Abstract
Background
Despite the wide use of indigenous browse plant species, there is almost no information on the rehabilitation of indigenous browse species following area exclosure (AE) established on communal grazing lands (CGL) in Southern Tigray. The objectives of this study were to assess the rehabilitation of browse plant species following AE establishment on CGL. A total of 61 and 59 plots of 10 × 10 m2 size were laid down at 50-m intervals along parallel line transects at AE and GCL, respectively. Data collected on vegetation attributes were subjected to analysis of t test (unequal variances) using R-software.
Results
The Shannon diversity index of the browse plant species was 1.25 and 0.81 in AE and CGL, respectively (P < 0.001). The overall population structure of browse plant species in the AE shows a reverse J-shaped population curve and “good” regeneration status, which reveals that the future communities may be sustained. Leaf biomass and basal area of browse plant species were significantly higher in the AE than in CGL (P < 0.001). After exclusion of grazing, AE was found to have positive effects on diversity and aboveground biomass of browse plant species.
Conclusions
The study gives an understanding of the diversity, the pattern of population and regeneration of the browse plant species, which may help in the management and conservation of the species. Our results indicate that grazing exclusion is an effective management strategy to restore browse plant species. We concluded that the establishment of AE had a positive effect on the rehabilitation of browse plant species diversity and improved population structure and regeneration potentials of degraded grazing lands. Long-term monitoring and evaluation systems will be required to gain an informed understanding of the roles played by area exclosures in the rehabilitation and conservation of browse palnt species diversity.
Similar content being viewed by others
Background
Browse plants, beside grasses, constitute one of the cheapest sources of feed for ruminants (Ahamefule et al. 2006). Particularly, fodder trees and shrubs could play both ecological and economic roles in livestock production systems (Karki and Goodman 2009). However, the extensive utilization of the browse plants has increased pressure on the native vegetation, resulting in degradation of the resources due to over-utilization by the communities and over-browsing by livestock and game animals (Abule et al. 2005). As a result, encroachment by weeds and undesirable plants, which are plant species that are classified as undesirable, noxious, injurious or poisonous like Lantana camara L. and Prosopis juliflora (Sw.) DC (Allen and Allen 1981; Asfaw and Thulin 1989), has become a threat to the livestock production systems in the Horn of Africa, especially in Ethiopia (Amaha 2003; Gemedo et al. 2006). Gebremedhin (2000) stated that the misuse of natural resources has resulted in very serious land degradation in most places. Improving the management of the natural resources providing ecological services (soil formation, nutrient cycling, and primary production (Alcamo, 2003; Zerihun et al. 2002); gully stabilization, controlling accelerated soil erosion, reducing flood damage (Nedessa et al. 2005; Wolde et al. 2007) and immediate economic needs are the major research and development challenges for the degraded areas of northern Ethiopia in particular and drylands of East Africa in general.
A common restoration practice in Ethiopia is the use of area exclosure (AE) (Tekle 2001; Tekle and Bekele 2000; Asefa et al. 2003; Tefera et al. 2005). In the current study, we distinguished between small-scale exclosures used for protecting livestock grazing (Desta and Oba 2004) and the large-scale multi-purpose degraded areas protected from human and livestock exploitation. Restoration of biodiversity using AE has not been evaluated in terms of the effects of management factors (i.e. AE versus communal grazing land (CGL)) on indigenous browse plant species (BPS) diversity. We evaluated the restoration of BPS diversity in highly degraded mountain landscapes in Southern Tigray, which were previously used for farming and communal grazing.
In the places where AE are established, particularly in the southern part of the region, area exclosures are among the green spots with considerable species diversity (Tefera et al. 2005; Betru et al. 2005). In exclosures, it is generally believed that all the land resources will be protected from degradation. Although the restoration effects of AE have been well studied (Kindeya 2003; Aerts et al. 2004; Descheemaker et al. 2006), chemical composition and digestibility of some browse plant species (Ammar et al. 2004; Ahamefule et al. 2006; Melaku et al. 2010; Boufennara et al. 2012; Girma et al. 2015; Weldemariam and Gebremichael 2015) and use and availability of tree and shrub resources (John and Moses 2005; Teferi et al. 2008), whereas there is no study that offers quantitative information that helps to compare AE with the unprotected areas with respect to browsing plant species restoration. In addition, in the Tigray region, Northern Ethiopia, some of the studies conducted earlier (Gebremedhin 2000; Emiru et al. 2002; Kindeya 2003; Aerts et al. 2004; Tefera et al. 2005; Muluberhan et al. 2006; Yayneshet et al. 2009; Wolde and Aynekulu 2011, Wolde et al. 2011b, 2011c; Yayneshet 2011; Wolde and Mastewal 2013; Tsegay et al. 2019; Tesfay et al. 2019) were specifically trying to estimate the role of AE in the recovery of woody vegetation diversity without considering specifying indigenous browse species. It is very important to have basic information to browse plant species diversity and biomass production, as these may facilitate the efficient and effective use of rangeland resources as livestock feed. Therefore, this study was initiated to generate quantitative information and thereby to evaluate whether AE has an impact on the density and diversity of BPS. Thus, the objectives of this study were to assess rehabilitation of indigenous browse species following exclosure established on communal grazing lands in South Tigray, Ethiopia.
Materials and methods
Description of the study area
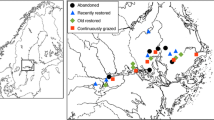
The study was conducted in the South zone of Tigray selected districts. It is located at 680 km North of Addis Ababa, the capital city of Ethiopia and 180 km South of Mekele, the capital city of the Tigray regional state. The zone consists of five administrative districts, namely Raya Alamata, Alaje, Endamohoni, Ofla and Raya Azebo. The study was conducted at five sites (Raya Alamata, Emba Alaje, Endamohoni, Ofla and Raya Azebo) (Fig. 1). The sample of different sites was selected with the aim of encompassing a wide range of ecoregional diversity. The Southern Tigray Zone is one of the seven zones of the region bordering to the South and West with Amhara Regional State, to the North with a South Eastern zone of Tigray and to the East with Afar Regional State. Geographically, it is located between 12° 15′ and 13° 41′ north latitude and 38° 59′ and 39° 54′ east longitude with an altitudinal range of 1350–3925 m.a.s.l. Based on the traditional classification system, Southern zone covers Kola, Weynadega and Dega agro-ecologies that enable to grow varied types of crops, livestock and tree species. It covers an area of 9446 km2 with a total population of 538,422. The Southern zone has experienced two rainfall seasons: the short rainy season locally known as “Belgi” that occurs usually from February to April and the main rain season locally described as “Kiremti” that comes during June to September. On average, the area receives annually about 600 mm rainfall with a mean annual temperature of 25 °C. The dominant soil types of the area are Vertisol, Fluvisols, Luvisols and Cambisols, which implies the need for site-specific management practices to improve the productivity of the land (Kidane et al. 2016).
Site selection and sampling procedure
A reconnaissance survey was conducted in the second week of September 2018, to collect baseline information, observe vegetation distribution and determine the number of transect lines to be laid. The CGL was located 0.5–1 km away from the AE. It is assumed that the AE and CGL were homogenous in biophysical factors before the AEs are restricted for rehabilitation and are similar in topographic and climatic characteristics. The AE was established since 2004 and covers a total area of 500–1500 ha, while the CGL covered 200–1300 ha (Southern Zone BOARD 2018). Adjacent to this AE, there is CGL used by the community with no restriction to access resource. Therefore, this study was conducted in these two land-use systems: AE having the restriction and rules of resource access, in which people and grazing animals are permanently kept outside this area, and the nearby CGL having no restriction and rule of resource access. The CGL areas were considered controls, while the AE represented rehabilitation.
Data collection
After the identification of plant species, which are browsed by animals, data were collected for quantitative analysis. Since the study area has different formation types, stratified sampling design, as described by Krebs (1989) was used to collect data on vegetation. A total of 120 (10 m by 10 m as recommended by Kent and Coker (1992)) plots were established. The total number of plots inside AE and outside AE were 61 and 59, respectively. In each plot, all woody species with a diameter at breast height (DBH) ≥ 2.5 cm and height ≥ 1.5 m were recorded. Within the major plot of 100 m2, five sub-plots, each 5 m × 5 m (25 m2), were set up to collect vegetation data of shrubs with a diameter at stump height (DSH) ≥ 1.5 cm and height ≥ 0.5 m and the mean of these five sub-plots were used in the analysis.
Seedlings were recorded for analysis of the regeneration status of trees within each 25-m2 sub-plot. In this study, all individuals with a height between 0.01 and 1 m were considered seedlings of trees, and both height and diameter measurements were taken (DBH < 2.5 cm and height < 1 m). Within each 25-m2 sub-plot, five 1 m × 1 m sub-plots were used to collect data on seedlings (below a height of 0.1 m) of shrubs. For identification of species in the field, vernacular names from key informants were used and the specimens supported by voucher specimens by referring to Edwards et al. (2000) and Hedberg et al. (2006). The important quantitative analysis such as important value index (IVI) of indigenous browse species was determined as in Curtis and McIntosh (1950).
Basal area was calculated using DBH as follows: Basal area of a tree = D2/4휋, where D = diameter and 휋 = 3.14. Browse species diversity was calculated based on the number of species and their abundance, according to Kent and Coker (1992): \( {H}^{\prime }=-{\sum}_{i=1}^s Pi\ \mathit{\ln}(Pi), \) where H′ = Shannon diversity index, S = the number of species, Pi = proportion of individual species and InPi = natural log. \( \mathrm{Equitability}\ \left(\mathrm{evenness}\right)=\frac{H^{\prime }}{H\ \max } \) where H′ = Shannon diversity index, S = the number of species and Hmax = the maximum level of diversity possible within a given population.
The population structure of each of the browse plans species in each of the two study sites was assessed through grouped arbitrarily into height classes and diameter classes (Emiru et al. 2002). Regeneration status of the study area was analysed by comparing saplings and seedlings with the matured trees according to Dhaulkhandi et al. (2008) and Tiwari et al. (2010). The leaf biomass of a tree was estimated by using the model of Petmak (1983): - logW = 2.24 logDBH-1.50, where W = leaf biomass in kilograms of dry weight and DBH (diameter at breast height) at 130 cm height and the leaf biomass of a shrub was estimated by the equation: - logW = 2.62 logDS-2.46, where DS is the stem diameter (cm) at 30 cm height. The model was adopted because it is established in northeast Thailand with similar climatic and topographic conditions in the study area. The argument claims similarities between climate and topography because climate and topography are among the most important drivers of broad-scale patterns of species (Irl et al. 2015; Moura et al. 2016). Climate can affect species composition indirectly via their effects on vegetation (Stein et al. 2014), while topography can interplay with both climate and vegetation, also affecting species richness (Ruggiero and Hawkins 2008; Stein et al. 2014). In addition, this was developed in the woodland and agricultural trees with 98% of trees having a DBH less than 40 cm (similar with the study area).
Data analysis
t test unequal variance using R-software version 3.5.1 (The R Core Team 2018) was employed to test the significance of differences of species richness, diversity index, species evenness, basal area and estimation of leaf biomass of browse plant species of the two land-use systems.
Results
Browse species composition
The floristic composition can be described in terms of species richness, abundance, dominance and frequency. In the floristic analysis, 64 plant species belonging to 52 genera and 37 families were identified in AE. The life form distribution of these species were 22 (34.38%) mesophanerophyte, 25 (39.06%) microphanerophyte, 13 (20.31%) nanophanerophyte and 4 (6.25%) climbers. On the other hand, 43 plant species belonging to 34 genera and 25 families were identified in CGL. The life form distribution of these species were 16 (37.21%) mesophanerophyte, 17 (39.53%) microphanerophyte, 9 (20.93%) nanophanerophyte and 1 (2.33%) climbers (Fig. 2).
Species diversity, richness and evenness
The value of the Shannon diversity index of browse plant species in the AE and CGL was 1.25 and 0.81, respectively, indicating significantly lower browse species diversity in the CGL than the AE (p < 0.001). The average number of individuals per hectare (ind/ha) was also higher in the AE (3481) than in the CGL (2040) (p < 0.001). The species evenness also showed significant differences (p < 0.05) between the two land-use systems (Table 1).
Basal area (BA) and leaf biomass of browse plant species
The mean basal area (expressed as the basal area of stems per hectare) of browse plant species with DBH > 2.5 cm were 51.81 and 23.14 m2/ha in AE and CGL, respectively. Basal area (BA) of the browse plant species was significantly larger in the AE compared to the CGL (P < 0.001) (Table 2) (Additional file 1). The mean leaf biomass of browse plant species at the AE and CGL was 753.68 and 485.57 kg/ha, respectively. In addition, the mean leaf biomass of browse plant species was significantly lower in the CGL compared to the AE (P < 0.001) (Table 2).
Population structure of browse plant species
The diameter class distribution of browse plant species in the AE reflected reversed J-shape (L-shape). This means species frequency distribution had the highest frequency in the lower diameter and height classes and a gradual decrease towards the higher classes (Fig. 3). Similarly, the density distribution of browse individuals in different height classes also showed a similar pattern with diameter classes at the AE. However, the height distribution for the CGL shows a bell-shape, which showed there was a higher number of individuals in the middle height classes but decrease towards the lower and higher height classes (Fig. 4).
Regeneration status of browse plant species
The composition and density of seedlings and saplings indicate the status of regeneration in the study area. According to Dhaulkhandi et al. (2008), the density values of seedling and saplings are considered as regeneration potential of the species. According to Khumbongmayum et al. (2006), Dhaulkhandi et al. (2008) and Tiwari et al. (2010), the regeneration status of the browse plant species at AE is considered as good regeneration because density of individuals of species showed that the sapling (37.03%) > seedling (26.16%) < matures (36.82%). Based on the criteria of Khumbongmayum et al. (2006), Dhaulkhandi et al. (2008) and Tiwari et al. 2010, the CGL was categorized under fair regeneration (mature > sapling > seedling) (Fig. 5).
Discussion
Browse species composition
The wood species composition recorded in the AE site is higher than that in the CGL. This study revealed that the vegetation composition of the AE was much denser than that of the CGL. The difference observed in woody species composition between the two land-use systems showed the positive effect of AE through proper conservation strategies like rehabilitation. Similarly, encouraging results have been reported from studies made on exclosures established in Tigray, Ethiopia (Emiru et al. 2002; Tefera et al. 2005; Tesfay et al. 2019). Sisay et al. (2001) and Tessema et al. (2011) also suggested that heavy grazing might cause a reduction of plant species composition over time. Due to the high and frequent disturbance by animals and human interference, the number of seedlings of browse plant species was low in the CGL.
Species diversity, richness and evenness
The value of the Shannon diversity index of browses plant species in the AE and CGL was significantly different (p < 0.001). This could result from repeated habitat disturbances in the CGL due to frequent and intensive interference of both humans and livestock for grazing and other communal uses. Decline in browse species diversity in the grazing land could be a result of the loss of seedlings of some species unable to establish at an early stage of development, and selective defoliation and trampling by grazing herbivores (Belaynesh 2006) or either harvested at their early age by the local inhabitants or their domestic animals (Wondie et al. 2014). According to Kibret (2008), the reduction of species diversity in the CGL could be an indication of the increased vulnerability of the plant species by animals and/or human intervention at maturity or early stage of regeneration.
The vegetation composed of the browse plant species was denser in the AE than in the CGL. The density of woody vegetation was significantly affected by grazing, i.e. a higher density of woody species was observed on exclosure site than in the communal grazing sites (Augustine and McNaughton 2004; Teshome et al. 2009). This indicates that AE influenced both species richness and density. The present result agrees with the same previous study in Ethiopia, like Muluberhan et al. (2006), Yayneshet (2011) and Gebrewahd (2014). The species evenness also showed significant differences (p < 0.05) between the two land-use systems (Table 1). This might indicate that the existence of variations in species diversity was a result of the heterogeneous distribution of species due to protection vegetation establishment factors. A low evenness value means that there is the dominance of one or more species in the community, while high evenness means that there is a uniform distribution among the species in samples, demonstrating that individuals are well distributed (Cavalcanti and Larrazábal 2004). In agreement with the above statements, the browse plant species in the AE was more uniformly distributed than that in the CGL.
Basal area and leaf biomass of browse plant species
BA of browse plant species in the AE and CGL showed a significant difference (P < 0.001). This variation may come from the differences in the management practice of the study sites. The basal area of the browse plant species in the study area was low in CGL, indicating the browse plant species were thin in the CGL. The great difference in basal area between the AE and CGL could be due to the high number of multi-stemmed trees in the AE, leading to bigger diameters. The management practices, basal area, species richness, the number of individuals per hectare and species diversity were the probable reason for the differences in the leaf biomass among the two land-use systems. For instance, Raju (2012) found that the basal area is an important parameter, which determines the biomass by the species. The more basal area indicated the more biomass.
In the current study, AE had a significant difference in brows plant species biomass over the CGL. The lower leaf biomass in CGL compared to the AE in our study could be due to continuous heavy grazing, which negatively affects the growth of browse plant species. This finding is in line with Yayneshet et al. (2009) who reported that more than double biomass was produced under AE than CGL in the highlands of the Tigray region of Ethiopia. Overgrazing is one of the most important disturbances resulting in rangeland degradation and environmental problems (Bellows 2003; Amiri et al. 2008; Darkoh 2009; Suizi et al. 2019). It has been suggested as the main cause of biodiversity loss (Scholes and Biggs 2005). In particular, excessively heavy grazing has been indicated to contribute to decline in biodiversity (Biggs et al. 2008). Besides, Tsegay et al. (2019) also reported that accumulation of vegetation biomass declined with grazing land degradation and change in species composition, leading to a less biomass production at a higher grazing pressure compared to lower grazing pressure. Grazing is one of the main land uses of natural rangelands and affects the species composition and plant biomass (Klein et al. 2007; Post and Pedersen 2008). Both plant productivity and species diversity will increase under appropriate grazing intensity (Proulx and Mazumder 1998). However, overgrazing is considered to be the main cause of natural grazing land degradation (Ma et al. 2019). Grazing reduces the aboveground biomass of vegetation but quickly recover after implementation of AE (Sasaki et al. 2008; Frank et al. 2002). According to Witt et al. (2011), woody biomass increases with grazing exclusion due to the regeneration potentials of tree and shrub species. Likewise, some studies (Verdoodt et al. 2010; Wesche et al. 2010) showed that biomass of woody vegetation improved following the establishment of grazing exclosure on communal grazing lands, which might be due to the higher abundance and density of woody vegetation. The pressure of livestock grazing and overall utilization of rangelands has caused vegetation degradation (Amiri et al. 2008).
Population structure of browse plant species
Girth class frequency showed a reverse J-shaped population curve in AE which is dissimilar to CGL (a bell-shape). The possible reason for decreasing percentage of the number of individual browse species within the largest diameter class (> 25.1 cm) might be due to the illegal cutting system used by the local people for construction materials and fuelwood consumption. This result is in line with the study made by Getaneh (2007) and Tefera et al. (2015), who showed that woody species with large diameter were harvested by the local people for construction and charcoal preparation. The presence of heavy browsing leads to skewed age structure, small seedlings being dominant in the grazed areas (Butler and Kielland 2008).
Similarly, the density distribution of browse individuals in different height classes also showed a similar pattern with diameter classes at the AE. However, the height distribution for the CGL shows a bell-shape, according to Feyera et al. (2007), the bell-shape pattern indicates a poor reproduction and recruitment of species. Protection influenced the height class distribution of the woody vegetation attributes (Butler and Kielland 2008; Yayneshet 2011). The low proportion of lower and higher height classes is probably because of grazing/browsing or trampling and shows that the CGL had a lower potential for rehabilitation of browse plant species than the area enclosed. A reversed J-shape distribution of height/diameter classes in our study indicated a continuous and good regeneration and/or a stable population of browse plant species. In contrast, bell-shaped distribution of browse plant species in the CGL indicated a hampered regeneration status, which might be due to several disturbance factors, including deforestation by local people in addition to frequent browsing and trampling by livestock.
Regeneration status of browse plant species
The composition and density of seedlings and saplings indicate the status of regeneration in the study area. According to Dhaulkhandi et al. (2008), the density values of seedling and saplings are considered as regeneration potential of the species. In general, the regeneration of species is affected by various anthropogenic factors and natural phenomena (Iqbal et al. 2012). The overall regeneration status of the browse plant species of the AE and CGL site is satisfactory at community level showing good and fair regeneration status, respectively. These revealed that the high level of protection in the area helps the regeneration of browse species. Woody species’ appearance in AE indicates a long period of protection, allowing regeneration of shrubs and trees (Kebrom 2001; Tefera et al. 2005). However, in the CGL, a low level of protection in the area helped the regeneration of browse plant species. The low proportion of seedlings in the CGL showed the browse plant species rehabilitation has been continuously restricted by disturbance, such as, overgrazing and human activity. According to this data, most of the seedlings perished off before reaching sapling and mature stages for various reasons, which might be due to grazer and browser pressure (Teshome et al. 2015). The relatively better/lower regeneration of browse plant species might be attributed to the density of their mature trees, maybe due to the presence/absence of significant amounts of viable seeds in the soil and/or the wide dispersal of their seeds through ungulate feces (Anteneh et al. 2011). According to Taye et al. (2002), a tree species with no seedling and sapling in natural vegetation is under a risky condition and it is suggested that these species are under threat of local extinction.
Species under not regenerating condition might have occurred due to an existing disturbance at the study site like grazing, firewood collection and poor biotic potential of tree species, which either affect the fruiting or seed germination or successful conversion of seedling to sapling stage. Moreover, individuals in young stages of any species are more vulnerable to any kind of environmental stress and anthropogenic disturbance (Iqbal et al. 2012; Moumita and Ashalata 2014). Besides, the decline in species diversity in the communal grazing land could be a result of the loss of seedlings of some species unable to establish at an early stage of development, and selective defoliation and trampling by grazing herbivores (Belaynesh 2006).
Conclusion and recommendation
The overall population structure of tree species in the study site reveals that the contribution of seedlings to the total population was the highest, followed by saplings and adult trees. It shows regeneration of browse plant species in the AE is “good” and the future communities may be sustained unless there is any major environmental stress or interference exerted by human activities. However, considering the increasing anthropogenic pressure, there may be a spatial and temporal threat to the seedling establishment and growth of browse plant species in the study site. The growth, survival and reproductive potential of the browse plant species will be at risk in the near future if the present trend of anthropogenic continues at CGL. The present study has demonstrated that grazing prevention is an important means of rehabilitating and renovating browse plant species. Quantitative analysis of diversity, population structure and regeneration status of browse plant species recorded from the present study may provide baseline information for livestock feed resource projects, for evaluation of whether the AE establishment should be expanded and for policymakers to take into account the value of AE in their management decisions.
Availability of data and materials
The dataset(s) supporting the conclusions of this article is (are) available in the supplement materials.
Abbreviations
- AE:
-
Area exclosure
- BPS:
-
Browse plant species
- CGL:
-
Communal grazing land
References
Abule E, Smit GN, Snyman HA (2005) The influence of woody plants and livestock grazing on grass species composition, yield and soil nutrients in the Middle Awash Valley of Ethiopia. J Arid Environ 60(2):343–358
Aerts R, Wagendrop T, November E, Mintesinot B, Deckers J, Muys B (2004) Ecosystem thermal buffer capacity as an indicator of the restoration status of protected areas in the Northern Ethiopian Highlands. Restor Ecol 12:586–596
Ahamefule FO, Obua BE, Ibeawuchi JA, Udose NR (2006) The nutritive value of some plants browsed by cattle in Umudike, Southeastern Nigeri. Pak J Nutr 5(5):404–409
Alcamo J (2003) Ecosystems and human well-being: a framework for assessment. Island Press, Washington, DC, p 245
Allen ON, Allen EK (1981) The Leguminosae. University of Wisconsin, Madison
Amaha K (2003) Pastoralism and the need for future intervention in pastoral areas of Ethiopia. Annual review of National Dry Land Agricultural Research Systems, Addis Ababa
Amiri F, Ariapour A, Fadai S (2008) Effects of livestock grazing on vegetation composition and soil moisture properties in grazed and non-grazed range site. J Biol Sci 8(8):1289–1297
Ammar H, López S, González JS, Ranilla MJ (2004) Chemical composition and in vitro digestibility of some Spanish browse plant species. J Sci Food Agric 84(2):197–204
Anteneh B, Tamrat B, Sebsebe D (2011) The natural vegetation of Babile elephant sanctuary, eastern Ethiopia: implications for biodiversity conservation. Ethiopian J Biol Sci 10(2):137–152
Asefa DT, Oba G, Weladji RB, Coleman JE (2003) An assessment of restoration of biodiversity in degraded high mountain grazing lands in northern Ethiopia. Land Degrad Dev 14:25–38
Asfaw H, Thulin M (1989) Mimosoideae. In: Hedberg I, Edwards S (eds) Flora of Ethiopia, vol 3. National Herbarium, Addis Abeba University, Uppsala University, Addis Abeba, Uppsala, pp 71–73
Augustine DJ, McNaughton SJ (2004) Regulation of shrub dynamics by native browsing ungulates on East African rangeland. J Appl Ecol 41:45–58
Bellows BC (2003) Managed grazing in riparian areas. National Center for Appropriate Technology.
Biggs R, Simons H, Bakkenes M, Scholes RJ, Eickhout B, Van Vuuren D, Alkemade R (2008) Scenarios of biodiversity loss in southern Africa in the 21st century. Global Environ 18:296–309
Boufennara S, Lopez S, Bousseboua H, Bodas R, Bouazza L (2012) Chemical composition and digestibility of some browse plant species collected from Algerian arid rangelands. Span J Agric 10(1):88–98
Butler LG, Kielland K (2008) Acceleration of vegetation turnover and element cycling by mammalian herbivory in riparian ecosystems. J Ecol 96:136–144
Betru N, Ali J, Nyborg I (2005) Exploring Ecological and Socio-Economic Issues for the Improvement of Area Enclosure Management: A Case Study from Ethiopia. DCG (Drylands Coordination Group), Oslo, Norway
Belaynesh D (2006) Floristic composition and diversity of the vegetation, soil seed bank flora and condition of the rangelands of the Jijiga Zone, Somali Regional State, Ethiopia. MSc Thesis, Haramaya University, Haramaya, Ethiopia.
Cavalcanti EAH, Larrazábal MEL (2004) Macrozooplâncton da zona econômica exclusiva do nordeste do brasil (segunda expedição oceanográfica – revizee/ne II) com ênfase em CopepFicus sycomorous (Crustacea). Revista Brasileira de Zoologia 21(3):467–475
Curtis JT, Mcintosh RP (1950) The interrelations of certain analytic and synthetic phytosociological characters. Ecology 31(3):434–455
Darkoh MBK (2009) An overview of environmental issues in southern Africa. Afr J Ecol 47(Suppl. 1):93–98
Descheemaker K, Nyssen J, Rossi J, Poesen J, Mitiku H, Moeyerson J, Deckers J (2006) Sediment deposition and pedogenesis in exclosures in the Tigray Highlands, Ethiopia. Geoderma 132(3–4):291–314
Desta ZH, Oba G (2004) Feed scarcity and livestock mortality in enset farming systems in the Bale highlands of Southern Ethiopia. Outlook Agric 33:277–280
Dhaulkhandi M, Dobhal A, Bhatt S, Kumar M (2008) Community structure and regeneration potential of natural forest site in Gangotri, India. J Basic Appl Sci 4:49–52
Edwards S, Mesfin T, Sebsebe D, Hedberg I (eds) (2000) Flora of Ethiopia and Eritrea, Vol. 2. Part 1. The National Herbarium, Addis Ababa University, Addis Ababa and Department of Systematic Botany, Uppsala University, Uppsal
Emiru B, Demel T, Barklund P (2002) Actual and potential contributions of enclosure to enhance biodiversity in drylands of Eastern Tigray, with particular emphasis on woody plants. MSc Thesis, Swedish University, Sweden
Feyera S, Tadesse W, Sebsebe D, Denich M (2007) Floristic diversity and composition of Sheko forest, Southwest Ethiopia. Ethiopian J Biol Sci 6:11–42
Frank DA, Kuns MM, Guido DR (2002) Consumer control of grassland plant production. Ecology 83:602–606
Gebremedhin H (2000) A study on the ecology and management of the Dessa forest in the northeastern escarpment of Ethiopia. Dissertation, University of Addis Ababa.
Gebrewahd A (2014) Herbaceous vegetation restoration potential and soil physical condition in a mountain grazing land of Eastern Tigray, Ethiopia. JAEID 108(1):81–106
Gemedo D, Maass BL, Isselstein J (2006) Rangeland condition and trend in the semi-arid Borana lowlands, Southern Oromia, Ethiopia. Afr J Range For Sci 23(1):49–58
Getaneh B (2007) Floristic composition and structure in Beschillo and Abay (Blue Nile) riverine vegetation. Doctoral Dissertation. Addis Ababa University, Addis Ababa
Girma M, Animut G, Assefa G (2015) Chemical composition and in vitro organic matter digestibility of major indigenous fodder trees and shrubs in Northeastern drylands of Ethiopia. Livestock Res Rural Dev 27(2):26
Hedberg I, Ensermu K, Edwards S, Sebsebe D (eds) (2006) Flora of Ethiopia and Eritrea, Gentianaceae to Cyclocheilaceae, vol 5. The National Herbarium, Addis Ababa University and Uppsala University Department of Systematic Botany, Uppsala University, Sweden
Iqbal K, Pala NA, Bhat JA, Negi AK (2012) Regeneration status of trees around Khoh River in Garhwal Himalaya, India. Indian J For 35:471–476
Irl SD, Harter DE, Steinbauer MJ, Gallego Puyol D, Fernández-Palacios JM, Jentsch A, Beierkuhnlein C (2015) Climate vs. topography–spatial patterns of plant species diversity and endemism on a high-elevation island. J Ecol 103(6):1621–1633
John WK, Moses MO (2005) Use and availability of tree and shrub resources on Maasai communal rangelands near Amboseli, Kenya. Afr J Range For Sci 22(1):37–45
Karki U, Goodman MS (2009) Cattle distribution and behaviour in southern-pine silvopasture versus open-pasture. Agrofor Syst 78:159–168
Kebrom T (2001) Natural regeneration of degraded hill slopes in Southern Wello, Ethiopia: a study based on permanent plot. Appl Geogr 21:275–300
Kent M, Coker P (1992) Vegetation description and analysis: a practical approach. John Wiley and Sons, Chichester
Khumbongmayum AD, Khan ML, Tripathi RS (2006) Biodiversity conservation in sacred groves of Manipur, Northeast India: population structure and regeneration status of woody species. Biodivers Conserv 15:2439–2456
Kibret M (2008) Enclosure as a viable option for rehabilitation of degraded lands and biodiversity conservation: the case of Kallu Wereda, Southern Wello. MSc Thesis, Addis Ababa University, Addis Ababa
Kidane W, Tesfay A, Yemane N, Adehanom B, Hagos K, Ykaelo T, Hagos H, Dawit H, Giwergis A, Gebre H, Desalegn E (2016) Survey report on participatory agricultural production constraints appraisal in AGP-I districts of Sothern Tigray. Alamata Agricultural Research Center (unpublished).
Kindeya G (2003) Ecology and management of Boswellia papyrifera (Del.) Hochst. Dry forests in Tigray, Northern Ethiopia. Doctoral Dissertation. Georg-August University of Göttingen, Germany, p 182
Klein JA, Harte J, Zhao XQ (2007) Experimental warming, not grazing, decreases rangeland quality on the Tibetan plateau. Ecol Appl 17:541–557
Krebs CJ (1989) Ecological methodology. Harper and Row Cop, New York
Ma QQ, Chai LR, Hou FJ, Chang SH, Ma YS, Tsunekawa A, Cheng YX (2019) Quantifying grazing intensity using remote sensing in alpine meadows on Qinghai-Tibetan Plateau. Sustainability 11:417
Melaku S, Aregawi T, Nigatu L (2010) Chemical composition, in vitro dry matter digestibility and in sacco degradability of selected browse species used as animal feeds under semi-arid conditions in Northern Ethiopia. Agrofor Syst 80(2):173–184
Moura MR, Villalobos F, Costa GC, Garcia PC (2016) Disentangling the role of climate, topography and vegetation in species richness gradients. PLoS One 11(3):e0152468
Muluberhan H, Gufu O, Ayana A, Weladji RB (2006) The role of area enclosures and fallow age in the restoration of plant diversity in northern Ethiopia. Afr J Ecol 44:507–514
Moumita S, and Ashalata D (2014) Assessment of diversity, population structure and regeneration status of tree species in Hollongapar Gibbon Wildlife Sanctuary, Assam, Northeast India. Tropical plant research, 1(2): 26-36.
Nedessa B, Nyborg I, Ali J (2005) Exploring ecological and socio-economic issues for the improvement of area enclosure management: a case study from Ethiopia. DCG report
Petmak MV (1983) Primary productivity, nutrient cycling and OM turnover of tree plantation after agricultural intercropping practices in northeast Thailand. PhD Thesis, University of Philadelphia, Los Banos Philadelphia, p 228
Post E, Pedersen C (2008) Opposing plant community responses to warming with and without herbivores. Proc Natl Acad Sci U S A 105:12353–12358
Proulx M, Mazumder A (1998) Reversal of grazing impact on plant species richness in nutrient-poor vs. nutrient-rich ecosystems. Ecology 79:2581–2592
Raju J (2012) Comparative study of carbon assessment. A study in Kumvakarna Conservation Community Forest, Ghunsa, Lelep VDC, Taplejung District, Nepal. Doctoral Dissertation. Khwopa College, Bhaktapur
Ruggiero A, Hawkins BA (2008) Why do mountains support so many species of birds? Ecography 31:306–315
Sasaki T, Okayasu T, Jamsran U, Takeuchi K (2008) Threshold changes in vegetation along a grazing gradient in Mongolian rangelands. J Ecol 96:145–154
Scholes RJ, Biggs R (2005) A biodiversity intactness index. Nature 434:45–49
Sisay A, Baars R, Zinash S (2001) Herbaceous species composition, dry matter production and condition of the major grazing areas in the mid rift valley of Ethiopia. In: Proceedings of 9th annual conference of the Ethiopian Society of Animal Production (ESAP) held in Addis Ababa, Ethiopia, pp 267–283
Southern Zone BOARD (2018) Natural resources core process, annual report. Southern Zone of Office of Agriculture and Rural Development, Unpublished, Tigrigna version
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17(7):866–880
Suizi W, Jiangwen F, Yuzhe L, Lin H (2019) Effects of grazing exclusion on biomass growth and species diversity among various grassland types of the Tibetan Plateau. Sustainability 11:1705
Taye B, Getachew B, Elias T, Matheos E, Kumlachew Y (2002) Regeneration status of moist montane forests of Yayu Ethiopia: conservation, part I: Boginda, Bonga, Masha - Anderacha and forests. Walya 22:45–60
Tefera J, Minyishaw E, Kebenu F, Nurhusen A, Gufran M (2015) Woody species diversity, management and contribution of local community in protected forest of Kuneba District, Afar Region Ethiopia. Afr J Environ Sci Technol 8(4):172–179
Teferi A, Solomon M, Lisanework N (2008) Management and utilization of browse species as livestock feed in semi-arid district of North Ethiopia. Livestock Res Rural Dev 20(6):86
Tekle K (2001) Natural regeneration of degraded hill slopes in Southern Wello, Ethiopia: a study based on permanent plots. Appl Geogr 21:275–300
Tekle K, Bekele T (2000) The role of soil seed banks in the rehabilitation of degraded hill slopes in Southern Wello, Ethiopia. Biotropica 32:23–32
Tesfay A, Anteneh BD, Tessema Z (2019) Woody species diversity, population structure, and regeneration status in the Gra-Kahsu natural vegetation, Southern Tigray of Ethiopia. Heliyon 5(1):e01120
Teshome A, Abule E, Lisanework N (2009) Pastoralist’s perceptions and rangeland evaluation for livestock production in South Eastern Ethiopia. Livestock Res Rural Dev 21:7
Teshome G, Teshome S, Ensermu K (2015) Structure and regeneration of Gendo moist montane forest, East Wellega Zone, Western Ethiopia. J Environ Earth Sci 5:15
Tessema Z, De Boer W, Baars R, Prins H (2011) Changes in vegetation structure, herbaceous biomass and soil nutrients in response to grazing in semi-arid savannas in Ethiopia. J Arid Environ 75:662–670
The R Core Team (2018) A language and environment for statistical computing, version 3.3.3. R foundation for Statistical Computing
Tiwari K, Tadele K, Aramde F, Tiwari C (2010) Community structure and regeneration potential of Shorea robusta forest in sub-tropical submontane zone of Garhwal Himalaya, India. Nat Sci 8:70–74
Tsegay G, Tessema ZK, Negasi S, Emiru B (2019) Carbon sequestration and soil restoration potential of grazing lands under exclosure management in a semi-arid environment of northern Ethiopia. Ecol Evol 9:6468–6479
Tefera M, Demel T, Hulten H, and Yonas Y (2005) The role of enclosure in the recovery of woody vegetation in degraded dry land hillsides of central and northern Ethiopia. Journal of Arid Environ, 60(2): 259-281.
Verdoodt A, Mureithi SM, Van Ranst E (2010) Impacts of management and enclosure age on recovery of the herbaceous rangeland vegetation in semi-arid Kenya. J Arid Environ 74:1066–1073
Weldemariam B, Gebremichael D (2015) Identification and determination of chemical composition of some potential browses as livestock fodder in the Central and North Western zones of Tigray, Ethiopia. Livestock Res Rural Dev 27(4):75
Wesche K, Ronnenberg K, Retzer V, Miehe G (2010) Effects of large herbivore exclusion on Southern Mongolian desert steppes. Acta Oecol 36:234–241
Witt GB, Noël MV, Bird MI, Beeton RJS, Menzies NW (2011) Carbon sequestration and biodiversity restoration potential of semi-arid Mulga lands of Australia interpreted from long-term grazing exclosures. Agric Ecosyst Environ 141:108–118
Wolde M, Aynekulu E (2011) Enclosure land management for restoration of the soils in degraded communal grazing lands in northern Ethiopia. Land Degrad Dev 24:528–538
Wolde M, Mastewal Y (2013) Changes in woody species composition following establishing exclosures on grazing lands in the lowlands of Northern Ethiopia. Afr J Environ 7(1):30–40
Wolde M, Veldkamp E, Corre MD (2011b) Restoration of ecosystem carbon stocks following enclosure establishment in communal grazing lands in Tigray, Ethiopia. Soil Sci Soc Am J 75(1):246–256
Wolde M, Veldkamp E, Haile M, Nyssen J, Muys B, Gebrehiwot K (2007) Effectiveness of exclosures to restore degraded soils as a result of overgrazing in Tigray, Ethiopia. J Arid Environ 69(2):270–284
Wolde M, Veldkamp E, Mesfin T, Olschewski R (2011c) Economic valuation of land restoration: the case of exclosures established on communal grazing lands in Tigray, Ethiopia. Land Degrad Dev 22:334–344
Wondie M, Eyayu M, Temesgen G (2014) A comparative study of woody plant species diversity at Adey Amba enclosed forest and nearby open site in West Belessa district, northwestern Ethiopia. JBAH 4:15
Yayneshet T (2011) Restoration of degraded semi-arid communal grazing land vegetation using the enclosure model. Mekelle University, Mekelle, Ethiopia. Int J Water Res Environ 1(5):382–386
Yayneshet T, Eik LO, Moe SR (2009) The effects of exclosures in restoring degraded semi-arid vegetation in communal grazing lands in northern Ethiopia. J Arid Environ 73:542–549
Zerihun W, Dragan M, Feoli E, Fernetti M (2002) Reducing soil erosion in Northern Ethiopia, Adwa Zone, through a special decision support system (SDSS). Ethiopian J Biol Sci 1(1):1–12
Acknowledgements
We would like to acknowledge the Tigray Agricultural Research Institute for financial support to do the research. We would also like to thank the local community of the study area.
Funding
No funding was received
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of the concept and implementation of the study. TA, NG, TGe, and TGi carried out field data collection and data analysis and drafted the manuscript. All authors revised the manuscript and read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Table S1. Density (D), basal area (BA), biomass (kg/ha) and regeneration status of browse plant species of area exclosure in the south Tigray. Table S2. Density (D), basal area (BA), biomass (kg/ha) and regeneration status of browse plant species of communal grazing land in the south Tigray.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Atsbha, T., Wayu, S., Gebretsadkan, N. et al. Rehabilitation of indigenous browse plant species following exclosure established on communal grazing lands in South Tigray, Ethiopia, and implication for conservation. Ecol Process 8, 43 (2019). https://doi.org/10.1186/s13717-019-0197-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13717-019-0197-1