Abstract
Interleukin 18 (IL18) is a kind of proinflammatory cytokine that belongs to the interleukin-1 family. IL18 is associated with obesity and type 2 diabetes. To discover whether body composition parameters in IL18 deficient mouse are altered in single-housed condition, body weight, glucose tolerance, lipid profiles, fat masses, and size of white adipocytes were examined. Mice were housed singly and were divided as follows: C57BL/6 J male (B6-M), IL18 deficient male (IL18-M), C57BL/6 J female (B6-F), IL18 deficient female (IL18-M). Body weight statistically significantly increased in IL18-M at 9 months (p < 0.05). Glucose tolerance occurred in IL18-M at 6 and 9 months. Total cholesterol and HDL cholesterol were statistically significantly increased in IL18-F compared with B6-F at 9 and 12 months, respectively (p < 0.05). Also, total cholesterol of IL18-M was statistically significantly increased compared with B6-F and IL18-F at 9 months (p < 0.05). The perirenal and inguinal fat masses were statistically significantly increased in IL18-M at 9 months (p < 0.05). In addition, the size of white adipocytes was increased in IL18-M at 9 months. In single-housed condition, onset of obesity and glucose tolerance were delayed by 3 months in IL18-M. Taken together, these results suggest that housing condition is a very important factor for weight gain and onset of glucose tolerance in IL18 deficient male mouse.
Similar content being viewed by others
Introduction
Environmental and social factors are very important for understanding physiological differences in laboratory mice. To enhance the replicability and reliability of animal research, these factors should be regulated precisely. Inbred strains of mice are widely used for physiological research and are regulated for body composition by environmental and genetic factors [1, 2]. In single-housed mice, variance of body fat mass is less than that in group-housed mice [2]. Therefore, housing condition (single, group) should be considered in research into metabolic related diseases such as obesity and diabetes.
The mice’s body weight is determined by the metabolic rate. Obesity is excessive accumulation of body fat, caused by overeating and low metabolic rate [3]. Brown fat and white fat have functions for heat production and lipid storage, respectively [4, 5]. Obesity occurs with the progressive increase of white adipose tissues and is risk factor of type 2 diabetes [6, 7]. Type 2 diabetes is marked by insulin resistance and hyperinsulinemia. In addition, high blood glucose appears with type 2 diabetes and causes thirst, hunger, and polyuria [8].
Interleukin 18 (IL18) is associated with obesity and type 2 diabetes. Hyperphagia and weight gain appear at 6 months of age in IL18 deficient mouse [9, 10]. In addition, IL18 deficient mouse shows abnormal lipidemia in liver at 9 months [11]. All those studies achieved their results in group-housed condition. Therefore, our hypothesis is that body composition parameters in IL18 deficient mouse will be altered in single-housed condition. In this study, body weight was statistically significantly increased in IL18 deficient male mouse compared with C57BL/6 J male mouse at 9 months. Lipidemia and glucose tolerance appeared in IL18 deficient male mouse at 9 months. In addition, the size of white adipocytes was increased in IL18 deficient male mice at 9 months. The phenotypes of body weight gain and diabetes were delayed in IL18 deficient male mouse by single-housed condition. Our results suggest that housing condition is a very important factor for weight gain and onset of diabetes in IL18 deficient male mouse.
Materials and methods
Animals
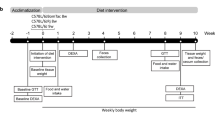
C57BL/6 J and interleukin 18 deficient mice were obtained from the Laboratory Animal Resources Center, Hallym University, Korea. The animal room retained at temperature (22 ± 2 °C), humidity (55 ± 10%) and light (8 a.m. to 8 p.m.). The animals were provided chow diet (Protein: 20%, Fat: 4.5%, Fiber: 6%, Calcium 0.5%, Phosphorus 1%, Cargill Agri Purina, Korea) and water ad libitum. The experimental groups were divided as follows: (1) C57BL/6 J-male (B6-M, n = 60), (2) C57BL/6 J female (B6-F, n = 48), (3) IL18 deficient male (IL18-M, n = 60), and (4) IL18 deficient female (IL18-F, n = 60). Mice were housed singly (13 × 24 × 12 cm, MJ Ltd, Korea). The animals were handled with Hallym University guidelines (Hallym2016-65).
Measurement of body weight, food intake, and fasting blood glucose
The body weight was measured at 3, 6, 9, and 12 months using an animal electronic scale. Food intake was measured at 3 (2 ~ 3 M), 6 (4 ~ 6 M), 9 (7 ~ 9 M), and 12 months (10 ~ 12 M). To measure fasting blood glucose, blood was collected from the retro-orbital plexus after fasting (16 h). Blood glucose monitoring meter (Accu-Chek, Roche, USA) was used to measure fasting blood glucose.
Measurement of lipid profiles and Hemoglobin A1C
Blood samples (300 μl) were collected at 3, 6, 9, and 12 months using the same method as described above. Then, total cholesterol, triglyceride (TG), low density lipoprotein cholesterol (LDL), high density lipoprotein cholesterol (HDL) were measured by blood biochemistry (Kornelab20XT, Thermo, USA). Hemoglobin A1C (HbA1C) was measured using whole blood by an HbA1C analyzer (HLC-723G7, Tosoh, Japan).
Oral glucose tolerance test (OGTT)
OGTT was performed at 3, 6, 9, and 12 months. All animals were fasted for 16 h. Mice were given 2 g/kg of d-glucose (Sigma, USA) by oral administration. After 0, 30, 60, and 120 min of d-glucose administration, blood were collected and measured glucose levels using a glucose meter (Accu-Chek, Roche, USA). Then, to determine whether glucose tolerance exists, the area under the curve (AUC) was calculated.
Histological analysis
Mice were anesthetized with isoflurane (gas flow with flowmeter 100–200 ml/min and vaporizer at 3%) and were perfused transcardially with 4% paraformaldehyde. Whole adipose tissues were dissected and weighed. The interscapular, epididymal, and ovary fat were embedded in paraffin and sectioned at 5 μm for hematoxylin–eosin staining. For histological analysis, the adipose tissue sections were blocked in blocking solution (1X TBS, 1% BSA, and 10% goat serum) at room temperature for 1 h. Then the adipose tissue sections were incubated with mitochondrial uncoupling protein-1 (UCP-1) antibody (1:500, Abcam, UK) at 4 °C overnight. After that, the adipose tissue sections were incubated with anti-rabbit secondary antibody (1:200, Cell Signaling, USA) at room temperature (1 h). At least 4 mice per group were used for the histological analysis.
Statistical analysis
All data were expressed as mean ± S.D. The significant differences were compared using one-way analysis of variance (one-way ANOVA). All results were analyzed by SPSS Statistics 22.0 (SPSS Inc., Chicago, USA).
Results
IL18 deficient male (IL18-M) mice show obesity independently of food intake at 9 months
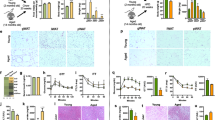
Body weight of IL18-M was statistically significantly increased compared with that of C57BL/6 J male (B6-M) at 9 months (p < 0.05). There was no difference in body weight between IL18 deficient female (IL18-F) and C57BL/6 J female (B6-F) at all ages. Body weight of IL18-M was statistically significantly increased compared with that of IL18-F at all ages (Fig. 1a, p < 0.005). Food intake of IL18-M was statistically significantly increased compared with that of IL18-F at 6 months (Fig. 1b, p < 0.05). Fasting blood glucose (FBG) was statistically significantly decreased in IL18-F compared with B6-F at 12 months (p < 0.05), but IL18-M had similar FBG levels compared with B6-M at 3, 6, 9, and 12 months (Fig. 1c). Hemoglobin A1C (HbA1C) was statistically significantly different in IL18-M compared with that of IL18-F at 3, 6, and 12 months (Fig. 1d, p < 0005).
Changes in body weight, food intake, fasting blood glucose, and HbA1c. a–b Body weight and food intake were measured at 3, 6, 9, and 12 months. c–d Fasting blood glucose and HbA1c were measured at 3, 6, 9, and 12 months after 16 h fasting. These data are expressed as mean ± S.D. *p < 0.05 compared with same sex. #p < 0.05, ##p < 0.01, ###p < 0.005 compared between male and female
IL18 deficient male (IL18-M) mice have glucose tolerance and dyslipidemia
To verify abnormal glucose metabolism, an OGTT (oral glucose tolerance test) was performed. At 3 and 12 months, there was no difference in area under the curve (AUC) of all groups (data not shown). AUC was statistically significantly increased in IL18-M compared with the other groups at 6 and 9 months (Fig. 2a, b, p < 0.05). These findings suggest that IL18 deficient male (IL18-M) had glucose tolerance at 6 and 9 months.
Results of oral glucose tolerance test (OGTT). a, b OGTT was performed at 6, and 9 months. Area under the curve (AUC) was calculated to determine whether glucose tolerance exists. These data are expressed as mean ± S.D. *p < 0.05, ***p < 0.005 compared with same sex. ###p < 0.005 compared between male and female
Dyslipidemia is a major risk factor of abnormal glucose metabolism. At 9 months, total cholesterol was statistically significantly increased in IL18-F compared with B6-F (Table 1, p < 0.05). Total cholesterol of IL18-M was statistically significantly increased compared with B6-F and IL18-F at 9 months (p < 0.05). HDL cholesterol showed no significant differences among all groups. LDL cholesterol of IL18-F was statistically significantly increased compared with that of B6-F at 12 months (p < 0.05). In B6-F and IL18-F, LDL cholesterol was statistically significantly increased compared with B6-M and IL18-M at 12 months (Table 1, p < 0.05). TG in IL18-M was statistically significantly increased compared with that in B6-M at 9 months (p < 0.05), but IL18-F showed a similar TG level compared with B6-F at 6, 9, and 12 months.
IL18 deficient male (IL18-M) mice promote adipose tissue accumulation
Adipose tissue weight at 9 months is shown in Table 2. To reduce variation, the weight of adipose tissue was expressed as a ratio of body weight. The total fat mass of B6-M and IL18-M was 10.85 ± 5.43 and 12.45 ± 6.22, respectively. The perirenal and inguinal fat masses statistically significantly increased in IL18-M compared with B6-M at 9 months (Table 2, p < 0.05). The total fat mass of B6-F and IL18-F was 9.89 ± 4.95 and 8.66 ± 4.33, respectively. There was no difference in fat mass between B6-F and IL18-F at 9 months.
Histological analysis was performed with interscapular and epididymal (ovary) fat at 9 months (Fig. 3a). The expression of UCP-1 protein, brown adipose tissue marker, was observed in interscapular fat, but not in epididymal (ovary) fat. UCP-1 protein was expressed in the mitochondria of brown adipose tissue. The size of the adipocytes was measured because the size of adipocytes increases with obesity (Fig. 3b). The mean size of epididymal adipocytes was statistically significantly increased in IL18-M compared with that of B6-M at 9 months (Fig. 3b, p < 0.05). In IL18 F, the mean size of ovary adipocytes increased slightly compared with that of the B6-F group at 9 months. These findings indicate that IL18-M have obesity via adipose tissue accumulation at 9 months.
Morphological observation of adipose tissues and mean size of adipocytes. a Interscapular and epididymal (ovary) fats were stained with hematoxylin and eosin or UCP-1 antibody at 9 months. b Adipocyte size was measured in epididymal (ovary) fat at 9 months. Scale bar = 50 μm. These data are expressed as mean ± S.D. *p < 0.05 compared with same sex. #p < 0.05 compared between male and female
Discussion
This study hypothesized that body composition parameters might be changed in IL18 deficient mouse in accordance with single-housed condition. IL18 deficient male mice showed obesity at 9 months, dependent on adipose tissue accumulation (Figs. 1, 3). These male mice also developed dyslipidemia at 9 months and glucose tolerance from 6 to 9 months (Table 1, Fig. 2). In addition, IL18 deficient male mice had more beige body fat compared with control mice (Table 2). Netea et al. reported that IL18 deficient mice showed diet-induced obesity and glucose tolerance at 6 months [9]. IL18 deficient male mice develop dyslipidemia and steatohepatitis in group condition housing [11]. There are two differences between our results and those of previous studies [9, 10]. First, the onset of obesity in our experiment was delayed by 3 months compared with Netea’s results. Second, obesity in our experiment was induced by fat accumulation rather than increased food intake. There are two possible reasons for these discrepancies: (1) housing condition as group-housed or single-housed and (2) difference of microbiome composition
Mice are social animals; therefore, they are affected by an isolated environment such as single housing [12]. Group-housed mice show a considerable increase in body weight compared with single-housed mice [1]. Increased body weight in the group-housed mice is due to the differences in thermoregulation [13]. Therefore, in group-housing, IL18 deficient male mice showed rapid increases in weight gain because of increased food intake and differences in thermoregulation [13]. Ultimately, this leads to obesity and type 2 diabetes at 6 months [9, 10, 14]. On the other hand, single-housed mice show genetic effects on body composition compared with group-housed mice [2]. With single-housed mice, depression-like behavior is induced [15]. The multi-housed mice are more active compared to the single-housed mouse [16]. Thus, the single-housed mouse need more calories to keep it’ s body temperature compared to group housed mouse. Therefore, body weight gain and onset of diabetes were delayed in IL18 deficient male mouse by single-housed condition.
Second, recent studies demonstrated that the gut microbiome in very important in human metabolism such as obesity and diabetes [17, 18]. When the microbiota (obese microbiota) of obese mice transplant to normal mice, obesity occurs in the normal mice [19, 20]. Also, obese microbiota influence lipid metabolism such as triglyceride [21]. In group housing, the microbiome of mice is homogenized by the cage effect [22]. IL18 deficient mice used in our study were purchased from Jackson Laboratory several years ago and were maintained at the Laboratory Animal Resources Center, Hallym University. Therefore, the microbiota of the IL18 deficient mice used in our study might have a different composition compared with that of Netea’s IL18 deficient mice [9]. To confirm this speculation, microbiota analysis of the IL18 deficient mice of the two institutions is needed.
In our results, IL18 deficient female mice did not show any statistically significant differences in body weight and size of adipocytes in ovary fat (Figs. 1a, 3b). These mice also showed no statistically significant differences in the OGTT at 3, 6, 9, and 12 months (Fig. 2). The prevalence of diabetes in woman is lower compared with that in man [23]. Females are protected from β cell death in rodent diabetic models [24]. Therefore, gender differences in our study might be caused by estrogen, which modulates glucose homeostasis and insulin resistance.
In conclusion, IL18 deficient male mice showed delayed onset of obesity and glucose tolerance in the single-housed condition. This result indicates that the housing condition and gender are key factors in metabolic studies such as body composition and glucose tolerance.
References
Voikar V, Polus A, Vasar E, Rauvala H (2005) Long-term individual housing in C57BL/6 J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav 4:240–252
Nagy TR, Krzywanski D, Li J, Meleth S, Desmond R (2002) Effect of group vs. single housing on phenotypic variance in C57BL/6 J mice. Obes Res 10:412–415
Misra A, Singhal N, Khurana L (2010) Obesity, the metabolic syndromes, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr 29:289S–301S
Bartelt A, Heeren J (2014) Adipose tissue browning and metabolic health. Nat Rev Endocrinol 10:24–36
Yamashita H, Wang Z, Wang Y, Segawa M, Kusudo T, Kontani Y (2008) Induction of fatty acid-binding protein 3 in brown adipose tissue correlates with increased demand for adaptive thermogenesis in rodents. Biochem Biophys Res Commun 377:632–635
Guilherme A, Virbasius JV, Puri V, Czech MP (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9:367–377
Lee HE, Yang G, Han SH, An TJ, Jang JK, Lee JY (2018) Anti-obesity potential of glycyrrhiza uralensis and licochalcone A through induction of adipocyte browning. Biochem Biophys Res Commun 503:2117–2123
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN (2009) Hyperglycemic crises in adult patients with diabetes. Diabetes Care 32:1335–1343
Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, Krieken H, Kim SH, Stalenhoef AF, van de Loo FA, Verschueren I, Pulawa L, Akira S, Eckel RH, Dinarello CA, van den Berg W, van der Meer JW (2006) Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 12:650–656
Zorrilla EP, Sanche-Zlavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B (2007) Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA 104:11097–11102
Yamanishi K, Maeda S, Kuwahara-Otani S, Watanabe Y, Yoshida M, Ikubo K, Okuzaki D, El-Darawish Y, Li W, Nakasho K, Nojima H, Yamanishi H, Hatakawa T, Okamura H, Matsunaga H (2016) Interleukin-18-deficient mice develop dyslipidemia resulting in nonalcoholic fatty liver disease and steatohepatitis. Transl Res 173:101–114
Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J, Quimby FW, Turner PV, Wood GA, Würbel H (2011) Guide for the care and use of laboratory animals, 8th edn. The National Academies Press, Washington DC
van Leeuwen SD, Bonne OB, Avraham T, Berry EM (1997) Separation as a new animal model for self-induced weight loss. Physiol Behav 62:77–81
Lindegarrd B, Matthews VB, Brandt C, Hojman P, Allen TL, Estevez E, Watt MJ, Bruce CR, Mortensen OH, Syberg S, Rudnicka C, Abildgaard J, Pilegaard H, Hidalgo J, Ditlevesen S, Alsted TJ, Madsen AN, Pedersen BK, Febbraio MA (2013) Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes 62:3064–3074
Martin AL, Brown RE (2010) The lonely mouse: verification of a separation-induced model of depression in female mice. Behav Brain Res 207:196–207
Gordon CJ, Becker P, Ali JS (1998) Behavioral thermoregulatory responses of single- and group-housed mice. Physiol Behav 65:255–262
Bevaraj S, Hemarajata P, Versalovic J (2013) The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem 59:617–628
Dalby MJ, Ross AW, Walker AW, Morgan PJ (2017) Dietary uncoupling of gut microbiota and energy harvesting from obesity and glucose tolerance in mice. Cell Rep 21:1521–1533
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102:11070–11075
Turnbaugh PJ, Ley RE, Mahowld MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031
Matey-Hernandez ML, Williams FMK, Potter T, Valdes AM, Spector TD, Menni C (2018) Genetic and microbiome influence on lipid metabolism and dyslipidemia. Physiol Genomics 50:117–126
Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P (2016) Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev 40:117–132
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F (2006) Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103:9232–9237
Authors’ contribution
BK, YYK, HJ, and HN carried out the animal experiment. BK wrote the first draft of the manuscript. JGS initiated the project, supervised the experiment, and critically reviewed the manuscripts. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by Basic Science Research Program (NRF-2016R1D1A2B02011858) and Mouse Phenotyping Project (KMPC:2014M3A9D5A01075129) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
Competing interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kim, B., Kim, Y.Y., Jung, H. et al. Delayed onset of obesity and glucose tolerance in interleukin 18 deficient mice by single housed condition. Appl Biol Chem 62, 1 (2019). https://doi.org/10.1186/s13765-019-0414-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-019-0414-8