant connection between liver dysfunction and mortality of COVID-19 patients with a pooled OR of 1.98 (95% CI 1.39–2.82; P = 0.0002). There was a significant association between AST and severity of COVID-19 with a pooled OR of 4.48 (95% CI 3.24–7.21; P < 0.001), and a pooled WMD of 3.35 (95% CI, 2.07 to 4.64; P < 0.001). In addition, there was a significant difference between TBIL and severity of COVID-19, with a pooled OR of 1.91 (95% CI 1.40–2.60; P < 0.001), and with a pooled WMD of 1.18 (95% CI, 0.78 to 1.58; P < 0.001).
Conclusion
The mortality and severity of COVID-19 patients are significantly associated with liver dysfunction. The non-survivors and severe COVID-19 patients have elevated serum AST levels than the survivors and non-severe COVID-19 patients. The results of this study form a basis for better clinical liver management of patients with COVID-19.
Similar content being viewed by others
Background
Coronavirus (CoV) is an enveloped positive single-stranded RNA virus. It is widely distributed in humans and animals. It is known to cause human respiratory infections [1]. The ongoing COVID-19 pandemic caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is rapidly developing [2]. COVID-19 is caused by a virus, SARS-COV-2, and its major symptoms are fever, cough, and dyspnea, and minor symptoms are alteration of the smell and taste, gastrointestinal symptoms, headache, and cutaneous manifestations [3,4,5]. SARS-CoV-2 enters cells through the angiotensin converting enzyme 2 (ACE2) which is its intended receptor [6]. Currently, there are no specific/targeted drugs or vaccines for SARS-CoV-2. In many parts of the world, the number of SARS-CoV-2 positive patients are increasing exponentially. As of 18th April, 2020, there were 2,251,633 positive cases and 154,329 deaths worldwide. This suggested that the total death rate of COVID-19 was 6.85%. Clinical symptoms of COVID-19 are pneumonia and acute respiratory symptoms. However, COVID-19 patients also have varying levels of liver injuries or liver dysfunction though the results are controversial. Yang et al. reported that there were no differences in liver function among survivors and non-survivors of COVID-19 [7]. A recent study reported that 318 out of the 417 COVID-19 patients tested during hospitalization had aberrant liver test results while 90 patients had liver injury. The use of ritonavir/lopinavir also increased the occurrence of liver injury [8]. Chen et al. reported that there were liver enzyme abnormalities in 99 COVID-19 patients in Wuhan. Among the 99, 43 (43.4%) had elevated aspartate aminotransferase (AST) and serum alanine aminotransferase (ALT) [9]. However, in a cohort of 548 COVID-19 patients from mainland China, there were no significant differences in ALT levels between non-severe (n = 279) and severe (n = 269) patients (P = 0.683) [10]. Similarly, Wan et al. retrospectively studied 135 hospitalized COVID-19 patients and reported that there were no significant differences in ALT (P = 0.73) and total bilirubin (TBIL, P = 0.07) between mild cases (n = 95) and severe cases (n = 40) [11]. These contradicting reports have now attracted widespread attention. As such, this meta-analysis aimed to explore the probable clinical severity and mortality of COVID-19 patients and their liver dysfunction. The study further analyzed the likelihood of COVID-19 patients to have bad liver injuries. The results of this study will help researchers and clinicians in formulating stronger prevention guidelines as well as have effective responses to COVID-19. Ultimately, this will contribute to effective management and treatment of liver injuries.
Methods
Search summary
Studies involving SARS-CoV-2 or COVID-19 or liver injury/dysfunction were included. In order to find relevant original articles, we conducted a comprehensive search in the database, involving Medline through PubMed, EMBASE and Web of Science, and using the following words: "COVID-19", "SARS-CoV-2", "Wuhan virus", "Chinese virus", "novel coronavirus 2019", "2019 nCoV", "Wuhan coronavirus", ``2019 Coronavirus", "Characteristics", "Characteristics" and "Liver". As of April 17, 2020, the papers have been searched in the language range. We also refer to the recognized literature to find other qualified research subjects. We first screened the article title and abstract, as well as publications that may involve data on COVID-19 or SARS-CoV-2 and liver injury/dysfunction.
Inclusion and exclusion standard
This study had no national restrictions. All studies reporting on COVID-19 non-survivors/survivors, non-severe/severe and laboratory confirmed COVID-19 patient data were included in the study. Moreover, the studies had to be limited to humans, include raw data, be published in English and be in either abstract form or full text. Repeated studies, letters, case reports, abstracts, and comments were excluded from the study. Data collected from each study included the year of publication, name of first author, sample size, and age of COVID-19 patients.
Statistical analysis
The quality of each study was evaluated using the Newcastle–Ottawa scale while the meta-analysis was conducted through the Review Manager 5.3 software. Heterogeneity was evaluated by calculating the I2 index. I2values less than 25%, 25–50%, 50–75% and 75–100% were homogeneous, or had low, medium, and high heterogeneity levels, respectively. The random effect model (REM) was applied if the I2 value is > 50% while the fixed effect model (FEM) was applied if the I2 value is < 50%. The combined odds ratio (OR) and weighted mean difference (WMD) of different studies with corresponding 95% confidence intervals (CI) were used to assess the relationship between liver dysfunction and COVID-19. The sensitivity analysis was repeated to evaluate the impact of each study in the analysis by subsequently excluding different individual studies each time. Based on the method of Hozo et al. [12], the mean and standard deviation were inferred from the sample size, median and interquartile range (IQR) when the continuous variables were not available.
Results
Study processing
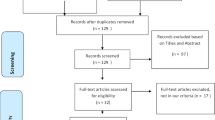
Five hundred and eighty-five relevant articles and one from the reference list were identified. Exclusion of duplicates brought down the number to 326 studies. The 326 studies were further screened if they meet the inclusion criteria. This resulted in the exclusion of 270 studies. Forty-three more studies were excluded after scanning of the entire body text of the 56 remaining studies. The remaining 13 articles meet the eligibility criteria [7, 8, 10, 11, 13,14,15,16,17,18,19,20,21]. The steps of document retrieval are shown in Fig. 1. Similarly, the study characteristics are shown in Tables 1 and 2. The meta-analysis involved 3,722 cases (285 dead vs 748 alive; 1968 non-severe vs 721 severe). All studies were conducted and published in 2020. One of the studies (Reference 4) was conducted in an ICU setting while the remaining were conducted in isolation wards. In the same line, there was one study (Reference 5) that had categorized patients based on abnormal liver test (Group A) and liver injury (Group B). All articles had a NOS score of six and above. This meant that they were all of high quality.
Pooled analysis
Survivors vs non-survivors
The forest plot outcome showing the association between liver dysfunction and the mortality of patients with COVID-19 is displayed in Fig. 2a. There was a significant connection between liver dysfunction and mortality of COVID-19 patients with a pooled OR of 1.98 (95% CI 1.39–2.82; P = 0.0002). The pooled data were calculated under the FEM as a low heterogeneity within the studies. Pooled results of five studies revealed that AST was significantly lower in COVID-19 survivors (WMD 3.72; 95% CI 2.74 to 4.71; P < 0.001) (Fig. 2c). However, there were no significant differences in ALT levels between COVID-19 survivors and non-survivors (WMD 1.34; 95% CI -0.47 to 3.16; P = 0.15) (Fig. 2b).
Non-severe vs severe based on ALT, AST and TBIL
Dichotomous and continuous analysis revealed that there was no significant association between ALT and severity of COVID-19 with a pooled OR of 1.38 (95% CI 0.84–2.27; P = 21). However, ALT was significantly lower in patients with non-severe COVID-19 (WMD 2.56; 95% CI 1.34 to 3.78; P < 0.001) (Fig. 3). In the same line, there was a significant association between AST and severity of COVID-19 with a pooled OR of 4.48 (95% CI 3.24–7.21; P < 0.001), and a pooled WMD of 3.35 (95% CI, 2.07 to 4.64; P < 0.001) (Fig. 4). Similarly, there was a significant association between TBIL and severity of COVID-19 with a pooled OR of 1.91 (95% CI 1.40–2.60; P < 0.001), and a pooled WMD of 1.18 (95% CI 0.78 to 1.58; P < 0.001) (Fig. 5).
Subgroup analysis and sensitivity analysis
Subgroup analysis was used to reveal the sources of heterogeneity. Age and the number of non-severe patients were found to be potential sources of heterogeneity (Table 3). However, the impact of age stratification should be verified further using bigger data sets because this study had a small data set. The overall mortality of COVID-19 had low heterogeneity (I2 = 36%). However, when either Chen’s or Wang’s study was eliminated, the results significantly affect the pooled outcomes (I2 dropped from 36 to 25%). Similarly, when Chen’s study was removed from the AST group, the I2 dropped from 93 to 70%. In the same line, the severity of COVID-19 in AST (events) had moderate heterogeneity (I2 = 61%). However, when Li’s study was eliminated, the I2 dropped from 61 to 49%. Similarly, when Zhang’s study was removed in the AST (levels) group, the I2 dropped from 97 to 79%. When Zhang’s study was removed in the AST (events) group, the I2 dropped from 71 to 28%. In the TBIL (levels) group, removal of Zheng’s study led to reduction of the I2 from 71 to 67%. Cognizant of this, it was hypothesized that the studies of Zhang et al. 2020 and Zheng et al. 2020 were the sources of heterogeneity in this meta-analysis. The Egger’s regression test (P > 0.05) further revealed that the meta-analysis had no significant publication bias.
Discussion
Herein, the abnormal liver function was associated with mortality and severity of COVID-19. Subgroup analysis conducted to identify sources of heterogeneity further revealed that the overall outcome was acceptable. Similarly, sensitivity analysis revealed that when any study was excluded or REM was converted to FEM, the overall outcome was acceptable. These results strongly suggested that there in a correlation between liver injury and COVID-19.
Although it has been observed that the most severe and fatal cases of COVID-19 occur in the elderly individuals with liver injury, the relevant pathophysiology is still unclear. Xu et al [22] reported the pathological characteristics of the liver of a 50-year-old man who died from critical COVID-19. His liver had moderate microvascular steatosis as well as mild lobular and portal activity. However, no histological features of liver failure and bile duct injuries have been observed until now. SARS-CoV-2 is closely related to SARS-CoV by virtue of both sharing the same receptor (ACE2). However, there is no relevant study that has explored the role of ACE2 in liver injury especially in COVID-19 patients given that majority of them usually have elevated serum aminotransferase. The over-activated immune response caused by SARS-CoV-2 infection and systemic inflammation associated with cytokine storms may lead to organ (liver) damage [23]. Qin et al. [24] reported that most severe COVID-19 patients had elevated biomarkers associated with infection, increased inflammatory cytokine levels, and decreased number of T cells. In addition, stress (such as shock, ARDS, and septic) or drug toxicity-induced liver injury might be associated with hypoxia reoxygenation, oxidative stress, and imbalance of acid–base. This also contributes to very high aminotransferase concentrations. Nonetheless, studies have reported varying levels of aminotransferase. This meta-analysis revealed that there is a significant correlation between COVID-19 and liver dysfunction which provides a basis for better clinical management of COVID-19 and liver disease patients.
Patients aged above 65 years have higher comorbidities, more severe symptoms, and are more prone to multiple organ involvement and death compared to the younger patients [13]. For example, Feng et al. 2020 reported that the survival rate of patients aged above 75 years was significantly lower than that of young patients [25]. Factors leading to poor health outcomes include physiological changes in aging and various age-related complications. Moreover, older people's suspicion and detection thresholds for SARS-2 such as temperature, decreased function of cough, and shortness of breath are lower [26]. Cognizant of this, more attention should be paid to COVID-19 patients with a history of liver disease (especially elderly patients) by constantly observing liver changes, and carefully determining the cause of liver abnormalities. Age subgroup analysis revealed that the age ≥ 70 or age ≥ 60 subgroup is of great significance. However, further studies should be conducted on the effect of age stratification on the mortality and severity of patients with COVID-19 combined with liver injury.
Previous studies quantify the effects of COVID-19 on the digestive system and chronic liver disease [27, 28]. While this meta-analysis to prove that there is an association between severity and mortality of COVID-19 patients, and liver dysfunction. Nevertheless, this study was limited by several factors. Additional information such as liver disease types, drug use, nutritional levels, and other indicators to assess liver function was not present. As such, stratifying the risk in subgroup analysis of liver injury patient populations was not possible. In the same line, only the information regarding the age and gender of COVID-19 patients was collected. Other factors such as BMI, measurement methods and instruments for detecting SARS-CoV-2, and sample size could have as well affected the accuracy of the results. Moreover, patients diagnosed with COVID-19 could have had multiple chronic diseases such as intestinal diseases and pre-existing liver disease that could have affected the accuracy of the results. As such, large-scale prospective studies should be conducted to verify these results.
Conclusion
The mortality and severity of COVID-19 patients are significantly associated with liver dysfunction. The non-survivors and severe COVID-19 patients have elevated serum AST levels than the survivors and non-severe COVID-19 patients. The results of this study form a basis for better clinical liver management of patients with COVID-19.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ACE2:
-
Angiotensin converting enzyme 2
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COV:
-
Coronaviruses
- AST:
-
Aspartate aminotransferase
- ALT:
-
Serum alanine aminotransferase
- REM:
-
Random effect model
- FEM:
-
Fixed effect model
- OR:
-
Odds ratio
- WMD:
-
Weighted mean difference
- CI:
-
Confidence intervals
- ICU:
-
Intensive care unit
- TBIL:
-
Total bilirubin
References
Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–74.
Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–44.
Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, Ferrari M, Gagliardini L, Pipolo C, Deiana G, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42(7):1560–9.
Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, Babudieri S, Petrocelli M, Serra A, Bussu F, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. 2020;42(6):1252–8.
Guarneri C, Rullo EV, Pavone P, Berretta M, Ceccarelli M, Natale A, Nunnari G. Silent COVID-19: what your skin can reveal. Lancet Infect Dis. 2020;S1473–3099(20):30402–3.
Bindom SM, Lazartigues E. The sweeter side of ACE2: Physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2008;302(2):193–202.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–74.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13.
Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–8.
Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;20(5):13.
Wang L, He W, Yu X, Hu D, Bao M, Liu H, Zhou J, Jiang H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–45.
Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, Chen X, Luo M, Liang K, Gao S, Zhang Y, Deng L, Xiong Y. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a Single-Centered, Retrospective Study. J Gerontol A Biol Sci Med Sci. 2020;75(9):1788–95.
Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P, Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–9.
Zheng F, Tang W, Li H, et al. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24(6):3404–10.
Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40(9):2095–103.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73(2):451–3.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Li J, Fan JG, et al. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8(1):13–7.
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8.
Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–8.
Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42(2):505–14.
Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–78.
Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52(4):584–99.
Acknowledgements
Not applicable.
Funding
This work is not supported by grants.
Author information
Authors and Affiliations
Contributions
WZH designed and analyzed the research study. WZH wrote and revised the manuscript. WZH and YDL collected the data. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to public
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, Zh., Yang, D. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res 25, 54 (2020). https://doi.org/10.1186/s40001-020-00454-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-020-00454-x