Abstract
Objective
Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative Staphylococcus (MRCoNS) are among the main causes of nosocomial infections, which have caused major problems in recent years due to continuously increasing spread of various antibiotic resistance features. Apparently, vancomycin is still an effective antibiotic for treatment of infections caused by these bacteria but in recent years, additional resistance phenotypes have led to the accelerated introduction of newer agents such as linezolid, tigecycline, daptomycin, and quinupristin/dalfopristin (Q/D). Due to limited data availability on the global rate of resistance to these antibiotics, in the present study, the resistance rates of S. aureus, Methicillin-resistant S. aureus (MRSA), and CoNS to these antibiotics were collected.
Method
Several databases including web of science, EMBASE, and Medline (via PubMed), were searched (September 2018) to identify those studies that address MRSA, and CONS resistance to linezolid, tigecycline, daptomycin, and Q/D around the world.
Result
Most studies that reported resistant staphylococci were from the United States, Canada, and the European continent, while African and Asian countries reported the least resistance to these antibiotics. Our results showed that linezolid had the best inhibitory effect on S. aureus. Although resistances to this antibiotic have been reported from different countries, however, due to the high volume of the samples and the low number of resistance, in terms of statistical analyzes, the resistance to this antibiotic is zero. Moreover, linezolid, daptomycin and tigecycline effectively (99.9%) inhibit MRSA. Studies have shown that CoNS with 0.3% show the lowest resistance to linezolid and daptomycin, while analyzes introduced tigecycline with 1.6% resistance as the least effective antibiotic for these bacteria. Finally, MRSA and CoNS had a greater resistance to Q/D with 0.7 and 0.6%, respectively and due to its significant side effects and drug-drug interactions; it appears that its use is subject to limitations.
Conclusion
The present study shows that resistance to new agents is low in staphylococci and these antibiotics can still be used for treatment of staphylococcal infections in the world.
Similar content being viewed by others
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (MRCoNS) represent main causes of hospital- and community-acquired infections; because of their increasing numbers and elevated mortality, morbidity, and medical expenses, they have become a global concern in recent years [1, 2]. Staphylococci contain virulence factors and toxins that cause various diseases including blood, skin and soft tissues infections, nosocomial infections connected with the presence of medical devices, and toxic shock syndrome [3]. The mecA gene, located in the SCCmec region, is responsible for the expression of methicillin resistance through PBP2a—an altered penicillin-binding protein that is characterized by its low affinity to penicillin and other beta-lactam drugs [4]. For both MRSA and MRCoNS vancomycin is used as the first line drug for treatment. However, in recent years, decreased susceptibility and even resistance to vancomycin and other antibiotics, including aminoglycosides, tetracyclines, and lincosamides, have been reported in many parts of the world [5,6,7]. Therefore, for the treatment of severe infections caused by multi-drug resistant staphylococci, new antibiotics such as daptomycin, linezolid, tigecycline, and Quinupristin/Dalfopristin (Q/D) were introduced [8]. Daptomycin, a cyclic lipopeptide antibiotic, is the second most important anti-MRSA drug, which received FDA approval in 2003 and approval by the European Medicines Agency (EMA) in 2005. It is mostly used for the treatment of acute bacterial skin and soft tissues infections [9]. Daptomycin is still quite active against staphylococci and enterococci; however, resistance to this antibiotic has been reported over the past years due to mutation of various genes (dltABCD genes, mprF and rpoB), causing changes in membrane fluidity, cell wall thickness, and membrane charge [10, 11]. Tigecycline is an example of a new class of broad-spectrum antimicrobial agents known as glycylcyclines with activity against Gram-positive and Gram-negative organisms. This antibiotic was approved by FDA (2005–2009) for the treatment of skin infections, intra-abdominal infections and community-acquired bacterial pneumonia [12, 13]. Tigecycline provides an alternative treatment for complicated MRSA and vancomycin resistant enterococci (VRE) infections; due to mutations in mepR and mepA genes that result in overexpression of efflux pumps, resistant phenotypes have been reported in recent studies [13]. Linezolid is another new antibiotic that was approved in 2000 for the treatment of MRSA and MRCoNS infections and infections caused by VRE. Linezolid binds to the 50S ribosomal subunit of the 23S rRNA molecule and inhibits protein synthesis. Cfr gene encodes a methyltransferase that modifies the 23S rRNA site of the 50S ribosomal subunit and prevents linezolid from binding to it [14]. Q/D is composed of two streptogramins (70% dalfopristin (streptogramin A) and 30% quinupristin (streptogramin B)), which was approved in 1999 as a treatment option for VRE and MRSA infections. This drug consists of quinupristin that inhibits late-stage protein synthesis, while dalfopristin inhibits early-stage protein synthesis. It should be noted that, Synercid® (formerly RP59000; Rhone-Poulenc) is the first semisynthetic injectable streptogramin and it is used as a trade name for Q/D [15, 16]. The World Health Organization (WHO) has considered MRSA as important antibiotic-resistant bacteria and put them on their priority list. All organisms on that list require new treatment modalities and substantiate an urgent overall need for new antimicrobial drugs [17]. According to the authors’ knowledge, no comprehensive data are available on the resistance levels to daptomycin, Q/D, linezolid, and tigecycline among MRSA and MRCoNS strains. This study aims to investigate the prevalence of resistance to the mentioned antibiotics among staphylococcal strains isolated from clinical samples around the world.
Methods
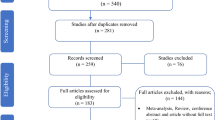
We conducted a literature search through databases, including web of science, EMBASE, and Medline (via PubMed), using the versions of September 2018. The historic publication year was unrestricted and the search was limited to original articles. The following search keywords were obtained from the National Library of Medicine’s medical subject heading (MeSH) terms or titles or abstracts with the help of Boolean operators (and, or): “staph”, “staphylococcus”, “staphylococci”, “staphylococcal”, “staphylococcaceae” and “Linezolid”, “Daptomycin”, “Tigecycline”, “Quinupristin/Dalfopristin”, and “Synercid”. Two independent reviewers screened the titles and abstracts of original articles and posters; if an article appeared relevant (Figs. 1 and 2), the full text was reviewed. We used the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for daptomycin, linezolid, Q/D resistance and tigecycline resistance in Staphylococci, respectively (there is no standard for tigecycline in staphylococci in the CLSI). The resistance cut-off rates are defined in the following ranges ≤1 mg/L, ≥8 mg/L, ≥4 mg/L, and > 5 mg/L, respectively. We considered all articles that evaluated antibiotic resistance by different methods such as broth microdilution (BMD), agar dilution, disk diffusion (DD), E-test and Vitek or Vitek 2 or any other automated instruments. It should be noted that, the final version of the CLSI (2018) states that staphylococci with resistant results to linezolid by DD should be confirmed by using an MIC method, therefore, studies that only used the DD method for susceptibility to the linezolid were excluded. Moreover, case reports, basic research on the resistance mechanism of the mentioned antibiotics, and review articles were excluded from this study.
Meta-analysis
Quality assessment
All reviewed studies were subjected to a quality assessment (designed by the Joanna Briggs Institute) and only high-quality investigations were evaluated in our final analysis [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116].
Data analysis
The analysis was performed by STATA (version 14.0) software. The data were pooled using a fixed effects model (FEM) [117] and a random effects model (REM) [118]. Statistical heterogeneity was assessed by statistical methods [119] and was evaluated using the Q-test and the I2 statistical methods [118]. P-value < 0.1 was regarded as statistically significant [120].
Results
This study identified 1813, 2222, 512, and 636 articles for daptomycin, linezolid, Q/D (Synercid), and tigecycline, respectively, in the first step. Then, upon secondary screening, a large number of articles were excluded on the basis of title and abstract evaluation because of the lack of relevance to the study principles, and the reasons for the deletion of these articles are presented in Figs. 1 and 2. Therefore, 477, 768, 124, and 214 articles for the mentioned antibiotics were reviewed with full text, and a number of papers were excluded from the study for the reasons listed in Figs. 1 and 2. Finally, 37, 51, 17, and 22 eligible studies for daptomycin, linezolid, Q/D, and tigecycline were chosen for final analysis, respectively. Resistance percentage in S. aureus, MRSA and CONS to the mentioned antibiotics is shown in Table 1. The characteristics of the included articles are summarized in Tables 2, 3, 4 and 5. All pertinent studies were included from around the world (25 different countries) (Tables 2, 3, 4 and 5). The USA was the most frequently represented country for all antibiotics followed by Canada and European countries (Italy and Spain). From the African continent, only one study from Nigeria, where tigecycline resistance in one isolate was reported (Fig. 3). Linezolid-resistant staphylococci from 15 countries were included in the present study, which was more widely distributed among antibiotics (Fig. 4). Strains were isolated from various clinical samples including blood, wound, skin, urine, respiratory tract, sputum, catheter, bone, etc. A majority of studies used BMD, E-test, agar dilution, disk diffusion, and Vitek or vitek 2. Our results showed that linezolid had the best inhibitory effect on S. aureus. Although resistance to the linezolid has been reported from different countries, due to the high volume of the samples and the low number of resistance, in terms of statistical analyzes, the resistance to this antibiotic is zero. Moreover, linezolid and tigecycline effectively (99.9%) inhibit MRSA (Table 1). Studies have shown that CoNS with 0.3% show the lowest resistance to linezolid and daptomycin, while analyzes introduced tigecycline with 1.6% resistance as the least effective antibiotic for these bacteria. Finally, MRSA and CoNS had a greater resistance to Q/D with 0.7 and 0.6%, respectively.
Discussion
MRSA is a frequent cause of skin and soft tissue infection, pneumonia, endocarditis, bone and joint infection in individuals with some risk factors such as indwelling devices, surgical interventions, long-term antibiotic use, intensive care admission, and dialysis [121, 122]. In recent years, this bacterium has had very high health costs for patients due to increased length of hospital stay and longer duration of antibiotic treatment [123]. Moreover, CoNS are opportunistic pathogens that lead to 30% of hospital-induced infections and 10% of uncomplicated urinary tract infections in young women and native valve endocarditis, especially in immunocompromised patients [124, 125]. Currently, the treatment of MRSA and CoNS is difficult due to the high antibiotic resistance to beta-lactams and other antibiotic classes, and newer agents such as linezolid, daptomycin, Q/D, and tigecycline can be used as alternative if available and deemed cost-effective. Accordingly, this study collected data from resistance to these antibiotics all over the world to determine the extent of their clinical application. The analysis of the results showed that linezolid had the highest inhibitory effect on S. aureus; due to the high volume of the samples in the studies and a small number of bacteria that have been reported as resistant (mostly in the United States), in terms of statistical analyses, the percentage of resistance to this antibiotic is zero (Table 1). It should be noted that the studies (20 studies) that used the DD method as an antibiotic susceptibility test for linezolid were removed from this study and not entered into statistical analyses. Furthermore, the most linezolid-resistance S. aureus isolates isolated from pneumonia and blood infections were the highest in number. In addition to the good effect of linezolid on S. aureus, this drug also had the efficient activity against MRSA, while the resistance of CoNS was higher to this antibiotic. One of the reasons for the increased resistance in CoNS is the ability of these bacteria to develop resistance quite easily following linezolid exposure, even though this has not been proven in vitro, to the best of our knowledge. Furthermore, more Linezolid-resistant CoNS (LRCoNS) were associated with outbreaks; 50% of those studies that analysed LRCoNS involved clonal LRCoNS across one or more patients and facilities. The studies that used MLST for typing of resistant-linezolid CoNS, ST5, ST22 and for S. aureus ST228, ST8 and ST5 were reported to be more sequence types related to linezolid resistance [25, 67].
Tigecycline had the best effect (equal to linezolid) on MRSA, and very low resistance in S. aureus was observed; however, CoNS with 1.6% showed the highest percentage of resistance to this antibiotic (Table 1). Since very few studies have reported the resistance of CoNS to tigecycline (Fig. 3), the high percentage of resistance noted by tigecycline cannot be deemed. The geographic diversity of the countries that reported the tigecycline resistance was higher than those with linezolid, thus showing more use of this antibiotic in different parts of the world. Recent MRSA infection treatment guidelines have not incorporated tigecycline. The reason is the FDA’s September 2010 safety statement, which describes increased overall mortality among severely infected patients who are treated with tigecycline; besides, cause of the excess deaths in these trials usually remains uncertain. However, it is likely that most cases of death among such patients were associated with the infection progression. Moreover, this antibiotic is not authorized for pneumonia or diabetic foot infections. Although tigecycline is recommended for treating skin and soft tissue infections, previous studies have shown no significant difference between this antibiotic and other new drugs, and tigecycline is referred to as the second or third line of treatment for infections caused by MRSA [126, 127]. Therefore, although the present study showed that S. aureus resistance to tigecycline is low, the use of this drug still has limitations in treating staphylococcal infections. Daptomycin is another new drug used to treat infections caused by Gram-positive bacteria including MRSA and VRE. It kills microorganisms by rapid membrane depolymerisation, loss of membrane potential and disruption of DNA, as well as RNA and protein-synthesis [128]. The daptomycin resistance among staphylococcal strains has been reported from around the world, although there has been no resistance report from the African continent. The United States had the highest rate of resistance (42.5% of studies); India, Taiwan, and Saudi Arabia reported resistance to this antibiotic from the Asian continent, and most of the bacteria were isolated from wounds and blood infections. In the United States and Europe, daptomycin is used for treating skin and soft tissue infections, bacteraemia, and endocarditis caused by S. aureus [129]. Previous studies have reported that it is not very practical to use daptomycin for the treatment of pneumonia, because it is deactivated by pulmonary surfactants. Therefore, vancomycin and linezolid are recommended to treat pneumonia caused by MRSA [130]. Our results have shown that daptomycin has the best performance with linezolid regarding CoNS, indicating that this antibiotic can be used for a therapeutic approach to infections caused by these bacteria. Furthermore, the present study showed that resistance to daptomycin has been very low (0.1–0.3%); considering that this antibiotic shortens the duration of the treatment of soft-tissue infections due to MRSA compared to vancomycin [131], it can be used to a greater degree for treating the mentioned infections. However, spontaneous resistance to daptomycin seems to occur rarely [132], and vancomycin can also decrease the function of this drug [130, 133]. Therefore, it is possible to isolate daptomycin-resistant strains from the areas where this antibiotic is not even used, and physicians usually use alternative agents (linezolid and vancomycin) instead of daptomycin, which can be considered as a factor. Daptomycin can be one of the choices for treating staphylococci-induced infections if there is a strong possibility based on local microbiological data or recent treatment history of vancomycin in an infected patient with MIC of > 1 μg/mL.
Q/D comprises quinupristin and dalfopristin in a 30:70 ratio, which prevents protein synthesis in bacteria [134]. Studies have shown that Q/D with 0.7% has the highest resistance rate amongst MRSA strains (Table 1). Resistance reports were gathered from the continents of America, Asia, and Europe, although more studies have been carried out in European countries. This antibiotic is used for the treatment of VRE bloodstream infection and complicated skin and soft tissues infections caused by MRSA and Streptococcus pyogenes. However, the results of this study showed that Q/D had a weaker inhibitory effect than linezolid and daptomycin on S. aureus, MRSA, and CoNS (Table 1); on the other hand, it has significant side effects (myalgia, arthralgia, increased alkaline phosphatase, and nausea), high drug interactions, and treatment costs [135], which led to the limited use of this antibiotic. Therefore, it is better to use other new alternative antibiotics instead of Q/D for treating of staphylococcal infections. The present study showed that although linezolid, Q/D, daptomycin, and tigecycline are prescribed by clinicians for about 15 to 20 years, there is still very low resistance to these antibiotics around the world. On the other hand, with the increasing resistance of staphylococci to vancomycin and the high side effects of other drugs such as cotrimoxazole, it seems that these antibiotics have to be used more often in the future. The results of a recent study on the global prevalence of vancomycin-nonsusceptible MRSA showed that the prevalence of vancomycin-intermediate S. aureus (VISA) was 3.01% in 68,792 MRSA strains. Furthermore, the pooled prevalence of heterogeneous vancomycin-intermediate S. aureus (hVISA) was 6.05% and is highly dangerous, because these bacteria lead to higher rates of vancomycin treatment failure. It should be noted that this study reported that the rate of vancomycin-nonsusceptible MRSA has been increasing in recent years, and this is a danger to the international community [136]. It should be noted that, still, some diseases caused by Staphylococcus genus, such as pneumonia, are treated easier with older drugs, and more studies are needed to evaluate the effect of the newer agents. The higher rates of resistance to the mentioned antibiotics in the United States and European countries compared to other parts of the world do not imply higher resistance to these antibiotics in this areas and are related to microbial susceptibility testing programs that are regularly carried out in these countries, while there are no such reports in the African and Asian countries (may because of non-availability and elevated prices in these regions). Therefore, by performing such programs in other countries, the exact resistance rates of the staphylococcal strains to the newer Gram-positive cocci antibiotics can be determined.
Conclusion
The present study shows that resistance to new agents is low in staphylococci and these antibiotics can still be used for treatment of staphylococcal infections in the world. It should be noted that the development of resistance to these antibiotics should be prevented by appropriate antibiotic resistance testing programs.
Availability of data and materials
All data were included.
Abbreviations
- MRCoNS:
-
Methicillin-resistant coagulase-negative staphylococci
- PBP2:
-
Penicillin-Binding Protein 2
- FDA:
-
Food and Drug Administration
- EMA:
-
European Medicines Agency
- VRE:
-
Vancomycin resistant enterococci
- MeSH:
-
Medical subject heading
- CLSI:
-
Clinical and Laboratory Standards Institute
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- BMD:
-
Broth microdilution
- REM:
-
Random effects model
- FEM:
-
Fixed effects model
References
Braun T, Kahanov L, Dannelly K, Lauber C. CA-MRSA infection incidence and Care in High School and Intercollegiate Athletics. Med Sci Sports Exerc. 2016;48:1530–8.
Fooladvand S, Sarmadian H, Habibi D, van Belkum A, Ghaznavi-Rad E. High prevalence of methicillin resistant and enterotoxin gene-positive Staphylococcus aureus among nasally colonized food handlers in Central Iran. Eur J Clin Microbiol Infect Dis. 2019;38:87–92.
Yousefi M, Pourmand MR, Fallah F, Hashemi A, Mashhadi R, Nazari-Alam A. Characterization of Staphylococcus aureus biofilm formation in urinary tract infection. Iran J Public Health. 2016;45:485–93.
Lee L-H, Zainal N, Azman A-S, Eng S-K, Goh B-H, Yin W-F, Ab Mutalib N-S, Chan K-G. Diversity and antimicrobial activities of actinobacteria isolated from tropical mangrove sediments in Malaysia. Sci World J. 2014;2014.
Ghahremani M, Jazani NH, Sharifi Y. Emergence of vancomycin-intermediate and-resistant Staphylococcus aureus among methicillin-resistant S aureus isolated from clinical specimens in the northwest of Iran. J Global Antimicrob Resist. 2018;14:4–9.
Abbasian S, Farahani NN, Mir Z, Alinejad F, Haeili M, Dahmardehei M, Mirzaii M, Khoramrooz SS, Nasiri MJ, Darban-Sarokhalil D. Genotypic characterization of Staphylococcus aureus isolated from a burn Centre by using agr, spa and SCCmec typing methods. New Microbes New Infect. 2018;26:15–9.
Bijari A, Zade MH, Hatami S, Kalantar E, Sepehr MN, Kabir K, Zahmatkesh E, Naseri MH, Sarokhalil DD. High frequency of methicillin-resistant staphylococcus aureus in intensive care unit in Karaj, Iran. Arch Clin Infect Dis. 2018;13.
Kemung HM, Tan LT-H, Khan TM, Chan K-G, Pusparajah P, Goh B-H, Lee L-H. Streptomyces as a prominent resource of future anti-MRSA drugs. Front Microbiol. 2018;9.
Sader HS, Farrell DJ, Flamm RK, Jones RN. Daptomycin activity tested against 164 457 bacterial isolates from hospitalised patients: summary of 8 years of a worldwide surveillance Programme (2005–2012). Int J Antimicrob Agents. 2014;43:465–9.
Mishra NN, Bayer AS, Moise PA, Yeaman MR, Sakoulas G. Reduced susceptibility to host-defense cationic peptides and daptomycin coemerge in methicillin-resistant Staphylococcus aureus from daptomycin-naive bacteremic patients. J Infect Dis. 2012;206:1160–7.
Bæk KT, Thøgersen L, Mogenssen RG, Mellergaard M, Thomsen LE, Petersen A, Skov S, Cameron DR, Peleg AY, Frees D. Step-wise decrease in daptomycin susceptibility in clinical Staphylococcus aureus isolates associated with an initial mutation in rpoB and a compensatory inactivation of the clpX gene. Antimicrob Agents Chemother. 2015;(AAC):01303–15.
Noskin GA. Tigecycline: a new glycylcycline for treatment of serious infections. Clin Infect Dis. 2005;41:S303–14.
Peterson LR. A review of tigecycline—the first glycylcycline. Int J Antimicrob Agents. 2008;32:S215–22.
Quiles-Melero I, Gómez-Gil R, Romero-Gómez MP, Sánchez-Díaz AM, de Pablos M, García-Rodriguez J, Gutiérrez A, Mingorance J. Mechanisms of linezolid resistance among staphylococci in a tertiary hospital. J Clin Microbiol. 2013;51:998–1001.
Welte T, Pletz MW. Antimicrobial treatment of nosocomial meticillin-resistant Staphylococcus aureus (MRSA) pneumonia: current and future options. Int J Antimicrob Agents. 2010;36:391–400.
Abdel-Hamid ME, Phillips OA. LC-MS/MS determination of Synercid injections. J Pharm Biomed Anal. 2003;32:1167–74.
Organization WH. WHO publishes list of bacteria for which new antibiotics are urgently needed. Geneva: WHO; 2017.
Adam HJ, Baxter MR, Davidson RJ, Rubinstein E, Fanella S, Karlowsky JA, Lagacé-Wiens PR, Hoban DJ, Zhanel GG, Alliance CAR. Comparison of pathogens and their antimicrobial resistance patterns in paediatric, adult and elderly patients in Canadian hospitals. J Antimicrob Chemother. 2013;68:i31–7.
Anastasiou DM, Morgan M, Ruane PJ, Steenbergen JN, Katz BD, Alder JD, Thorne GM. In vitro activity of daptomycin against multidrug-resistant Staphylococcus aureus and S. aureus with known virulence factors, including community-acquired methicillin-resistant isolates. Diagn Microbiol Infect Dis. 2008;61:339–42.
Ayepola OO, Egwari L, Olasehinde GI. Antibiotic resistance profile of Staphylococcus aureus clinical isolates from Nigeria. Antimicrob Resist Infect Control. 2015;4:P195.
Ballow CH, Jones RN, Biedenbach DJ, Group NAZR. A multicenter evaluation of linezolid antimicrobial activity in North America. Diagn Microbiol Infect Dis. 2002;43:75–83.
Biedenbach DJ, Bell JM, Sader HS, Fritsche TR, Jones RN, Turnidge JD. Antimicrobial susceptibility of gram-positive bacterial isolates from the Asia-Pacific region and an in vitro evaluation of the bactericidal activity of daptomycin, vancomycin, and teicoplanin: a SENTRY program report (2003-2004). Int J Antimicrob Agents. 2007;30:143–9.
Biswas S, Watwani J, Vadwai V, Shetty A, Kelkar R, Rodrigues C. Comparative in vitro activities of daptomycin, vancomycin, teicoplanin and linezolid against resistant gram-positive bacterial isolates from two large centres in western India. Int J Antimicrob Agents. 2012;40:567–9.
Błażewicz I, Jaśkiewicz M, Piechowicz L, Neubauer D, Nowicki RJ, Kamysz W, Barańska-Rybak W. Activity of antimicrobial peptides and conventional antibiotics against superantigen positive Staphylococcus aureus isolated from patients with atopic dermatitis. Postepy Dermatol Alergol. 2018;35:74–82.
Bongiorno D, Mongelli G, Stefani S, Campanile F. Burden of rifampicin- and methicillin-resistant Staphylococcus aureus in Italy. Microb Drug Resist. 2018;24:732–8.
Brzychczy-Wloch M, Borszewska-Kornacka M, Gulczynska E, Wojkowska-Mach J, Sulik M, Grzebyk M, Luchter M, Heczko PB, Bulanda M. Prevalence of antibiotic resistance in multi-drug resistant coagulase-negative staphylococci isolated from invasive infection in very low birth weight neonates in two polish NICUs. Ann Clin Microbiol Antimicrob. 2013;12:41.
Campanile F, Bongiorno D, Perez M, Mongelli G, Sessa L, Benvenuto S, Gona F, Varaldo PE, Stefani S. Epidemiology of Staphylococcus aureus in Italy: first nationwide survey, 2012. J Glob Antimicrob Resist. 2015;3:247–54.
Cassettari M, Morrissey I. In vitro activity of Telavancin against staphylococci circulating in Europe during 2010 and 2011; 2011.
Castanheira M, Jones RN, Sader HS. Update of the in vitro activity of daptomycin tested against 6710 gram-positive cocci isolated in North America (2006). Diagn Microbiol Infect Dis. 2008;61:235–9.
Chen YH, Liu CY, Ko WC, Liao CH, Lu PL, Huang CH, Lu CT, Chuang YC, Tsao SM, Chen YS, et al. Trends in the susceptibility of methicillin-resistant Staphylococcus aureus to nine antimicrobial agents, including ceftobiprole, nemonoxacin, and tyrothricin: results from the Tigecycline in vitro surveillance in Taiwan (TIST) study, 2006-2010. Eur J Clin Microbiol Infect Dis. 2014;33:233–9.
Cui L, Wang Y, Li Y, He T, Schwarz S, Ding Y, Shen J, Lv Y. Cfr-mediated linezolid-resistance among methicillin-resistant coagulase-negative staphylococci from infections of humans. PLoS One. 2013;8:e57096.
Cuny C, Layer F, Werner G, Harmsen D, Daniels-Haardt I, Jurke A, Mellmann A, Witte W, Kock R. State-wide surveillance of antibiotic resistance patterns and spa types of methicillin-resistant Staphylococcus aureus from blood cultures in North Rhine-Westphalia, 2011-2013. Clin Microbiol Infect. 2015;21:750–7.
Decousser JW, Woerther PL, Soussy CJ, Fines-Guyon M, Dowzicky MJ. The tigecycline evaluation and surveillance trial; assessment of the activity of tigecycline and other selected antibiotics against gram-positive and gram-negative pathogens from France collected between 2004 and 2016. Antimicrob Resist Infect Control. 2018;7:68.
Decousser J-W, Pina P, Picot F, Delalande C, Pangon B, Courvalin P, Allouch P. Frequency of isolation and antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections: a French prospective national survey. J Antimicrob Chemother. 2003;51:1213–22.
Draghi DC, Benton BM, Krause KM, Thornsberry C, Pillar C, Sahm DF. Comparative surveillance study of Telavancin activity against recently collected gram-positive clinical isolates from across the United States. Antimicrob Agents Chemother. 2008;52:2383–8.
Draghi DC, Sheehan DJ, Hogan P, Sahm DF. In vitro activity of linezolid against key gram-positive organisms isolated in the United States: results of the LEADER 2004 surveillance program. Antimicrob Agents Chemother. 2005;49:5024–32.
Duncan LR, Smart JI, Flamm RK, Sader HS, Jones RN, Mendes RE. Telavancin activity tested against a collection of Staphylococcus aureus isolates causing pneumonia in hospitalized patients in the United States (2013-2014). Diagn Microbiol Infect Dis. 2016;86:300–2.
Farrell DJ, Mendes RE, Ross JE, Jones RN. Linezolid surveillance program results for 2008 (LEADER program for 2008). Diagn Microbiol Infect Dis. 2009;65:392–403.
Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. LEADER program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States. Antimicrob Agents Chemother. 2011;55:3684–90.
Flamm RK, Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. LEADER surveillance program results for 2010: an activity and spectrum analysis of linezolid using 6801 clinical isolates from the United States (61 medical centers). Diagn Microbiol Infect Dis. 2012;74:54–61.
Flamm RK, Mendes RE, Hogan PA, Ross JE, Farrell DJ, Jones RN. In vitro activity of linezolid as assessed through the 2013 LEADER surveillance program. Diagn Microbiol Infect Dis. 2015;81:283–9.
Flamm RK, Mendes RE, Hogan PA, Streit JM, Ross JE, Jones RN. Linezolid surveillance results for the United States (LEADER surveillance program 2014). Antimicrob Agents Chemother. 2016;60:2273–80.
Francia MV, Clewell DB. Transfer origins in the conjugative Enterococcus faecalis plasmids pAD1 and pAM373: identification of the pAD1 nic site, a specific relaxase and a possible TraG-like protein. Mol Microbiol. 2002;45:375–95.
Fuchs PC, Barry AL, Brown SD. In vitro bactericidal activity of daptomycin against staphylococci. J Antimicrob Chemother. 2002;49:467–70.
Gales AC, Sader HS, Ribeiro J, Zoccoli C, Barth A, Pignatari AC. Antimicrobial susceptibility of gram-positive bacteria isolated in Brazilian hospitals participating in the SENTRY program (2005-2008). Braz J Infect Dis. 2009;13:90–8.
Gallon O, Guillet-Caruba C, Lamy B, Laurent F, Doucet-Populaire F, Decousser JW. In vitro activity of daptomycin against staphylococci isolated from bacteremia and community-onset skin and soft tissue infections in France: data from two nationwide studies. Eur J Clin Microbiol Infect Dis. 2009;28:1209–15.
Gandra S, Mojica N, Klein EY, Ashok A, Nerurkar V, Kumari M, Ramesh U, Dey S, Vadwai V, Das BR, Laxminarayan R. Trends in antibiotic resistance among major bacterial pathogens isolated from blood cultures tested at a large private laboratory network in India, 2008-2014. Int J Infect Dis. 2016;50:75–82.
Garza-González E, Dowzicky MJ. Changes in Staphylococcus aureus susceptibility across Latin America between 2004 and 2010. Braz J Infect Dis. 2013;17:13–9.
Haley RW, Hightower AW, Khabbaz RF, Thornsberry C, Martone WJ, Allen JR, Hughes JM. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals: possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982;97:297–308.
Hellmark B, Unemo M, Nilsdotter-Augustinsson A, Soderquist B. Antibiotic susceptibility among Staphylococcus epidermidis isolated from prosthetic joint infections with special focus on rifampicin and variability of the rpoB gene. Clin Microbiol Infect. 2009;15:238–44.
Hodille E, Delouere L, Bouveyron C, Meugnier H, Bes M, Tristan A, Laurent F, Vandenesch F, Lina G, Dumitrescu O. In vitro activity of ceftobiprole on 440 Staphylococcus aureus strains isolated from bronchopulmonary infections. Med Mal Infect. 2017;47:152–7.
Hsueh P-R, Chen W-H, Teng L-J, Luh K-T. Nosocomial infections due to methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci at a university hospital in Taiwan from 1991 to 2003: resistance trends, antibiotic usage and in vitro activities of newer antimicrobial agents. Int J Antimicrob Agents. 2005;26:43–9.
Isnard C, Dhalluin A, Malandain D, Bruey Q, Auzou M, Michon J, Giard JC, Guerin F, Cattoir V. In vitro activity of novel anti-MRSA cephalosporins and comparator antimicrobial agents against staphylococci involved in prosthetic joint infections. J Glob Antimicrob Resist. 2018;13:221–5.
Jain S, Gaind R, Chugh TD. In vitro activity of vancomycin and daptomycin against clinical isolates of Staphylococcus aureus and enterococci from India. Int J Antimicrob Agents. 2013;42:94–5.
Jain S, SenGupta M, Jindal N, Ghosh S, Ghosh C. Phylogenetic analysis of cfr mediated linezolid resistance in clinical isolates of MRSA isolated from eastern India. Int J Adv Biotechnol Res. 2015;16:450–8.
Jan E, Camou F, Texier-Maugein J, Whinnett Z, Caubet O, Ploux S, Pellegrin JL, Ritter P, Metayer PL, Roudaut R, et al. Microbiologic characteristics and in vitro susceptibility to antimicrobials in a large population of patients with cardiovascular implantable electronic device infection. J Cardiovasc Electrophysiol. 2012;23:375–81.
Jevitt LA, Smith AJ, Williams PP, Raney PM, McGowan JE Jr, Tenover FC. In vitro activities of Daptomycin, linezolid, and Quinupristin-Dalfopristin against a challenge panel of staphylococci and enterococci, including vancomycin-intermediate staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Microb Drug Resist. 2003;9:389–93.
John MA, Pletch C, Hussain Z. In vitro activity of quinupristin/dalfopristin, linezolid, telithromycin and comparator antimicrobial agents against 13 species of coagulase-negative staphylococci. J Antimicrob Chemother. 2002;50:933–8.
Jones RN, Ballow CH, Biedenbach DJ, Hospital ECM, Hospital SM, Hospital FC, Hospital GM, Pettis Memorial V, Center LLM, Center UM. Multi-laboratory assessment of the linezolid spectrum of activity using the Kirby-Bauer disk diffusion method: report of the Zyvox® antimicrobial potency study (ZAPS) in the United States. Diagn Microbiol Infect Dis. 2001;40:59–66.
Jones RN, Fritsche TR, Sader HS, Ross JE. LEADER surveillance program results for 2006: an activity and spectrum analysis of linezolid using clinical isolates from the United States (50 medical centers). Diagn Microbiol Infect Dis. 2007;59:309–17.
Jones RN, Kohno S, Ono Y, Ross JE, Yanagihara K, ZAAPS International Surveillance Program. For linezolid resistance: results from 5591 gram-positive clinical isolates in 23 countries. Diagn Microbiol Infect Dis. 2007;2009(64):191–201.
Jones RN, Ross JE, Bell JM, Utsuki U, Fumiaki I, Kobayashi I, Turnidge JD. Zyvox annual appraisal of potency and Spectrum program: linezolid surveillance program results for 2008. Diagn Microbiol Infect Dis. 2009;65:404–13.
Jones RN, Ross JE, Castanheira M, Mendes RE. United States resistance surveillance results for linezolid (LEADER program for 2007). Diagn Microbiol Infect Dis. 2008;62:416–26.
Kao TM, Wang JT, Weng CM, Chen YC, Chang SC. In vitro activity of linezolid, tigecycline, and daptomycin on methicillin-resistant Staphylococcus aureus blood isolates from adult patients, 2006-2008: stratified analysis by vancomycin MIC. J Microbiol Immunol Infect. 2011;44:346–51.
Karlowsky JA, Walkty AJ, Baxter MR, Arhin FF, Moeck G, Adam HJ, Zhanel GG. In vitro activity of Oritavancin against gram-positive pathogens isolated in Canadian hospital laboratories from 2011 to 2015. Diagn Microbiol Infect Dis. 2017;87:349–56.
Khan MM, Faiz A, Ashshi AM. Clinically significant coagulase negative staphylococci and their antibiotic resistance pattern in a tertiary care hospital. J Pak Med Assoc. 2014;64:1171–4.
Li X, Arias CA, Aitken SL, Galloway Pena J, Panesso D, Chang M, Diaz L, Rios R, Numan Y, Ghaoui S, et al. Clonal emergence of invasive multidrug-resistant Staphylococcus epidermidis Deconvoluted via a combination of whole-genome sequencing and microbiome analyses. Clin Infect Dis. 2018;67:398–406.
Limoncu MH, Ermertcan S, Cosar G, Tunger O. In vitro effectiveness of quinupristin/dalfopristin against Staphylococcus aureus strains. Int J Antimicrob Agents. 2003;21:493–5.
Luh K-T, Hsueh P-R, Teng L-J, Pan H-J, Chen Y-C, Lu J-J, Wu J-J, Ho S-W. Quinupristin-dalfopristin resistance among gram-positive bacteria in Taiwan. Antimicrob Agents Chemother. 2000;44:3374–80.
Martinez-Melendez A, Morfin-Otero R, Villarreal-Trevino L, Camacho-Ortiz A, Gonzalez-Gonzalez G, Llaca-Diaz J, Rodriguez-Noriega E, Garza-Gonzalez E. Molecular epidemiology of coagulase-negative bloodstream isolates: detection of Staphylococcus epidermidis ST2, ST7 and linezolid-resistant ST23. Braz J Infect Dis. 2016;20:419–28.
Mathai D, Biedenbach DJ, Jones RN, Bell JM, Turnidge J, Sader HS. Activity of daptomycin against gram-positive bacterial isolates from Indian medical centres (2006-2007). Int J Antimicrob Agents. 2009;34:497–9.
McDonald LC, Lauderdale T-L, Shiau Y-R, Chen P-C, Lai J-F, Wang H-Y, Ho M. The status of antimicrobial resistance in Taiwan among gram-positive pathogens: the Taiwan surveillance of antimicrobial resistance (TSAR) programme, 2000. Int J Antimicrob Agents. 2004;23:362–70.
Mendes RE, Sader HS, Deshpande L, Jones RN. Antimicrobial activity of tigecycline against community-acquired methicillin-resistant Staphylococcus aureus isolates recovered from north American medical centers. Diagn Microbiol Infect Dis. 2008;60:433–6.
Mendes RE, Sader HS, Farrell DJ, Jones RN. Telavancin activity tested against a contemporary collection of gram-positive pathogens from USA hospitals (2007-2009). Diagn Microbiol Infect Dis. 2012;72:113–7.
Mendes RE, Sader HS, Janechek M, Jones RN: Antimicrobial Spectrum of activity of Telavancin and comparator agents tested against methicillin-resistant Staphylococcus aureus recovered from United States and European hospitals over a 3-year sampling period (2007–2009).
Morrissey I, Seifert H, Canton R, Nordmann P, Stefani S, MacGowan A, Janes R, Knight D. Activity of oritavancin against methicillin-resistant staphylococci, vancomycin-resistant enterococci and β-haemolytic streptococci collected from western European countries in 2011. J Antimicrob Chemother. 2012;68:164–7.
Mutnick AH, Enne V, Jones RN, Linezolid resistance since. SENTRY Antimicrobial Surveillance Program. Ann Pharmacother. 2001;2003(37):769–74.
Pedroso S, Sandes SHC, Filho RAT, Nunes AC, Serufo JC, Farias LM, Carvalho MAR, Bomfim MRQ, Santos SG. Coagulase-negative staphylococci isolated from human bloodstream infections showed multidrug resistance profile. Microb Drug Resist. 2018;24:635–47.
Petrelli D, Repetto A, D'ercole S, Rombini S, Ripa S, Prenna M, Vitali LA. Analysis of meticillin-susceptible and meticillin-resistant biofilm-forming Staphylococcus aureus from catheter infections isolated in a large Italian hospital. J Med Microbiol. 2008;57:364–72.
Pfaller MA, Mendes RE, Sader HS, Jones RN. Telavancin activity against gram-positive bacteria isolated from respiratory tract specimens of patients with nosocomial pneumonia. J Antimicrob Chemother. 2010;65:2396–404.
Pfaller MA, Mendes RE, Streit JM, Hogan PA, Flamm RK. Five-Year Summary of In Vitro Activity and Resistance Mechanisms of Linezolid against Clinically Important Gram-Positive Cocci in the United States from the LEADER Surveillance Program (2011 to 2015). Antimicrob Agents Chemother. 2017:61.
Picazo JJ, Betriu C, Culebras E, Rodríguez-Avial I, Gómez M, López F, Group VS. Activity of daptomycin against staphylococci collected from bloodstream infections in Spanish medical centers. Diagn Microbiol Infect Dis. 2009;64:448–51.
Picazo JJ, Betriu C, Rodriguez-Avial I, Culebras E, Lopez F, Gomez M. Comparative activity of daptomycin against clinical isolates of methicillin-resistant Staphylococcus aureus and coagulase-negative staphylococci. Enferm Infecc Microbiol Clin. 2010;28:13–6.
Picazo JJ, Betriu C, Rodriguez-Avial I, Culebras E, Lopez-Fabal F, Gomez M. Comparative activities of daptomycin and several agents against staphylococcal blood isolates. Glycopeptide tolerance. Diagn Microbiol Infect Dis. 2011;70:373–9.
Picazo JJ, Betriu C, Rodríguez-Avial I, Culebras E, López-Fabal F, Gómez M, Group VS. Comparative activities of daptomycin and several agents against staphylococcal blood isolates. Glycopeptide tolerance. Diagn Microbiol Infect Dis. 2011;70:373–9.
Putnam SD, Sader HS, Farrell DJ, Jones RN. Sustained antimicrobial activity of tigecycline against methicillin-resistant Staphylococcus aureus (MRSA) from United States medical centers from 2004 through 2008. J Chemother. 2010;22:13–6.
Richter SS, Heilmann KP, Dohrn CL, Riahi F, Costello AJ, Kroeger JS, Biek D, Critchley IA, Diekema DJ, Doern GV. Activity of ceftaroline and epidemiologic trends in Staphylococcus aureus isolates collected from 43 medical centers in the United States in 2009. Antimicrob Agents Chemother. 2011;55:4154–60.
Rolston KV, Kapadia M, Tarrand J, Coyle E, Prince RA. Spectrum of gram-positive bacteraemia and in vitro activities of daptomycin, linezolid and vancomycin against organisms isolated from cancer patients. Int J Antimicrob Agents. 2013;41:516–20..
Rosenthal ME, Mediavilla J, Chen L, Sonnenfeld J, Pierce L, Shannon A, Boucher H, Pearlmutter M, Kreiswirth B, Kuo YH, et al. Molecular epidemiology of Staphylococcus aureus in post-earthquake northern Haiti. Int J Infect Dis. 2014;29:146–51.
Ross JE, Anderegg TR, Sader HS, Fritsche TR, Jones RN. Trends in linezolid susceptibility patterns in 2002: report from the worldwide Zyvox annual appraisal of potency and Spectrum program. Diagn Microbiol Infect Dis. 2005;52:53–8.
Rouse MS, Steckelberg JM, Patel R. In vitro activity of ceftobiprole, daptomycin, linezolid, and vancomycin against methicillin-resistant staphylococci associated with endocarditis and bone and joint infection. Diagn Microbiol Infect Dis. 2007;58:363–5.
Sader H. Five-year trend of antimicrobial susceptibility rates and daptomycin activity among Staphylococcus aureus isolates collected in Latin American medical eenters (2005-2009). Int J Infect Dis. 2010;14:e191–2.
Sader HS, Farrell DJ, Flamm RK, Jones RN. Variation in potency and spectrum of tigecycline activity against bacterial strains from U.S. medical centers since its approval for clinical use (2006 to 2012). Antimicrob Agents Chemother. 2014;58:2274–80.
Sader HS, Farrell DJ, Flamm RK, Jones RN. Activity of ceftaroline and comparator agents tested against Staphylococcus aureus from patients with bloodstream infections in US medical centres (2009-13). J Antimicrob Chemother. 2015;70:2053–6.
Sader HS, Flamm RK, Jones RN. Antimicrobial activity of ceftaroline tested against staphylococci with reduced susceptibility to linezolid, daptomycin, or vancomycin from U.S. hospitals, 2008 to 2011. Antimicrob Agents Chemother. 2013;57:3178–81.
Sader HS, Flamm RK, Mendes RE, Farrell DJ, Jones RN. Antimicrobial activities of Ceftaroline and comparator agents against bacterial organisms causing bacteremia in patients with skin and skin structure infections in U.S. medical centers, 2008 to 2014. Antimicrob Agents Chemother. 2016;60:2558–63.
Sader HS, Fritsche TR, Jones RN. Frequency of occurrence and daptomycin susceptibility rates of gram-positive organisms causing bloodstream infections in cancer patients. J Chemother. 2008;20:570–6.
Sader HS, Fritsche TR, Jones RN. Antimicrobial activity of daptomycin and selected comparators tested against bloodstream Staphylococcus aureus isolates from hemodialysis patients. Int J Infect Dis. 2009;13:291–5.
Sader HS, Fritsche TR, Streit JM, Jones RN. Daptomycin in vitro activity tested against gram-positive strains collected from European and Latin American medical centers in 2003. J Chemother. 2005;17:477–83.
Sader HS, Jones RN. Antimicrobial susceptibility of gram-positive bacteria isolated from US medical centers: results of the Daptomycin surveillance program (2007-2008). Diagn Microbiol Infect Dis. 2009;65:158–62.
Sader HS, Jones RN, Stilwell MG, Dowzicky MJ, Fritsche TR. Tigecycline activity tested against 26,474 bloodstream infection isolates: a collection from 6 continents. Diagn Microbiol Infect Dis. 2005;52:181–6.
Sader HS, Moet GJ, Farrell DJ, Jones RN. Antimicrobial susceptibility of daptomycin and comparator agents tested against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: trend analysis of a 6-year period in US medical centers (2005-2010). Diagn Microbiol Infect Dis. 2011;70:412–6.
Sader HS, Streit J, Fritsche T, Jones R. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin surveillance Programme (2002–2004). Clin Microbiol Infect. 2006;12:844–52.
Sader HS, Watters AA, Fritsche TR, Jones RN. Daptomycin antimicrobial activity tested against methicillin-resistant staphylococci and vancomycin-resistant enterococci isolated in European medical centers (2005). BMC Infect Dis. 2007(7):29.
Sahm DF, Deane J, Bien PA, Locke JB, Zuill DE, Shaw KJ, Bartizal KF. Results of the surveillance of Tedizolid activity and resistance program: in vitro susceptibility of gram-positive pathogens collected in 2011 and 2012 from the United States and Europe. Diagn Microbiol Infect Dis. 2015;81:112–8.
Song Y, Lv Y, Cui L, Li Y, Ke Q, Zhao Y. cfr-mediated linezolid-resistant clinical isolates of methicillin-resistant coagulase-negative staphylococci from China. J Glob Antimicrob Resist. 2017;8:1–5.
Stuart JI, John MA, Milburn S, Diagre D, Wilson B, Hussain Z. Susceptibility patterns of coagulase-negative staphylococci to several newer antimicrobial agents in comparison with vancomycin and oxacillin. Int J Antimicrob Agents. 2011;37:248–52..
Tekin A, Dal T, Deveci O, Tekin R, Ozcan N, Atmaca S, Dayan S. In vitro susceptibility to methicillin, vancomycin and linezolid of staphylococci isolated from bloodstream infections in eastern Turkey. Braz J Microbiol. 2014;45:829–33.
Vamsimohan A, Gupta S, Muralidharan S. Daptomycin resistance in methicillin-resistant Staphylococcus aureus: a report from southern India. Germs. 2014;4:70–2.
Vega S, Dowzicky MJ. Antimicrobial susceptibility among gram-positive and gram-negative organisms collected from the Latin American region between 2004 and 2015 as part of the Tigecycline evaluation and surveillance trial. Ann Clin Microbiol Antimicrob. 2017;16:50.
Wang W-Y, Chiueh T-S, Lee Y-T, Tsao S-M. Correlation of molecular types with antimicrobial susceptibility profiles among 670 mecA-positive MRSA isolates from sterile sites (TIST Study, 2006–2010). J Microbiol Immunol Infect. 2015;48:S39.
Xi C, Liao W-M, Gong F-Y, Min X, Xiong W, Lai X-Q. Pathogen infection distribution and drug resistance analysis of patients with severe liver disease. Med J Chin Peoples Liberation Army. 2018;43:28–32.
Yousefi M, Fallah F, Arshadi M, Pourmand MR, Hashemi A, Pourmand G. Identification of tigecycline- and vancomycin-resistant Staphylococcus aureus strains among patients with urinary tract infection in Iran. New Microbes New Infect. 2017;19:8–12.
Zhanel GG, Adam HJ, Baxter MR, Fuller J, Nichol KA, Denisuik AJ, Lagacé-Wiens PR, Walkty A, Karlowsky JA, Schweizer F. Antimicrobial susceptibility of 22746 pathogens from Canadian hospitals: results of the CANWARD 2007–11 study. J Antimicrob Chemother. 2013;68:i7–i22.
Zhanel GG, Adam HJ, Low DE, Blondeau J, DeCorby M, Karlowsky JA, Weshnoweski B, Vashisht R, Wierzbowski A, Hoban DJ. Antimicrobial susceptibility of 15,644 pathogens from Canadian hospitals: results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis. 2011;69:291–306.
Zhanel GG, DeCorby M, Nichol KA, Wierzbowski A, Baudry PJ, Karlowsky JA, Lagace-Wiens P, Walkty A, Mulvey MR, Hoban DJ. Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada: Canadian National Intensive Care Unit Study, 2005/2006. Diagn Microbiol Infect Dis. 2008;62:67–80.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60.
Xiao G, Chen Z, Lv X. Chlorhexidine-based body washing for colonization and infection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an updated meta-analysis. Infect Drug Resist. 2018;11:1473–81.
Darban-Sarokhalil D, Khoramrooz SS, Marashifard M, Hosseini SAAM, Parhizgari N, Yazdanpanah M, Gharibpour F, Mirzaii M, Sharifi B, Haeili M. Molecular characterization of Staphylococcus aureus isolates from southwest of Iran using spa and SCCmec typing methods. Microb Pathog. 2016;98:88–92.
Khan TM, Kok YL, Bukhsh A, Lee LH, Chan KG, Goh BH. Incidence of methicillin resistant Staphylococcus aureus (MRSA) in burn intensive care unit: a systematic review. Germs. 2018;8:113–25.
Xu Z, Shah HN, Misra R, Chen J, Zhang W, Liu Y, Cutler RR, Mkrtchyan HV. The prevalence, antibiotic resistance and mecA characterization of coagulase negative staphylococci recovered from non-healthcare settings in London, UK. Antimicrob Resist Infect Control. 2018;7:73.
Zong Z, Peng C, Lü X. Diversity of SCCmec elements in methicillin-resistant coagulase-negative staphylococci clinical isolates. PLoS One. 2011;6:e20191.
Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother. 2011;55:1162–72.
Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58:1221–9.
Steenbergen JN, Alder J, Thorne GM, Tally FP. Daptomycin: a lipopeptide antibiotic for the treatment of serious gram-positive infections. J Antimicrob Chemother. 2005;55:283–8.
Drees M, Boucher H. New agents for Staphylococcus aureus endocarditis. Curr Opin Infect Dis. 2006;19:544–50.
Boucher HW, Sakoulas G. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis. 2007;45:601–8.
Davis SL, McKinnon PS, Hall LM, Delgado G Jr, Rose W, Wilson RF, Rybak MJ. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27:1611–8.
Silverman JA, Oliver N, Andrew T, Li T. Resistance studies with daptomycin. Antimicrob Agents Chemother. 2001;45:1799–802.
Sakoulas G, Eliopoulos GM, Fowler VG, Moellering RC, Novick RP, Lucindo N, Yeaman MR, Bayer AS. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother. 2005;49:2687–92.
Gostelow M, Gonzalez D, Smith PB, Cohen-Wolkowiez M. Pharmacokinetics and safety of recently approved drugs used to treat methicillin-resistant Staphylococcus aureus infections in infants, children and adults. Expert Rev Clin Pharmacol. 2014;7:327–40.
Micek ST. Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2007;45:S184–90.
Zhang S, Sun X, Chang W, Dai Y, Ma X. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS One. 2015;10.
Acknowledgments
This study is related to the project No. IR.IUMS.REC.1398.1137 from Student Research Committee, Iran University of Medical Sciences, Tehran, Iran.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Davood Darban-Sarokhalil and Aref Shariati conceived and designed the study. Masoud Dadashi, Mehdi Mirzaii and Seyed Sajjad Khoramrooz contributed in comprehensive research. Zahra chegini designed the Figures. Aref Shariati, Masoud Dadashi and Davood Darban-Sarokhalil wrote the paper. Alex van Belkum and Davood Darban-Sarokhalil participated in manuscript editing. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
AvB is a bioMerieux employee. bioMerieux is a company that design, develops and sells diagnostics in the field on infectious diseases. The company had no direct influence on the design and execution of the present study. Rest of the authors declare to have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shariati, A., Dadashi, M., Chegini, Z. et al. The global prevalence of Daptomycin, Tigecycline, Quinupristin/Dalfopristin, and Linezolid-resistant Staphylococcus aureus and coagulase–negative staphylococci strains: a systematic review and meta-analysis. Antimicrob Resist Infect Control 9, 56 (2020). https://doi.org/10.1186/s13756-020-00714-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-020-00714-9