Abstract
Background
The objective of this study was to evaluate the ability of endothelial biomarkers to early predict clinical deterioration of patients admitted to the emergency department (ED) with a suspected sepsis. This was a prospective, multicentre, international study conducted in EDs. Adult patients with suspected acute bacterial infection and sepsis were enrolled but only those with confirmed infection were analysed. The kinetics of biomarkers and organ dysfunction were collected at T0, T6 and T24 hours after ED admission to assess prognostic performances of sVEGFR2, suPAR and procalcitonin (PCT). The primary outcome was the deterioration within 72 h and was defined as a composite of relevant outcomes such as death, intensive care unit admission and/or SOFA score increase validated by an independent adjudication committee.
Results
After adjudication of 602 patients, 462 were analysed including 124 who deteriorated (27%). On admission, those who deteriorated were significantly older (73 [60–82] vs 63 [45–78] y-o, p < 0.001) and presented significantly higher SOFA scores (2.15 ± 1.61 vs 1.56 ± 1.40, p = 0.003). At T0, sVEGFR2 (5794 [5026–6788] vs 6681 [5516–8059], p < 0.0001), suPAR (6.04 [4.42–8.85] vs 4.68 [3.50–6.43], p < 0.0001) and PCT (7.8 ± 25.0 vs 5.4 ± 17.9 ng/mL, p = 0.001) were associated with clinical deterioration. In multivariate analysis, low sVEGFR2 expression and high suPAR and PCT levels were significantly associated with early deterioration, independently of confounding parameters (sVEGFR2, OR = 1.53 [1.07–2.23], p < 0.001; suPAR, OR = 1.57 [1.21–2.07], p = 0.003; PCT, OR = 1.10 [1.04–1.17], p = 0.0019). Combination of sVEGFR2 and suPAR had the best prognostic performance (AUC = 0.7 [0.65–0.75]) compared to clinical or biological variables.
Conclusions
sVEGFR2, either alone or combined with suPAR, seems of interest to predict deterioration of patients with suspected bacterial acute infection upon ED admission and could help front-line physicians in the triage process.
Similar content being viewed by others
Background
Sepsis is a major health burden with considerable economic consequences [1]. Recently, epidemiological studies performed on large cohorts reported that sepsis was present in 6% of adult hospitalizations [2]. Over the last decade, a decrease in the mortality rate has been observed [3] in particular thanks to improved management, and more appropriate intervention approaches in the emergency department (ED) [4]. Although the recently proposed qSOFA score [5] aims to help front-line clinicians detecting severe patients with a higher risk of mortality [6], it fails to get decisive support for discharge decision, especially in patients without initial organ dysfunction [7, 8], that could help to reduce ED crowding and cost.
Even if widely used as an infection biomarker and diagnostic of severity, procalcitonin (PCT) has not been fully validated for deterioration assessment, and no other biological marker has yet been validated to accurately early predict clinical deterioration in unselected patients admitted to the ED with infection or sepsis [9,10,11]. Asymptomatic endothelial injury participates in the development of organ failure with poor outcome [12, 13]. Endothelial biomarkers have been presented as predictors of death and/or organ dysfunction during sepsis [14,15,16,17,18,19,20,21,22]. Of those, soluble vascular endothelial growth factor receptors 2 (sVEGFR2, growth factor for vascular endothelial cells) and soluble urokinase plasminogen activator receptor (suPAR, pro-inflammatory activation of the immune system) were proposed. VEGFR2, which is selectively expressed in the endothelium, mediates endothelial growth, proliferation and permeability and pathological angiogenesis, and bound to VEGF increases microvascular permeability resulting in oedema and hypotension [23]. The uPAR receptor is expressed on different cell types including vascular endothelial cells [24]. After cleavage from the cell surface, the soluble receptor, suPAR, can be found in the blood and other organic fluids. Increased activation of the immune system caused by different types of infections results in increased suPAR concentrations. These biomarkers have been shown to be associated with initial severity and subsequent clinical worsening [25,26,27,28,29,30,31,32], but their ability to early predict deterioration on ED admission remains to be determined.
This study aimed to evaluate the ability of sVEGFR2 and suPAR biomarkers to early predict the clinical deterioration of patients with infection upon ED admission and compare them to conventional clinical and biological parameters (qSOFA and SOFA score, lactates, PCT, CRP). Second, we assessed the prognostic performance of biomarkers according to the presence of sepsis or not in accordance with the new definitions of Sepsis-3.
Methods
Population
We conducted a prospective, multicentre, international study in 14 EDs from 2015 to 2018. Inclusion criteria included adult patients (age ≥ 18 years) with an acute suspected bacterial community-acquired infection (≤ 3 days), evolution time window being checked with the patient and/or relatives, associated with at least two systemic inflammatory response syndrome (SIRS) criteria [33], which were currently the most sensitive criteria for sepsis [34, 35]. All patients admitted to the ED with a suspected infection, based on fever and/or any other infectious symptom reported by referral practitioner were screened 24/7 by emergency physicians for eligibility and treated following the Surviving Sepsis Campaign guidelines [36]. Exclusion criteria were patients with septic shock (based on ACCP/SCCM criteria), patients with a healthcare-associated infection, immunosuppression (e.g. human immunodeficiency virus (HIV), transplant, ongoing chemotherapy, steroid treatment > 20 mg/day of prednisone or equivalent for more than a week), non-infectious diseases potentially associated with SIRS (cancer), patients with a prior episode of infection within the 30 days before ED admission and onset of symptoms greater than 72 h and absence of consent. The protocol was recorded on ClinicalTrials.gov (N°: NCT02739152), approved by the Ethics Committee for Clinical Research (CPP SOOM IV: CPP15-004).
Endpoints
The primary endpoint was the occurrence of early clinical deterioration within 72 h following ED admission. Deterioration was determined by an independent adjudication committee (including one experienced emergency physician and two intensive care physicians) who were blinded to biomarker results, and followed a pre-defined adjudication charter. Patients were then classified according to their initial course during the first 72 h of hospitalization, as exhibiting an early deterioration defined by a composite endpoint (increase SOFA score of at least 1 point, or ICU admission directly related to the initial infectious disease because of documented sustained hypotension requiring vasopressors or ventilation support requirement, or death) or not. The same adjudication committee also confirmed the bacterial origin of infection according to available clinical, biological and microbiological data and based on pre-defined criteria for every different type of infection [37]. Patients without confirmed infection were excluded from the analysis.
Study design and measured variables
All the patients were included and received their first care and blood collection in ED. Clinical criteria, biological data, lactates [38], qSOFA score, SOFA score [39] and studied biomarkers were measured at three time points: the first within emergency room (T0) and the others at 6 ± 2 h (T6) and 24 ± 2 h (T24) after ED admission. The following data were prospectively collected by the study team blinded to biomarker results during ED stage: demographics, Charlson score, site of infection, antimicrobial therapy and initiation time, traditional biological parameters (leukocytes, CRP, platelets) and orientation after ED discharge. Pathogens, length of stay and mortality at day 28 were collected during hospitalization or at the end of follow-up.
Biomarker measurements
Serum sVEGFR2 concentrations (soluble vascular endothelial growth factor receptors 2) were measured using the enzyme-linked fluorescent assay (ELFA) technique. The results were automatically analysed by VIDAS® and expressed in relative fluorescence intensity or RFV (relative fluorescent value). Plasma suPAR (soluble urokinase plasminogen activator receptor) levels were analysed using the commercially available CE/IVD-labelled suPARnostic® AUTO Flex ELISA kit, according to the manufacturer’s instructions (Virogates, Birkeroed, Denmark). For sUPAR ELISA test, the inter-assay coefficient of variation (CV) given by the manufacturer is below 6%. For sVEGFR2, the inter-assay coefficient of variation was calculated at 3.09%. Serum PCT (procalcitonin) levels were measured using VIDAS BRAHMS PCT assay (Biomerieux, Marcy l’Etoile, France) according to the manufacturer’s instructions.
Analysis
The prognostic performance of studied biomarkers was evaluated in the entire cohort at T0. Following the current definitions and to analyse the prognostic performance of biomarkers according to the severity, the Sepsis-3 criteria were applied to define 2 groups: infected patients (SOFA score < 2) and septic patients (SOFA score ≥ 2) [5]. A model of risk of early deterioration based on the value of biomarkers on ED admission was proposed on non-septic patients. Data were censored after deterioration. No patient was lost to follow-up until T24.
Statistics
Data are presented either as means ± SD, median with interquartile range or as box and whisker plots with representation of the median value, 25th, 75th and 90th percentiles, and outliers. Parameters and biomarkers were compared between the two groups of patients according to their initial course (i.e., early deterioration or not), using the nonparametric Mann–Whitney U test for continuous variables, while categorical variables were compared with the Pearson χ2 test or the Fisher’s exact test when appropriate. The level of significance was set at 5% and results of regression analyses were presented with their 95% CI. All analyses were computed using the R version 3.4.0.
Logistic regressions were fit using single or both biomarkers. Association with clinical variables was independently evaluated, and clinical parameters with a p value below 0.1 in the univariate analyses were selected as adjustment covariates for multivariate analyses. Among significant clinical parameters, a selection was made to avoid collinearity and limit the number of variables introduced in multivariate models. Strength of association was reported using inter-quartile range (IQR) adjusted odds ratios (OR). Areas under the ROC curve and their 95% confidence interval (CI) were computed and compared using the DeLong’s method. Predictive performances were calculated/evaluated under constraint of a sensitivity higher than 90% (rule-out test). In a complementary approach, a decision tree was built. The thresholds used to partition the data were chosen to optimize sensitivity (> 90%).
Results
Study population
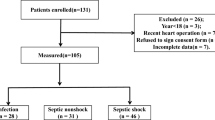
Of 602 patients enrolled in the 14 participating EDs between 2015 and 2018, 28 patients were secondarily excluded due to the presence of concomitant cancer potentially responsible for SIRS. Independent adjudication was performed in 574 patients, out of whom 112 patients were classified without bacterial infection nor confirmed infection (10%). Finally, 462 patients with a bacterial acute community-acquired infection were kept for the analysis (Fig. 1a). Baseline characteristics are described in Table 1. Infections were mainly of pulmonary (29.2%), urinary (27.5%) and abdominopelvic (25.3%) origin. Microorganisms were isolated from 51% of patients (cocci = 13%, bacilli = 34% and both = 4%). At baseline, the median lactatemia was 1.7 [1.2–2.4] mmol/L, median leukocyte was 14.6 [11,12,13,14,15,16,17,18] G/L and median CRP was 115 [43–219] mg/L (Table 1). Median time of antibiotic treatment was 4.1 h [2.3–6.7]. After ED management, 87% of patients were hospitalized in a conventional ward while the remaining 13% were admitted to the ICU. Median hospital stay was 5 [3,4,5,6,7,8,9] days and 28-day mortality was 2.6% (Table 1). Mean SOFA score was 1.72 ± 1.48. Based on the new Sepsis-3 definition, 229 patients (49.6%) had sepsis (SOFA ≥ 2 points) while the remaining 233 patients (50.4%) had infection (SOFA < 2 points) (Fig. 1b).
Study flowchart. a A total of 602 patients admitted to the emergency department and suspected of bacterial infection were enrolled. After adjudication, 462 patients were included in the analysis. b Infected non-septic patients according to Sepsis-3 definition; SOFA under 2 point at admission to the emergency department
Deterioration group
Among the 462 analysed patients, 124 patients (27%) were assigned to the “deterioration” group by the adjudication committee. Deterioration occurred within the first 6 h of inclusion for 64% of them. Thirty-nine patients progressed to severe sepsis and 11 patients to septic shock [32]. When compared with the “no deterioration” group (n = 338 patients, 73%), patients in the “deterioration” group were older (73 [60–82] vs 63 [45–78] years, p < 0.001), had a higher Charlson score (4 [2.75–5] vs 2 [0–5], p < 0.001), more frequent pulmonary infections (37.1 vs 26.3%, p = 0.049) and greater SOFA score (2.15 ± 1.61 vs 1.56 ± 1.40, p < 0.001). Patients with early deterioration were more frequently admitted to ICU (13 vs 3%, p < 0.001) and had a higher length of stay (8 [6,7,8,9,10,11,12,13] vs 5 [3,4,5,6,7] days, p < 02.001). In contrast, there was no statistical difference between the two groups on SIRS criteria count (3.0 ± 0.7 vs 2.9 ± 0.7, p = 0.073), lactates (2.04 ± 1.35 vs 2.09 ± 1.41 mmol/L, p = 0.999), CRP level (160 ± 127 vs 141 ± 126 mg/L, p = 0.087) and time to antibiotic treatment initiation (5.3 ± 4.8 vs 6.1 ± 7.5 h, p = 0.675) (Table 1). Interestingly, we did not find any difference between the groups “deterioration” and “no deterioration” regarding initial adequate antibacterial treatment (20 vs 15%, p = 0.246).
Biomarkers: predictive performances in the global cohort
At T0 in univariate analysis, the age, the Charlson score, the qSOFA score and the SOFA score were associated with the early clinical deterioration, but not traditional biological markers. On the global cohort, sVEGFR2 and suPAR level were, respectively, 6402 [5386–7715] ng/mL and 5.00 [3.67–6.86] ng/mL. Low sVEGFR2 level (5794 [5026–6788] vs 6681 [5516–8059], p < 0.0001), high circulating suPAR (6.04 [4.42–8.85] vs 4.68 [3.50–6.43], p < 0.0001) and high PCT level (7.8 ± 25.0 vs 5.4 ± 17.9 ng/mL, p = 0.001) were associated with early clinical deterioration (Table 2). In multivariate logistic regression analyses, only low sVEGFR2 expression, high suPAR and PCT levels were significantly associated with deterioration (sVEGFR2: OR [95% CI] = 1.53 [1.07–2.23], p < 0.001; suPAR: OR = 1.57 [1.21–2.07], p = 0.003 and PCT: OR = 1.10 [1.04–1.17], p = 0.0019), independently of covariates (Charlson, qSOFA, age, SOFA). The sVEGFR2 and sUPAR combination showed the higher predictive performances compared to other variable (AUC = 0.70 [0.65–0.75]) (Table 2). We also observed differences regarding site of infections, with the best performances found in abdominal infections (AUC = 0.81 [0.73–0.89], NPV 93%), then urinary (AUC = 0.7 [0.59–0.80], NPV = 95%) and finally pulmonary (AUC = 0.66 [0.56–0.76], NPV = 79%).
Biomarker association with clinical deterioration was also observed in secondary excluded patients (Additional file 1: Figure 1).
Levels of sVEGFR2, PCT and suPAR at inclusion were significantly associated with the degree of organ dysfunction, as reflected by the SOFA score on ED admission (Fig. 2).
Biomarkers: predictive performances in infected patients without sepsis (SOFA < 2)
Among the 233 patients considered with infection but no sepsis at enrolment (SOFA < 2 at admission), 48 of them (21%) deteriorated within 72 h (Fig. 1b). The clinical characteristics of this derivative cohort at admission were not different from those of the overall cohort (Table 3). In univariate logistic regression analyses, age and Charlson score were significantly associated with deterioration. Low sVEGFR2 expression and high suPAR level, either taken alone or combined, were also significantly associated with deterioration (sVEGFR2, IQR OR [95% CI] = 2.76 [1.68–4.75], p = 0.0002; suPAR IQR OR = 1.49 [1.06–2.10], p = 0.022 and combination IQR OR = 1.74 [1.28–2.41], p = 0.0005). In multivariate analysis, only sVEGFR2 alone or combined with suPAR remained significantly associated with deterioration (IQR OR [95% CI] = 2.35 [1.41–4.12], p = 0.0017 and IQR = 1.52 [1.07–2.19], p = 0.019, respectively) (Table 4). At T6, only sVEGFR2 and sUPAR were found significantly associated with worsening (p < 0.01) (Additional file 1: Figure 2b.). As data were censored after deterioration, the low number of patients deteriorating after T6 and T24 did not allow further analysis. The prognostic value of biomarkers on septic patients (SOFA ≥ 2) is presented in Additional file 1: Table 1.
Proposal of a stratification model in non-severe patients on ED admission
The best prognostic model including sVEGFR2 and suPAR combination and using cut-off values optimized to yield a high sensitivity allowed identifying distinct levels of risk (i.e. low and high) for deterioration. When comparing risk groups, we found that the low-risk group had a 15-fold lower risk of worsening than the high-risk group (OR = 14.50 [4.97–61.85]; p < 0.0001) (Additional file 1: Figure 3). This model proved a modest clinical deterioration performance (AUC = 0.73 [0.66–0.80]; p < 0.0001) with a promising negative predicting value (96%). The AUC of this model was significantly higher than that of lactate (AUC = 0.49 [0.39–0.60]; p > 0.05), qSOFA score (AUC = 0.54 [0.46–0.62]; p > 0.05), CRP (AUC = 0.61 [0.52–0.70]; p < 0.05), and PCT (AUC = 0.62 [0.53–0.70]; p < 0.05) (Fig. 3).
Comparison of predictive performance of biomarkers measured at inclusion (T0) on non-severe-infected patients. Sensitivity, specificity and predictive values of biomarkers according to optimized cut-offs to minimal sensitivity of 90%. Abbreviations: AUC [IC] area under the curve and confidence interval (two-sided p value < 0.05), se sensitivity (%), sp specificity (%), NPV negative predictive value (%), PPV positive predictive value (%)
Discussion
In this ED-based multicentre study, the endothelial biomarker sVEGFR2, used either alone or combined with suPAR, proved the best early predictor of patient deterioration, independently of potential confounders. High medical value biomarkers are those that are able to predict outcomes even before any clinical evidence of deterioration to help front-line physicians to better anticipate the complicated course. Since early prediction of patient deterioration is crucial to allow safe rule-out, over-triage reduction and better allocation of hospital resources, the high negative value of these potential endothelial biomarkers appears particularly useful in the ED settings with inherent peaks of activity and overcrowded units.
In the present study, 27% of the entire cohort deteriorated within 72 h of ED admission. Importantly, 21% of non-sepsis patients, without any severity criteria on ED admission, did deteriorate within the first 72 h of hospitalization. Intrinsically, these patients presented to the ED with low SOFA (i.e., SOFA < 2) and non-qualifying qSOFA score. This proportion is similar to that reported in previous studies [18,19,20]. About 20 to 25% of patients progressed to severe sepsis whereas they had no sign of seriousness at first medical contact [7]. Saeed et al. reported that early clinical deterioration occurred in more than 16% of patients presenting to the ED with sepsis, even when patients were non-severe with low lactate level (< 2 mmol/L) or low clinical score (qSOFA < 2) [40]. Recently, Cleek et al. confirmed that in predicting 28-day in-hospital mortality among infected ED patients, qSOFA did not outperform or improve physician judgment [41]. Overall, the clinical deterioration occurred very early after ED admission since two-third of the patients deteriorated within the first 6 h of inclusion. Although information on the delay between ED admission and deterioration are scare, some authors have reported that it may occur within 48 h [42], even within the first 12 h following ED arrival [43].
Due to various presentations of infected patients on ED admission, determining the severity early in the disease course remains challenging since clinical scoring systems have limited prognostic accuracy [44, 45]. Many conventional biomarkers reflecting end-organ compromise are not informative until significant clinical deterioration has occurred [46, 47]. The Sepsis-3 definition underlines organ dysfunction as the mainstay of sepsis and the value of the SOFA score to identify patients with a higher risk of subsequent death [48]. However, 21% of our patients with a SOFA score < 2, i.e. non-sepsis according to Sepsis-3, deteriorated within 72 h after ED arrival. In these circumstances, assessment of endothelial injury could be a good predictor of deterioration [49]. Likewise Fang et al. [20] described a relationship between endothelial biomarkers and variations of the SOFA score during the first week after admission. Liu et al. [50] also showed an association between the presence of endothelial injury on admission and severity of sepsis. More recently, Henning et al. also confirmed that biomarkers of endothelial activation and inflammation in combination with emergency department physician judgment improved prediction in hospital mortality [51, 52]. These observations are concordant with our findings showing that the level of sVEGFR2 and suPAR is associated with that of the SOFA score. Importantly, we have shown that sVEGFR2 alone or combined with suPAR is the best predictor of patient deterioration, independently of potential confounding factors. If confirmed, this result could allow safe rule out of patients who have low risk of deterioration, hence leading to a decrease in hospital admissions.
This prospective, multicentre, international, observational study presents several strengths, such as (i) a biological collection of biomarkers combining with the evolving clinical criteria/in line with the requirements of the new Sepsis-3 definition of sepsis, and (ii) the appointment and careful evaluation by an independent adjudication committee. We also demonstrated that circulating markers of endothelial activation, at the earliest time in ED, have a potential of risk stratification and could help emergency physicians better manage patients with sepsis.
Our study, however, has several limitations, the first one being the limited possibility to fully investigate the heterogeneity of the different subtypes of infections. Indeed, patients with pneumonia may differ from patients with abdo-pelvic infections. The population may be biased against deterioration, as it requires decompensation from a less ill state. Half of the cohort having a SOFA ≥ 2 at ED baseline may have been already quite ill. Therefore, the study may have been stronger if focused on a light-/middle-severity sepsis cohort, using new definition of sepsis if it had been available when designing the study. Also, the design and purpose of the study did not allow analysis of patients with septic shock, while they could have been used as a control group of severity. In addition, as the number of patients with non-confirmed or viral infection was low, no prediction analysis was done. The entire analysis has somewhere a modest sample size (n = 462 patients, of which 127 experienced deterioration) and do not support strong conclusions but serve as a robust early basis for future validation. Finally, we did not perform health economics and outcome research that could have brought useful information on the potential cost savings for hospitals.
Conclusion
The current findings highlight the potential interest of the sVEGFR2 protein, alone or in combination with suPAR, to diagnose initial endothelium stress and to predict/anticipate subsequent organ dysfunction. Such tool, suitable for routine test measurement, with time-to-results within 1 h and only one-time measurement required, could be used together with other laboratory findings and clinical assessments, to help in early prediction of the risk of deterioration and safely ruling out infected patients after ED admission.
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- ED:
-
Emergency department
- ELFA:
-
Enzyme-linked fluorescent assay
- HIV:
-
Human immunodeficiency virus
- ICU:
-
Intensive care unit
- IQR:
-
Inter-quartile range
- OR:
-
Odds ratio
- PCT:
-
Procalcitonin
- RFV:
-
Relative fluorescent value
- SD:
-
Standard deviation
- SIRS:
-
Systemic inflammatory response syndrome
- SOFA:
-
Sequential organ failure assessment
- sUPAR:
-
Soluble urokinase plasminogen activator receptor
- sVEGFR2:
-
Soluble vascular endothelial growth factor receptors 2
References
Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet Lond Engl. 2018;392:75–877.
Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–9.
Vincent J-L, Lefrant J-Y, Kotfis K, Nanchal R, Martin-Loeches I, Wittebole X, et al. Comparison of European ICU patients in 2012 (ICON) versus 2002 (SOAP). Intensive Care Med. 2018;44:337–44.
Miller RR, Dong L, Nelson NC, Brown SM, Kuttler KG, Probst DR, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188:77–82.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–10.
Freund Y, Lemachatti N, Krastinova E, Van Laer M, Claessens Y-E, Avondo A, et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the Emergency Department. JAMA. 2017;317:301–8.
Glickman SW, Cairns CB, Otero RM, Woods CW, Tsalik EL, Langley RJ, et al. Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad Emerg Med Off J Soc Acad Emerg Med. 2010;17:383–90.
Holder AL, Gupta N, Lulaj E, Furgiuele M, Hidalgo I, Jones MP, et al. Predictors of early progression to severe sepsis or shock among emergency department patients with nonsevere sepsis. Int J Emerg Med. 2016;9:10.
Filbin MR, Lynch J, Gillingham TD, Thorsen JE, Pasakarnis CL, Nepal S, et al. Presenting symptoms independently predict mortality in septic shock: importance of a previously unmeasured confounder. Crit Care Med. 2018;46:1592–9.
Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–2.
Almansa R, Martín S, Martin-Fernandez M, Heredia-Rodríguez M, Gómez-Sánchez E, Aragón M, et al. Combined quantification of procalcitonin and HLA-DR improves sepsis detection in surgical patients. Sci Rep. 2018;8(1):11999.
Xing K, Murthy S, Liles WC, Singh JM. Clinical utility of biomarkers of endothelial activation in sepsis–a systematic review. Crit Care Lond Engl. 2012;16:R7.
Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–45.
Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, et al. Biomarkers of endothelial cell activation in early sepsis. Shock Augusta Ga. 2013;39:427–32.
Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, et al. The endothelium in sepsis. Shock. 2016;45:259–70.
Amalakuhan B, Habib SA, Mangat M, Reyes LF, Rodriguez AH, Hinojosa CA, et al. Endothelial adhesion molecules and multiple organ failure in patients with severe sepsis. Cytokine. 2016;88:267–73.
Vassiliou AG, Mastora Z, Orfanos SE, Jahaj E, Maniatis NA, Koutsoukou A, et al. Elevated biomarkers of endothelial dysfunction/activation at ICU admission are associated with sepsis development. Cytokine. 2014;69:240–7.
de Pablo R, Monserrat J, Reyes E, Díaz D, Rodríguez-Zapata M, de la Hera A, et al. Circulating sICAM-1 and sE-Selectin as biomarker of infection and prognosis in patients with systemic inflammatory response syndrome. Eur J Intern Med. 2013;24:132–8.
Pauly D, Hamed S, Behnes M, Lepiorz D, Lang S, Akin I, et al. Endothelial cell-specific molecule-1/endocan: Diagnostic and prognostic value in patients suffering from severe sepsis and septic shock. J Crit Care. 2016;31:68–75.
Fang Y, Li C, Shao R, Yu H, Zhang Q. The role of biomarkers of endothelial activation in predicting morbidity and mortality in patients with severe sepsis and septic shock in intensive care: a prospective observational study. Thromb Res. 2018;171:149–54.
Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–77.
Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care Lond Engl. 2019;23:16.
Yang K-Y, Liu K-T, Chen Y-C, Chen C-S, Lee Y-C, Perng R-P, et al. Plasma soluble vascular endothelial growth factor receptor-1 levels predict outcomes of pneumonia-related septic shock patients: a prospective observational study. Crit Care Lond Engl. 2011;15:R11.
Donadello K, Scolletta S, Covajes C, Vincent J-L. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012;10:2.
Aslan A, van Meurs M, Moser J, Popa ER, Jongman RM, Zwiers PJ, et al. Organ-specific differences in endothelial permeability-regulating molecular responses in mouse and human sepsis. Shock. 2017;48:69–77.
Mutunga M, Fulton B, Bullock R, Batchelor A, Gascoigne A, Gillespie JI, et al. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med. 2001;163:195–200.
Ni W, Han Y, Zhao J, Cui J, Wang K, Wang R, et al. Serum soluble urokinase-type plasminogen activator receptor as a biological marker of bacterial infection in adults: a systematic review and meta-analysis. Sci Rep. 2016;6:39481.
Zeng M, Chang M, Zheng H, Li B, Chen Y, He W, et al. Clinical value of soluble urokinase-type plasminogen activator receptor in the diagnosis, prognosis, and therapeutic guidance of sepsis. Am J Emerg Med. 2016;34:375–80.
Rasmussen LJH, Ladelund S, Haupt TH, Ellekilde G, Poulsen JH, Iversen K, et al. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: a strong marker of disease presence and severity, readmission and mortality. A retrospective cohort study. Emerg Med J. 2016;33:769–75.
Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38:1418–28.
Pal E, Korva M, Resman Rus K, Kejžar N, Bogovič P, Strle F, et al. Relationship between circulating vascular endothelial growth factor and its soluble receptor in patients with hemorrhagic fever with renal syndrome. Emerg Microbes Infect. 2018;7:89.
Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, et al. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic Fever. J Virol. 2007;81:1592–600.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55.
Serafim R, Gomes JA, Salluh J, Póvoa P. A comparison of the quick-SOFA and systemic inflammatory response syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153:646–55.
Prasad PA, Fang MC, Abe-Jones Y, Calfee CS, Matthay MA, Kangelaris KN. Time to recognition of sepsis in the Emergency Department using electronic health record data: a comparative analysis of systemic inflammatory response syndrome, sequential organ failure assessment, and quick sequential organ failure assessment. Crit Care Med. 2020;48:200–9.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77.
Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;67:e1–94.
Singer AJ, Taylor M, Domingo A, Ghazipura S, Khorasonchi A, Thode HC, et al. Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Acad Emerg Med Off J Soc Acad Emerg Med. 2014;21:853–7.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Saeed K, Wilson DC, Bloos F, Schuetz P, van der Does Y, Melander O, et al. The early identification of disease progression in patients with suspected infection presenting to the emergency department: a multi-centre derivation and validation study. Crit Care Lond Engl. 2019;23:40.
Cleek WR, Johnson NJ, Watsjold BK, Hall MK, Henning DJ. Comparing mortality prediction by quick sequential organ failure assessment with Emergency physician judgment. Shock. 2019;54:213–7.
Capp R, Horton CL, Takhar SS, Ginde AA, Peak DA, Zane R, et al. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med. 2015;43:983–8.
Linder A, Arnold R, Boyd JH, Zindovic M, Zindovic I, Lange A, et al. Heparin-binding protein measurement improves the prediction of severe infection with organ dysfunction in the Emergency Department. Crit Care Med. 2015;43:2378–86.
Song J-U, Sin CK, Park HK, Shim SR, Lee J. Performance of the quick Sequential (sepsis-related) Organ Failure Assessment score as a prognostic tool in infected patients outside the intensive care unit: a systematic review and meta-analysis. Crit Care Lond Engl. 2018;22:28.
Tusgul S, Carron P-N, Yersin B, Calandra T, Dami F. Low sensitivity of qSOFA, SIRS criteria and sepsis definition to identify infected patients at risk of complication in the prehospital setting and at the emergency department triage. Scand J Trauma Resusc Emerg Med. 2017;25:108.
Contenti J, Occelli C, Lemoel F, Ferrari P, Levraut J. Blood lactate measurement within the emergency department: a two-year retrospective analysis. Am J Emerg Med. 2019;37:401–6.
Hohenstein C, Bernhardt M. Elevated serum lactate in emergency department patients predicts hospital admission unrelated to diagnosis—but not more. Biomark Biochem Indic Expo Response Susceptibility Chem. 2018;23:88–96.
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:775–87.
Coletta C, Módis K, Oláh G, Brunyánszki A, Herzig DS, Sherwood ER, et al. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Crit Care Lond Engl. 2014;18:511.
Liu X-W, Ma T, Liu W, Cai Q, Wang L, Song H-W, et al. Sustained increase in angiopoietin-2, heparin-binding protein, and procalcitonin is associated with severe sepsis. J Crit Care. 2018;45:14–9.
Henning DJ, Bhatraju PK, Johnson NJ, Kosamo S, Shapiro NI, Zelnick LR, et al. Physician judgment and circulating biomarkers predict 28-day mortality in Emergency Department patients. Crit Care Med. 2019;47:1513–21.
Simpson SQ. Sepsis biomarkers and physician judgment in the emergency room. Crit Care Med. 2019;47:1656–7.
Acknowledgements
Members of the TRIAGE study group:
bioMérieux SA, Marcy l’Etoile, France: Julien Textoris, Karine Kaiser and Laurence Barbier.
University Hospital Edouard Herriot—HCL, Lyon, France: Laurent Jacquin, Marion Douplat, Amélie Nicol, Marine Delaroche, Landry Jacquet, Nathalie Chapelle-Petit and Abdel Chekaoui.
Cliniques Universitaires Saint Luc, UCL, Brussels, Belgium: Franck Verschuren and Manon Fabry.
University Hospital, Tours, France: Valérie Gissot and Julie Magnan.
University Hospital Dupuytren, Limoges, France : Thomas Daix, Arnaud Desachy, Philippe Vignon, Marine Goudelin, Bruno Evrard and Anne Laure Fedou.
University Hospital Jean Minjoz, Besançon, France: Thibault Desmettre, François Jardin and Anne Claire Montini.
General Hospital, Montauban, France: Anaïs Colonna and Lionel Bertrand.
University Hospital, Grenoble, France: Maxime Maignan and Damien Viglino.
University Hospital Lapeyronie, Montpellier, France: Mustapha Sebbane.
General Hospital, Brive la Gaillarde, France: Jacques Remize.
General Hospital, La-Roche-sur-Yon, France: Caroline Anoot and Jérome Frey.
General Hospital Henri Mondor, Aurillac, France: Agathe Pancher.
University Hospital Cochin—APHP, Paris, France: Khalil Takun and Florence Dumas.
General Hospital, Versailles, France: Yves Lambert, Mehrsa Koukabi, Sabrina Measso and Ludovic Dalle.
General Hospital, Angoulême, France: Olivier Dupeux and Antoine Duconge.
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
TL included patients and drafted the manuscript. MAC was in the scientific committee and drafted the manuscript. CV and KT were in the scientific committee, included patients and critically reviewed the manuscript. SB performed the statistical analyses and critically reviewed the manuscript. AP, PFL, BF were in the scientific committee and critically reviewed the manuscript. SL included patients and critically reviewed the manuscript. The members of the TRIAGE study group included patients. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was recorded on ClinicalTrials.gov (N°: NCT02739152) on 15 April 2016—Retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT02739152?term=TRIAGE and approved by the Ethical Committee for Clinical Research (CPP SOOM IV: CPP15-004). All patients gave informed consent.
Consent for publication
NA.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
MA Cazalis, S Blein and A Pachot are employees of bioMerieux SA. The other authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Additional figures and table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lafon, T., Cazalis, MA., Vallejo, C. et al. Prognostic performance of endothelial biomarkers to early predict clinical deterioration of patients with suspected bacterial infection and sepsis admitted to the emergency department. Ann. Intensive Care 10, 113 (2020). https://doi.org/10.1186/s13613-020-00729-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-020-00729-w