Abstract
Background
The effect of statin treatment on circulating coenzyme Q10 (CoQ10) has been studied in numerous randomized controlled trails (RCTs). However, whether statin treatment decreases circulating CoQ10 is still controversial.
Methods
PubMed, EMBASE, and the Cochrane Library were searched to identify RCTs to investigate the effect of statin treatment on circulating CoQ10. We calculated the pooled standard mean difference (SMD) using a fixed effect model or random effect model to assess the effect of statin treatment on circulating CoQ10. The methodological quality of the studies was determined according to the Cochrane Handbook. Publication bias was evaluated by a funnel plot, the Egger regression test, and the Begg–Mazumdar correlation test.
Results
Twelve RCTs with a total of 1776 participants were evaluated. Compared with placebo, statin treatment resulted in a reduction of circulating CoQ10 (SMD, − 2.12; 95% CI, − 3.40 to − 0.84; p = 0.001), which was not associated with the duration of statin treatment (Exp, 1.00; 95% CI, 0.97 to 1.03; p = 0.994). Subgroup analysis demonstrated that both lipophilic statins (SMD, − 1.91; 95% CI, − 3.62 to 0.2; p = 0.017) and hydrophilic statins (SMD, − 2.36; 95% CI, − 4.30 to − 0.42; p = 0.028) decreased circulating CoQ10, and no obvious difference was observed between the two groups (SMD, − 0.20; 95% CI, − 0.208 to 0.618; p = 0.320). In addition, both low-middle intensity statins (SMD, − 2.403; 95% CI, − 3.992 to − 0.813; p < 0.001) and high intensity statins (SMD, − 1.727; 95% CI, − 2.746 to − 0.709; p < 0.001) decreased circulating CoQ10. Meta-regression showed that the effect of statin on decreasing circulating CoQ10 was not closely associated with the duration of statin treatment (Exp, 1.00; 95% CI, 0.97 to 1.03; p = 0.994).
Conclusions
Statin treatment decreased circulating CoQ10 but was not associated with the statin solution, intensity, or treatment time. The findings of this study provide a potential mechanism for statin-associated muscle symptoms (SAMS) and suggest that CoQ10 supplementation may be a promising complementary approach for SAMS.
Similar content being viewed by others
Introduction
Statins are widely used in the prevention and treatment of coronary heart disease [1]. Numerous large-scale studies have demonstrated that statins substantially reduce cardiovascular morbidity and mortality in both primary and secondary prevention, in both genders and in all age groups [2,3,4]. However, statin-associated muscle symptoms (SAMS), covering a broader range of muscle symptoms following statin treatment, are an important reason for statin discontinuation [5]. A previous study demonstrated that SAMS result in significantly high discontinuation rates of statin treatment (up to 75%) within 2 years of initiation [6], and in 65% of former statin users, the main reason for statin non-adherence or discontinuation was the onset of side effects, predominantly SAMS [7]. Non-adherence or discontinuation of statin treatment contributes to adverse cardiovascular outcomes. A meta-analysis showed a 15% lower cardiovascular risk in patients who adhered to statin treatment compared with those with low adherence [8]. Studying the possible mechanism and therapeutic approach of SAMS could decrease the cardiovascular risk in patients who are intolerant to statins due to SAMS [9]. The European Atherosclerosis Society has proposed four strategies for treating SAMS, including re-challenge with alterative statin therapy, lower or intermittent statin therapy, non-statin-based lipid-lowering therapy, and complementary therapies. Complementary therapies, such as coenzyme Q10 (CoQ10) supplementation, might be a promising method to manage SAMS [9]. The mechanism of SAMS is currently unclear, but changes in circulating coenzyme Q10 concentration may be involved in the pathological process. Exploring the level of circulating CoQ10 following statin treatment may explain potential mechanisms and suggest possible complementary approaches for SAMS.
CoQ10 is a naturally occurring, fat-soluble quinone located in the hydrophobic portions of cellular membranes [10], and it plays an important role in mitochondrial energy metabolism and stabilization of muscle cell membranes [11, 12]. A previous animal study demonstrated that statin treatment could lead to the reduction of circulating CoQ10 [13]. However, the findings concerning changes in circulating CoQ10 following statin therapy have been inconsistent in clinical studies. A previous meta-analysis [14] only included 6 clinical studies, and several RCTs, including statin treatment for a period of 24 weeks, have been published since, and these studies have provided new evidence. Therefore, the present meta-analysis of RCTs was designed to reassess the effect of statin treatment on circulating CoQ10.
Methods
This study was performed according to the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISM) (Additional file 1: Table S1) [15].
Data source and search strategies
Two reviewers (Hua Qu and Hua Chai) searched PubMed, EMBASE, and the Cochrane Library with no language restrictions from inception to January 2018 to identify all existing literature. Mesh terms and free-text terms were used in each database with the following relevant keywords: “statin treatment” AND “coenzyme Q10” AND “randomized controlled trials.” A manual search was also performed to identify relevant references from the selected articles and published reviews. The studies were eligible if they met the following inclusion criteria: (1) randomized, controlled, parallel, or crossover trial, (2) the intervention group received statin and the comparison group received placebo, or the intervention group received lipophilic statin and the comparison group received hydrophilic statin, and (3) the outcome regarding circulating CoQ10 (plasma CoQ10 or serum CoQ10) was available.
Data extraction and assessment of study quality
Two reviewers (Hua Qu and Yan-yan Meng) extracted data independently. If a disagreement occurred, it was resolved by consulting with a third investigator (Da-zhuo Shi). We contacted the authors if the article was only published with an abstract, and the studies without original data were excluded. The following data were extracted from each individual eligible study: (1) the first author’s name and publication year, (2) intervention duration, (3) inclusion criteria, (4) participant number, (5) participants’ age, (6) percentage of males, and (7) clinical outcomes. The methodological quality of eligible studies was determined according to the recommendation of the Cochrane Handbook [16].
Statistical analysis
In this meta-analysis, continuous data were used to analyze the standard mean difference (SMD) with a 95% confidence interval (CI) for the effect size. Heterogeneity in the eligible studies was evaluated using the Chi-square test based on Cochran’s Q test and I2 statistic at the p < 0.10 level of significance, and quantification of heterogeneity was calculated using the I2 metric, which describes the percentage of total variation estimated to be due to heterogeneity rather than chance. When P for the heterogeneity was < 0.1 and I2 ≥ 50%, the inter-study heterogeneity was considered statistically significant. The selection of the random or fixed effect model was based on the heterogeneity analysis. The fixed effect model was applied if I2 < 50%, and the random effect model was chosen if I2 ≥ 50%. We performed subgroup analysis and meta-regression to detect the potential sources of heterogeneity in the condition of I2 ≥ 50%. Sensitivity analysis was performed to assess the robustness of the pooled SMDs by eliminating one study at a time. The publication bias was evaluated by funnel plot, Egger regression, and the Begg–Mazumdar correlation test. Statistical analysis was performed using Stata (version 12.0). There is no registered protocol for the present meta-analysis.
Results
Description of included studies
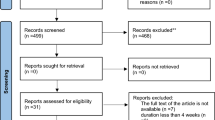
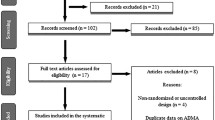
Six hundred and twenty-nine studies (298 from PubMed, 304 from EMBASE, and 27 from the Cochrane Library) were identified, and 191 articles were excluded as duplicated records. After the titles and abstracts of the articles were screened, 397 articles were excluded due to review format, improper study type, and/or improper comparisons. After the remaining 41 full-text articles were reviewed, 29 articles were excluded due to improper comparisons, irrelevant outcomes, and/or unavailable outcomes. Finally, 12 articles [17,18,19,20,21,22,23,24,25,26,27,28] with 1776 participants published in English from 1993 to 2018, with sample sizes ranging from 19 to 1103 participants and intervention durations ranging from 14 days to 26 weeks, were entered into our meta-analysis (Fig. 1, Table 1). Nine RCTs [17,18,19,20,21, 23,24,25], including 11 study arms (822 participants in the statin treatment group vs. 830 participants in the placebo group), evaluated the effect of statins (statins vs. placebo) on circulating CoQ10, and 4 RCTs [22, 25, 26, 28], including 7 study arms (94 participants in a lipophilic statin group vs. 115 participants in a hydrophilic statin group), evaluated the effect of different soluble statins (lipophilic statins vs. hydrophilic statins) on circulating CoQ10.
Quality assessment
“Low risk,” “high risk,” or “unclear risk” was categorized for all 12 included studies according to 7 risk biases presented in sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias (Additional file 2: Figure S1) [16]. No obvious attrition bias or reporting bias was observed. Additionally, the randomization and blinding in the included articles were considered adequate in the present study according to the Cochrane Handbook [16].
The effect of statin treatment on circulating CoQ10
When compared with placebo, statin treatment decreased circulating CoQ10 (SMD, − 2.12; 95% CI, − 3.40 to − 0.84; p = 0.001) with a significant heterogeneity (I2 = 98%, p < 0.001) (Fig. 2). The subgroup analysis demonstrated that statins could decrease circulating CoQ10 with both lipophilic statins (SMD, − 1.91; 95% CI, − 3.62 to 0.2; p = 0.017) and hydrophilic statins (SMD, − 2.36; 95% CI, − 4.30 to − 0.42; p = 0.028) (Fig. 3), and no obvious difference was observed between hydrophilic statins and lipophilic statins in the efficacy of decreasing circulating CoQ10 (SMD, − 0.20; 95% CI, − 0.208 to 0.618; p = 0.320) (Fig. 4). In addition, both low-middle intensity statins (SMD, − 2.403; 95% CI, − 3.992 to − 0.813; p < 0.001) and high intensity statins (SMD, − 1.727; 95% CI, − 2.746 to − 0.709; p < 0.001) could decrease circulating CoQ10 (Fig. 5). The meta-regression showed that the effect of statins on decreasing circulating CoQ10 was not closely associated with the statin treatment time (Exp, 1.00; 95% CI, 0.97 to 1.03; p = 0.994) (Fig. 6).
Sensitivity analysis
To ensure the reliability of the present meta-analysis, we performed sensitivity analysis to evaluate the robustness of the pooled SMDs by eliminating each study one at a time sequentially, which indicated that the heterogeneity among the studies did not significantly change regarding the effect of statins on circulating CoQ10. Thus, no one study showed a significant impact on the results of the present meta-analysis.
Publication bias
Three methods, including funnel plot, Egger regression test and the Begg–Mazumdar correlation test, were used to evaluate publication bias regarding the effect of statin treatment on circulating CoQ10, which suggested potential publication bias (Begg–Mazumdar correlation test, Kendall’s score = 0, continuity corrected z = 0.08, continuity corrected p = 1; Egger regression test, Coef., 9.81; 95% CI, 2.52 to 0.17.11; p = 0.014) (Additional file 3: Figure S2). Using a “trim and fill” correction, 5 potentially missing studies were imputed leading to a corrected effect size (SMD, − 4.01, 95% CI, − 5.38 to − 2.63, p < 0.0001), which was consistent with the previous effect size.
Discussion
In the previous meta-analysis performed by Banach et al. [14], only 6 studies were included. Additionally, subgroup analysis based on the intensity of statins and comparisons between lipophilic statins and hydrophilic statins were not performed due to the limited number of enrolled studies and their small sample sizes. In the present meta-analysis, the results from 12 RCTs validated a reduction of circulating CoQ10 following statin treatment. We also showed, for the first time, that both lipophilic statins and hydrophilic statins could decrease circulating CoQ10, and there was no significant difference between the two groups. In addition, a significant effect was observed for both low-middle intensity statins and high intensity statins in terms of decreasing the level of circulating CoQ10, and the effect of statins on circulating CoQ10 was not closely associated with the statin treatment time (from 14 days to 26 weeks).
The present meta-analysis demonstrated that statin treatment decreased the level of circulating CoQ10, which is consistent with some previous clinical studies [23, 24]. The mechanisms of the reduction of circulating CoQ10 following statin treatment remain unclear though some hypotheses have been offered. First, statin treatment may decrease the biosynthesis of CoQ10. Farnesyl pyrophosphate, a precursor in the synthesis of CoQ10, was blocked during statin treatment, which might contribute to a reduction of circulating/intramuscular CoQ10 [13]. Second, statin treatment may decrease absorption of dietary CoQ10. A recent study demonstrated that statin treatment could cause gut dysbiosis in mice through activating the PXR-dependent pathway, which might influence the absorption of CoQ10 in the gut [29]. CoQ10 participates in electron transport during oxidative phosphorylation in mitochondria, protects against oxidative stress produced by free radicals, and regenerates active forms of the antioxidants ascorbic acid and tocopherol, which play important roles in maintaining mitochondrial energy metabolism and stabilizing muscle cell membranes [30,31,32,33,34]. CoQ10 deficiency presents as increased oxidative stress, increased inflammatory responses, and an imbalanced serotonergic system, which may contribute to SAMS [35]. The present meta-analysis validated the effect of statin in decreasing circulating CoQ10, which will be helpful for studying the mechanism of SAMS and may provide a complementary approach for SAMS treatment.
Previous studies have suggested that hydrophilic statins may have a lower rate of SAMS compared with lipophilic statins and that clinicians should consider switching to a hydrophilic statin to manage SAMS [36, 37]. However, in the present meta-analysis, no obvious difference was observed between lipophilic statins and hydrophilic statins in decreasing circulating CoQ10. Shi et al. also found that patients with SAMS who are intolerant to some hydrophilic statins may be successfully managed with simvastatin (lipophilic statin) monotherapy [38]. Therefore, whether hydrophilic statins have a lower rate of SAMS compared with lipophilic statins deserves further study. In addition, a lower dose of statin is always recommended to manage patients who tolerate statins because of SAMS [9]. However, significant effects of statins were observed in both low-moderate intensity statins and high intensity statins by decreasing circulating CoQ10 in the present study. Therefore, performing large-scale trials is necessary to compare the rate of SAMS between low-moderate intensity statins and high intensity statins. Several limitations in the present study should be noted. First, the eligible studies were heterogeneous because of certain factors, such as population characteristics, study design, and duration of statin treatment. Thus, we performed subgroup analysis, sensitivity analysis, and meta-regression to minimize the effect of heterogeneity on estimated effect size and assure the reliability of the outcomes. Second, there was potential publication bias for the effect of statin treatment on circulating CoQ10, so we used the trim and fill method to assure the robustness of the pooled results.
In conclusion, statin treatment decreases circulating CoQ10, regardless of statin solution, intensity, or treatment time. The findings provide a potential mechanism for SAMS and suggest that CoQ10 supplementation might be a promising complementary approach for SAMS.
References
Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen M-R, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–818. https://doi.org/10.1093/eurheartj/ehr158.
Thavendiranathan P. Primary prevention of cardiovascular diseases with statin therapy. Arch Intern Med. 2006;166:2307. https://doi.org/10.1001/archinte.166.21.2307.
Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170 255 patients from 76 randomized trials. QJM. 2011;104:109–24. https://doi.org/10.1093/qjmed/hcq165.
Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events. Arch Intern Med. 2012. https://doi.org/10.1001/archinternmed.2012.2145.
Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:S52–60.
Chodick G, Shalev V, Gerber Y, Heymann AD, Silber H, Simah V, et al. Long-term persistence with statin treatment in a not-for-profit health maintenance organization: a population-based retrospective cohort study in Israel. Clin Ther. 2008;30:2167–79.
Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6:208–15.
Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–8. https://doi.org/10.1093/eurheartj/eht295.
Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–22.
Hernández-Camacho JD, Bernier M, López-Lluch G, Navas P. Coenzyme Q10 supplementation in aging and disease. Front Physiol. 2018. https://doi.org/10.3389/fphys.2018.00044/full.
Eriksson EK, Agmo Hernández V, Edwards K. Effect of ubiquinone-10 on the stability of biomimetic membranes of relevance for the inner mitochondrial membrane. Biochim Biophys Acta Biomembr. 2018;1860:1205–15.
Xu Z, Huo J, Ding X, Yang M, Li L, Dai J, et al. Coenzyme Q10 improves lipid metabolism and ameliorates obesity by regulating CaMKII-mediated PDE4 inhibition. Sci Rep. 2017;7:8253. http://www.nature.com/articles/s41598-017-08899-7.
Wang LW, Jabbour A, Hayward CS, Furlong TJ, Girgis L, Macdonald PS, et al. Potential role of coenzyme Q10 in facilitating recovery from statin-induced rhabdomyolysis. Intern Med J. 2015;45:451–3. https://doi.org/10.1111/imj.12712.
Banach M, Serban C, Ursoniu S, Rysz J, Muntner P, Toth PP, et al. Statin therapy and plasma coenzyme Q10 concentrations—a systematic review and meta-analysis of placebo-controlled trials. Pharmacol Res. 2015;99:329–36.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Jula A, Marniemi J, Huupponen R, Virtanen A, Rastas M, Rönnemaa T. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: a randomized controlled trial. JAMA. 2002;287:598–605. http://www.ebscohost.com.
Oranje WA, Sels JP, Rondas-Colbers GJ, Lemmens PJ, Wolffenbuttel BH. Effect of atorvastatin on LDL oxidation and antioxidants in normocholesterolemic type 2 diabetic patients. Clin Chim Acta. 2001;311:91–4.
Ashton E, Windebank E, Skiba M, Reid C, Schneider H, Rosenfeldt F, et al. Why did high-dose rosuvastatin not improve cardiac remodeling in chronic heart failure? Mechanistic insights from the UNIVERSE study. Int J Cardiol. 2011;146:404–7. https://doi.org/10.1016/j.ijcard.2009.12.028.
Strey CH, Young JM, Molyneux SL, George PM, Florkowski CM, Scott RS, et al. Endothelium-ameliorating effects of statin therapy and coenzyme Q10 reductions in chronic heart failure. Atherosclerosis. 2005;179:201–6.
Päivä H, Thelen KM, Van Coster R, Smet J, De Paepe B, Mattila KM, et al. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78:60–8.
Mortensen SA, Leth A, Agner E, Ronde M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects Med. 1997;18:137–44.
Morrison JT, Longenecker CT, Mittelsteadt A, Jiang Y, Debanne SM, McComsey GA. Effect of rosuvastatin on plasma coenzyme Q10 in HIV-infected individuals on antiretroviral therapy. HIV Clin Trials. 2016;17:140–6. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L610835401.
McMurray JJV, Dunselman P, Wedel H, Cleland JGF, Lindberg M, Hjalmarson K, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (Controlled Rosuvastatin Multinational Study in Heart Failure). J Am Coll Cardiol. 2010;56:1196–204. https://doi.org/10.1016/j.jacc.2010.02.075.
Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L. Evidence of Plasma CoQ10—lowering effect by HMG-CoA reductase inhibitors: a double blind, placebo-controlled study. Clin Pharmocol. 1993;33:226–9.
Chitose T, Sugiyama S, Sakamoto K, Shimomura H, Yamashita T, Hokamaki J, et al. Effect of a hydrophilic and a hydrophobic statin on cardiac salvage after ST-elevated acute myocardial infarction—a pilot study. Atherosclerosis. 2014;237:251–8. https://doi.org/10.1016/j.atherosclerosis.2014.08.053.
Berthold HK, Naini A, Di Mauro S, Hallikainen M, Gylling H, Krone W, et al. Effect of ezetimibe and/or simvastatin on coenzyme Q10 levels in plasma: a randomised trial. Drug Saf. 2006;29:703–12.
Bleske BE, Willis RA, Anthony M, Casselberry N, Datwani M, Uhley VE, et al. The effect of pravastatin and atorvastatin on coenzyme Q10. Am Heart J. 2001;142:2.
Caparrós-Martín JA, Lareu RR, Ramsay JP, Peplies J, Reen FJ, Headlam HA, et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017;5:95. https://doi.org/10.1186/s40168-017-0312-4.
Paunović MG, Matić MM, Ognjanović BI, Saičić ZS. Antioxidative and haematoprotective activity of coenzyme Q10 and vitamin E against cadmium-induced oxidative stress in Wistar rats. Toxicol Ind Health. 2017;33:746–56. https://doi.org/10.1177/0748233717725480.
Alkholy UM, Abdalmonem N, Zaki A, Elkoumi MA, Hashim MIA, Basset MAA, et al. The antioxidant status of coenzyme Q10 and vitamin E in children with type 1 diabetes. J Pediatr (Rio J). 2018. https://doi.org/10.1016/j.jped.2017.12.005.
James AM, Smith RAJ, Murphy MP. Antioxidant and prooxidant properties of mitochondrial coenzyme Q. Arch Biochem Biophys. 2004;423:47–56.
Yubero D, Adin A, Montero R, Jou C, Jiménez-Mallebrera C, García-Cazorla A, et al. A statistical algorithm showing coenzyme Q10 and citrate synthase as biomarkers for mitochondrial respiratory chain enzyme activities. Sci Rep. 2016;6:15. http://www.nature.com/articles/s41598-016-0008-1.
Ong S-B, Kalkhoran SB, Hernández-Reséndiz S, Samangouei P, Ong S-G, Hausenloy DJ. Mitochondrial-shaping proteins in cardiac health and disease—the long and the short of it! Cardiovasc Drugs Ther. 2017;31:87–107. https://doi.org/10.1007/s10557-016-6710-1.
Alcocer-Gómez E, Sánchez-Alcázar JA, Cordero MD. Coenzyme Q10 regulates serotonin levels and depressive symptoms in fibromyalgia patients. J Clin Psychopharmacol. 2014;34:277–8. https://insights.ovid.com/crossref?an=00004714-201404000-00023.
Mampuya WM, Frid D, Rocco M, Huang J, Brennan DM, Hazen SL, et al. Treatment strategies in patients with statin intolerance: the Cleveland Clinic experience. Am Heart J. 2013;166:597–603.
Rallidis LS, Fountoulaki K, Anastasiou-Nana M. Managing the underestimated risk of statin-associated myopathy. Int J Cardiol. 2012;159:169–76.
Qu H, Guo M, Kou N, Wu H-T, Zhang Y, Gao Z-Y, et al. Simvastatin monotherapy as a potential option for statin-associated muscle symptoms: a case report. J Clin Pharm Ther. 2016;41:568–71. https://doi.org/10.1111/jcpt.12419.
Authors’ contributions
D-zS and Z-yG designed the review and provided methodological perspectives. HQ and Y-yM developed the search strategy, performed the literature search, and wrote the manuscript. HC, FL, and J-yZ performed the study selection, data extraction, and data analyses. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors. Our study group worked under the oversight of the institutional ethics committee and followed all ethical recommendations. Before submitting our article, all authors agreed to the publication of our work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81030063), the National Sci-Tech Support Plan of China (No. 2013BAI02B01), the Major National Research and Development Project of China (No. 2018ZX09201009-002-006), the National Natural Science Foundation of China (No. 81303150), and the project of China Academy of Chinese Medical Sciences (No. ZZ11-066).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist [15].
Additional file 2: Figure S1.
Risk of bias.
Additional file 3: Figure S2.
Publication bias.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Qu, H., Meng, Yy., Chai, H. et al. The effect of statin treatment on circulating coenzyme Q10 concentrations: an updated meta-analysis of randomized controlled trials. Eur J Med Res 23, 57 (2018). https://doi.org/10.1186/s40001-018-0353-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-018-0353-6