Abstract
The bedside hemodynamic assessment of the critically ill remains challenging since blood volume, arterial–venous interaction and compliance are not measured directly. Mean circulatory filling pressure (Pmcf) is the blood pressure throughout the vascular system at zero flow. Animal studies have shown Pmcf provides information on vascular compliance, volume responsiveness and enables the calculation of stressed volume. It is now possible to measure Pmcf at the bedside. We performed a systematic review of the current Pmcf measurement techniques and compared their clinical applicability, precision, accuracy and limitations. A comprehensive search strategy was performed in PubMed, Embase and the Cochrane databases. Studies measuring Pmcf in heart-beating patients at the bedside were included. Data were extracted from the articles into predefined forms. Quality assessment was based on the Newcastle–Ottawa Scale for cohort studies. A total of 17 prospective cohort studies were included. Three techniques were described: Pmcf hold, based on inspiratory hold-derived venous return curves, Pmcf arm, based on arterial and venous pressure equilibration in the arm as a model for the entire circulation, and Pmcf analogue, based on a Guytonian mathematical model of the circulation. The included studies show Pmcf to accurately follow intravascular fluid administration and vascular compliance following drug-induced hemodynamic changes. Bedside Pmcf measures allow for more direct assessment of circulating blood volume, venous return and compliance. However, studies are needed to determine normative Pmcf values and their expected changes to therapies if they are to be used to guide clinical practice.
Similar content being viewed by others
Background
It is difficult to determine the cause for hemodynamic instability in patients and to predict the best treatments. Currently, cardiovascular resuscitation options are triggered by arterial pressure and cardiac output (CO) measures, focusing on the oxygen delivery side of the circulation. However, the primary determinants of CO reside on the venous side. Veins are 30–50 times more compliant than arteries and contain approximately 75% of the total blood volume [1,2,3,4,5]. Mean circulatory filling pressure (Pmcf) provides vital information on this “forgotten venous side of the circulation” [6].
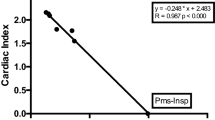
In 1894, Pmcf was defined as the equilibrium pressure throughout the circulation during circulatory arrest [7]. In the 1950s, Guyton and colleagues described a linear relationship between venous return (VR) and right atrial pressure (Pra), described as: VR = (Pmcf − Pra)/(RVR) [8, 9]. RVR is resistance to VR and defines the slope of the VR curve. This linearity has been confirmed in intact circulations in animal studies and is not affected by hypo- or hypervolemia [10,11,12,13,14,15]. VR curves enable to determine the equilibrium point of the circulation, which is the intersection between the CO and VR curve. Central venous pressure (CVP) is a surrogate of Pra used in clinical practice. CVP at zero flow equals Pmcf (Fig. 1).
Vascular volume requires a minimal volume before its distending pressure becomes positive. The amount of blood not causing pressure on the vessels is called unstressed volume (Vu) and reflects intravascular volume present with Pmcf of zero. Stressed volume (Vs) is the additional blood causing a distending pressure on the vascular walls and reflects the effective circulating volume. Vu and Vs together define the total blood volume. Vs is approximately 25% of the total blood volume [3,4,5]. Vs and vascular compliance (Csys) define Pmcf [16]. An increase in Vs increases Pmcf, and an increase in Csys decreases Pmcf. Fluid loading should increase Pmcf, but VR only increases if the pressure gradient for VR (i.e., Pmcf CVP) increases, RVR decreases, or both. Since in the steady state VR = CO, knowing the determinants of VR is relevant to understanding cardiovascular state.
Recently, methods have emerged to enable clinicians to estimate Pmcf at the bedside. Our objectives for this review were to describe the techniques and to highlight their clinical applicability, precision, accuracy and limitations in critically ill patients.
Materials and methods
Publication selection
This review was performed according to PRISMA guidelines [17] (Additional file 1) and methodology outlined in the Cochrane Handbook for systematic reviews [18]. No study protocol was published. A PubMed, Embase and Cochrane Library database search was performed with help of a clinical librarian with no restriction on publication date. The search was performed up to May 18, 2017. The search strategy combined the following concepts: (1) “mean systemic filling pressure” or “mean circulatory filling pressure” or “static filling pressure” and (2) “intensive care” or “critical care” or “perioperative” or “intraoperative” (Additional file 1). Titles, abstracts and full-texts were independently screened by two reviewers for relevance (MW and DPS), and discrepancies were resolved by a third reviewer (BFG). The references of the selected articles were examined for additional eligible articles. Studies were included when available in English and full-text, described prospective studies in which Pmcf estimation methods were examined in heart-beating ICU patients and contained a description of their clinical applicability, precision and accuracy or limitations.
Data extraction and analysis
Data were extracted into predefined forms. No additional analyses were performed. Critical appraisal was based on the Newcastle–Ottawa Scale for cohort studies [19] to assess the quality of non-randomized studies at study level. A modified version of the scale was used since only five out of nine questions were applicable, resulting in a possible highest score of five stars (Additional file 1).
Results
Study selection and characteristics
The initial search identified 369 articles, of which 300 were excluded after screening title and abstract. A total of 53 articles were excluded based on full-text. Two relevant articles were found by citation tracking. Consequently, 17 prospective cohort studies estimating Pmcf in heart-beating ICU patients were included (Additional file 1). Three different bedside measurement techniques were found. Eight studies estimated Pmcf applying inspiratory hold maneuvers (Pmcf hold), three studies during a circulatory stop-flow in the arm (Pmcf arm) and four studies using a mathematical algorithm (Pmcf analogue). Two studies compared multiple techniques.
Eleven studies were performed in postoperative cardiac surgery patients (Table 1). All patients were hemodynamically stable without alteration in vasopressor use or fluid therapy during the study protocol. All patients were sedated and mechanically ventilated. In one study, spontaneous breathing efforts were observed [20]. The number of included patients ranged from nine to 80. In all studies, CVP was measured via a catheter in the right internal jugular vein. CO measurement techniques differed between studies (Additional file 1).
P mcf hold
Technique description
Pmcf hold is based on the linear relation between CVP and VR (Pmcf = (VR − CVP)/RVR). CVP is raised by performing a series of end-inspiratory hold maneuvers. In 2009, the method was first studied in humans [21]. Inspiratory hold maneuvers at 5, 15, 25 and 35 cmH2O incremental ventilatory plateau pressures (Pvent) were performed, and CO was measured in the last 3 s of the 12 s inspiratory hold. They validated that after 7–10 s a steady state consists when VR = CO. By plotting the CVP and CO values, a VR curve is constructed and the zero-flow pressure (Pmcf) extrapolated. Seven studies [16, 21,22,23,24,25,26] estimated Pmcf hold using these four plateau pressures. Two studies [27, 28] used two points (Pvent 5 and 30 cmH2O) at 15-s inspiratory and expiratory hold plateau phase. Between the Pmcf hold measurements, either 1-min pauses were used to re-establish the initial hemodynamic steady state [16, 21, 22, 24, 28], or the consecutive inspiratory hold was performed when CO had returned to baseline [23, 26, 27].
Clinical applicability
The average baseline Pmcf hold values found in the eight included studies range from 19 to 33 mmHg with a wide standard deviation (Tables 2, 3). Five studies [21,22,23, 26, 28] demonstrated fluid administration caused an increase in Pmcf hold, confirming that in humans, as in animals before [14, 15], Pmcf hold follows hemodynamic changes (Table 2). One of these studies found passive leg raising (PLR) to significantly increase Pmcf hold values [28]. RVR was not significantly affected by different volumetric conditions nor by PLR. Vs was calculated from Pmcf as a measure for effective circulating volume [22]. In one study, Pmcf was used to assess the hemodynamic effects of arterial hyperoxia (FiO2 = 90% for 15 min) in ICU patients [26]. During this hyperoxia, left ventricular afterload increased and contractility remained similar; however, CO did not decrease. Both Pmcf and RVR increased significantly (Table 3), explaining why VR (thus CO) remained unaltered.
Studies have used Pmcf hold to describe hemodynamic changes caused by propofol [24] and norepinephrine [25, 27] (Table 3). In septic shock patients, decreasing the dose of norepinephrine decreased both Pmcf and RVR [27]. Further, after increasing norepinephrine CO decreased in ten patients and CO increased in six patients [25]. In all patients, Pmcf and RVR increased, though the “balance” between the two values determined whether CO increased. One study showed an increase in propofol caused a decrease in Vs without a change in CO [24]. These studies show Pmcf behaves within the framework of hemodynamic reasoning and lends itself to being used as a less invasive method to assess drug-induced physiology. Since Pmcf exists at the intersection of arterial and venous flow, it enables to calculate the true arterial and venous resistance by calculating the critical closing pressure (Pcc). Pcc is the mean arterial pressure (MAP) to zero CO-intercept. Arterial resistance is calculated as (MAP − Pcc)/CO [22].
Precision and accuracy
The technique precision has not yet been assessed in humans. However, in an animal study the averaged coefficient of variation for repeated measurements of Pmcf hold was 6% [29]. Comparing the techniques’ accuracy, no significant differences between Pmcf hold and Pmcf arm existed, whereas Pmcf analogue values were significantly lower [16, 30].
Limitations
The use of Pmcf hold is restricted to mechanically ventilated and sedated patients with a central venous catheter. The procedure of the inspiratory hold maneuvers is not yet automated and requires a direct link between monitor and ventilator, or advanced monitor analytics to detect the inspiratory holds and to perform the instantaneous CO calculations. Furthermore, it is not suitable during cardiac arrhythmia. This method is not suitable to measure rapid changes in hemodynamic status since it takes a couple of minutes to perform the multiple end-inspiratory (and end-expiratory) holds. Potentially, this technique is operator-dependent because a proper inspiratory plateau pressure is needed. CVP can be altered due to incorrect catheter placement. An absolute CO value is not necessary for Pmcf hold as the technique extrapolates to zero CO. If the trend measurements are accurate, the RVR slope might change, but the intersection Pmcf point remains constant. The latter holds only true for the Pmcf itself, the RVR is dependent of the slope of the curve. In clinical practice, a physician would use Pmcf together with RVR; therefore, for clinical use of the Pmcf an accurate CO value is needed.
Potentially, the inspiratory hold maneuver overestimates Pmcf by the blood translocation from the pulmonary into the systemic circulation [31,32,33]. However, the potential volume shifts relative to Csys suggest that this effect is minimal [10, 34]. During inspiratory hold maneuvers, arterial pressure decreases. If sustained, baroreflex-induced increased sympathetic tone may cause Pmcf to increase [35, 36]. Indeed one study performed in pigs found the Pmcf hold overestimating compared to a method using right atrial balloon occlusion in euvolemic conditions, in bleeding and hypervolemia; however, the values found between the two methods were similar [34]. Two clinical studies [16, 30] have shown Pmcf hold and Pmcf arm values not being significantly different, debating the former result found in pigs. Future studies in humans are needed. Moreover, all patients undergoing inspiratory holds are on neuro-humoral suppressive agents, probably dampening the baroreflex and other autonomic influences [37,38,39].
P mcf arm
Technique description
As Pmcf is defined as the steady-state blood pressure during no-flow conditions, instantaneously Pmcf should mainly be similar for different vascular compartments even though each compartment may have different Vu and Vs [2, 40]. Four studies [16, 41–43] used the arm to estimate Pmcf. For arm occlusion, a rapid cuff inflator (inflates in 0.3 s) [16, 43] or a pneumatic tourniquet (inflates in 1.4 s) [41, 42] was inflated around the upper arm to 50 mmHg above systolic blood pressure. Arterial and venous pressures were measured via a radial artery catheter and a peripheral venous cannula in the forearm. When these two pressures equalize, Pmcf arm values are achieved. An initial study determined that a 25–30 s stop-flow time was adequate to achieve this equilibration [16]. Following this, in two studies Pmcf arm was measured as the average radial arterial pressure at 30 s after stop-flow [16, 43]. One study found the smallest difference between venous and arterial pressure after 60 s of stop-flow [41]. This discrepancy could be explained by different inflation time, i.e., induction of stop-flow.
Clinical applicability
The average baseline Pmcf arm values found in the included studies range from 16 to 24 mmHg (Table 2). Pmcf arm can be performed in spontaneously breathing subjects and requires only one measure. In two studies, Pmcf arm was assessed as a predictor of fluid loading responsiveness (FLR) [16, 43]. One study showed that a low Pmcf arm (< 22 mmHg) predicts FLR with 71% sensitivity and 88% specificity, where responders were defined when CO increased > 10% after 500 mL colloid administration [43]. Another study showed changes in circulating volume (500 mL colloid) are tracked well by changes in Pmcf arm [16]. Finally, one study indicated a minimum of 4 mL/kg fluid challenge was needed to define FLR [42].
Precision and accuracy
Repeated measurements of Pmcf arm showed no significant differences [41]. The coefficient of variation for a single measurement was 5%, which reduced to 3% after four measurements. Bland–Altman analysis showed a bias of − 0.1 ± 1.68 mmHg for the first two measurements. The least significant change [44] for a single measurement was 14% (i.e., ± 3 mmHg for a Pmcf arm of 22 mmHg). One study observed a negligible bias of two Pmcf arm determinations at baseline position and after fluid expansion [16]. Two studies [16, 30] found no significant differences in Pmcf arm to Pmcf hold measures.
Limitations
Theoretically, a limitation of the technique is the influence of an auto regulatory hypoxia-induced response causing arterial vasodilation. The time of measuring Pmcf after arm occlusion should be enough for arterial and venous pressures to equilibrate, but before hypoxia-induced vasodilation causes an underestimation of Pmcf [45]. One study observed plateau pressures after 20–30 s and saw a further decrement after 35–40 s which indicates hypoxia-induced vasodilation [16]. Potentially, arm occlusion causes a small accumulation of blood volume because the venous outflow stops before the arterial inflow stops [16]. Though, this potential overestimation is negligible since the inflow is small compared to the total distal arm volume as long as cuff inflation is rapid. To note, Pmcf arm is only reliable when a stable plateau pressure is achieved [2].
In contrast to Pmcf hold, Pmcf arm measures can be made in non-sedated patients with cardiac arrhythmias. However, the possible influence of the rapid cuff inflator on reflex mechanisms needs to be studied. In septic patients, central and peripheral vasomotor tone might be altered differently [46]. Shortly after cardiac surgery differences between aortic and radial pressure can occur [47], still, the original validation studies were on postoperative cardiac surgery patients.
P mcf analogue
Technique description
Based on a Guytonian model of the systemic circulation (CO = VR = (Pmcf − CVP)/RVR), an analogue of Pmcf can be derived using a mathematical model: Pmcf analogue = axCVP + bxMAP + cxCO [5, 20, 48, 49]. In this formula, a and b are dimensionless constants (a + b = 1). Assuming a veno-arterial compliance ratio of 24:1, a = 0.96 and b = 0.04; c resembles arteriovenous resistance and is based on a formula including age, height and weight [5, 48,49,50].
Clinical applicability
The average baseline Pmcf analogue values found in the included studies range from 14 to 18 mmHg (Table 2). One study compared fluid replacement based on target Pmcf analogue compared to conventional treatment in continuous veno-venous hemodiafiltration [49]. Fluid replacement based on target Pmcf analogue led to significantly less fluid administration with stable cardiovascular variables (CVP, MAP, CO) and no complications. So, Pmcf analogue measurement adequately follows intravascular volume status in patients. Pmcf analogue measurements are automatic making it an attractive alternative to Pmcf hold and Pmcf arm.
More recently, the Pmcf analogue dynamics, measured with the Navigator™ device (Applied Physiology, Pty Ltd, Australia), were observed [20, 48, 51]. Patients were defined as responders with an increase in stroke volume or CO > 10% after 250 mL fluid administration. Pmcf analogue increased after fluid administration; however, baseline Pmcf analogue did not differ between responders and non-responders [20, 45, 48] (Table 2). This is contrary to results of another study [43] using Pmcf arm, possibly due to different fluid volume (250 vs. 500 mL) [42]. Although the driving pressure for VR (Pmcf CVP) was different between responders and non-responders, it showed low sensitivity (79%) and specificity (56%) to predict FLR [20, 48].
Precision and accuracy
Precision has not been assessed for Pmcf analogue (Table 4). Comparing measurement techniques revealed a lower Pmcf analogue value compared to Pmcf hold [16]. However, a significant regression of Pmcf analogue and Pmcf hold was observed enabling to adjust the Pmcf analogue value using calibration factor [5].
Limitations
The mathematical model is based on CVP, MAP and CO measurements. As CVP values vary during ventilation, usually end-expiratory CVP-recordings can be used. Furthermore, CVP values depend on the position of the transducer. Accurate CO values are needed for this method. The limitation of Pmcf analogue is that the algorithm is based on a mathematical model with mathematical coupling between CO and Pmcf and fixed Csys and resistance parameters [5], therefore presumably not applicable for all patient populations or clinical conditions. We are unable to assess the availability of the Navigator™ for routine care.
Discussion
We found three bedside techniques to measure Pmcf: Pmcf hold, Pmcf arm and Pmcf analogue. They were used to follow volumetric state and to study drug-induced hemodynamic changes in patients.
The interpretation of VR curves and Pmcf in clinical practice is subject to debate [52,53,54,55,56,57,58,59]. The values found in heart-beating ICU patients are higher (14–33 mmHg) than in deceased ICU patients (12.8 ± 5.6 mmHg, mean ± sd), probably because of alteration of vasomotor tone after dying [53]. Furthermore, ICU patients often receive vasopressors which increase Pmcf and the study populations differed making it not one-to-one comparable. It is also speculated that the pressure described by Guyton is not measurable in heart-beating patients and the extrapolated pressure of the curve represents a different physiological parameter. Nevertheless, in two studies Pmcf arm was interchangeable with Pmcf hold [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Furthermore, although Pmcf values may differ, the CVP values do as well, which may account for a similar driving pressure for VR. The reviewed studies illustrate the possible clinical benefits of using the bedside derived Pmcf values.
This review is limited since we were unable to pool the data because of the variety in used conditions and interventions. The 16 included studies were performed by only a few research groups with a limited amount of included patients. In most of the studies, each patient served as their own control since it is not clear what would be an appropriate outside control group.
Still, all studies testing the accuracy of Pmcf to follow intravascular changes and pharmacodynamics found significant results. Therefore, it is unlikely that a larger number of patients will show different outcomes. It is possible only positive studies were published, indicating publication bias. Pmcf values differ between the studies and have a wide range within studies (Table 2). Normal values for different patient populations need to be defined before Pmcf can be implemented into standard (ICU) care. The increase in Pmcf values after fluid administration depends on vascular redistribution, vasomotor tone and fluid loss into the interstitial space. Studies focusing on clinical decision-making based on Pmcf, driving pressure for VR, Vs or Csys have not yet been performed. Study designs need to be created to see if using these measures improves outcomes. Also, no precision studies examining Pmcf hold or Pmcf analogue exist yet.
Conclusions
Presently, three bedside Pmcf measurement techniques are available. All require invasive hemodynamic monitoring. Though Pmcf measures allow for more direct assessment of circulating blood volume, VR and Csys, studies are needed to determine cutoff values to allow Pmcf to trigger therapeutic interventions and to determine its value in clinical practice.
Abbreviations
CO: cardiac output; Csys: vascular compliance, CVP: central venous pressure; FiO2: fractional oxygen concentration; FLR: fluid loading responsiveness; ICU: intensive care unit; MAP: mean arterial pressure; RVR: resistance for venous return.
List of symbols
Pcc: critical closing pressure; Pmcf: mean circulatory filling pressure; Pra: right atrial pressure; VR: venous return; Vs: stressed volume; Vu: unstressed volume.
References
Hainsworth R. The importance of vascular capacitance in cardiovascular control. News Physiol Sci. 1990;5:250–4.
Maas JJ. Mean systemic filling pressure: its measurement and meaning. Neth J Crit Care. 2015;19:6–11.
Peters J, Mack GW, Lister G. The importance of the peripheral circulation in critical illnesses. Intensive Care Med. 2001;27:1446–58.
Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–48.
Parkin WG. Volume state control: a new approach. Crit Care Resusc. 1999;1:311–21.
Jansen JR, Maas JJ, Pinsky MR. Bedside assessment of mean systemic filling pressure. Curr Opin Crit Care. 2010;16:231–6.
Bayliss WM, Starling EH. Observations on venous pressures and their relationship to capillary pressures. J Physiol. 1894;16:159–318.
Guyton AC, Polizo D, Armstrong GG. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am J Physiol. 1954;179:261–7.
Guyton AC, Lindsey AW, Abernathy B, Richardson T. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol. 1957;189:609–15.
Versprille A, Jansen JR. Mean systemic filling pressure as a characteristic pressure for venous return. Pflugers Arch. 1985;405:226–33.
Pinsky MR. Instantaneous venous return curves in an intact canine preparation. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:765–71.
Hiesmayr M, Jansen JR, Versprille A. Effects of endotoxin infusion on mean systemic filling pressure and flow resistance to venous return. Pflugers Arch. 1996;431:741–7.
den Hartog EA, Versprille A, Jansen JR. Systemic filling pressure in intact circulation determined on basis of aortic vs. central venous pressure relationships. Am J Physiol. 1994;267:H2255–8.
Yamamoto J, Trippodo NC, Ishise S, Frohlich ED. Total vascular pressure–volume relationship in the conscious rat. Am J Physiol. 1980;238:H823–8.
Samar RE, Coleman TG. Measurement of mean circulatory filling pressure and vascular capacitance in the rat. Am J Physiol. 1978;234:H94–100.
Maas JJ, Pinsky MR, Geerts BF, de Wilde RB, Jansen JR. Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensive Care Med. 2012;38:1452–60.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions (version 5.1.0). 2011. http://handbook.cochrane.org. Accessed 16 March 2017.
Wells G, Shea B, O’Connell J, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 20 Feb 2017.
Gupta K, Sondergaard S, Parkin G, Leaning M, Aneman A. Applying mean systemic filling pressure to assess the response to fluid boluses in cardiac post-surgical patients. Intensive Care Med. 2015;41:265–72.
Maas JJ, Geerts BF, van den Berg PC, Pinsky MR, Jansen JR. Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med. 2009;37:912–8.
Maas JJ, de Wilde RB, Aarts LP, Pinsky MR, Jansen JR. Determination of vascular waterfall phenomenon by bedside measurement of mean systemic filling pressure and critical closing pressure in the intensive care unit. Anesth Analg. 2012;114:803–10.
Keller G, Desebbe O, Benard M, Bouchet JB, Lehot JJ. Bedside assessment of passive leg raising effects on venous return. J Clin Monit Comput. 2011;25:257–63.
de Wit F, van Vliet AL, de Wilde RB, Jansen JR, Vuyk J, Aarts LP, et al. The effect of propofol on haemodynamics: cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br J Anaesth. 2016;116:784–9.
Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41:143–50.
Helmerhorst HJ, de Wilde RB, Lee DH, Palmen M, Jansen JR, van Westerloo DJ, et al. Hemodynamic effects of short-term hyperoxia after coronary artery bypass grafting. Ann Intensive Care. 2017;7:20.
Persichini R, Silva S, Teboul JL, Jozwiak M, Chemla D, Richard C, et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med. 2012;40:3146–53.
Guerin L, Teboul JL, Persichini R, Dres M, Richard C, Monet X. Effects of passive leg raising and volume expansion on mean systemic pressure and venous return in shock in humans. Crit Care. 2015;19:411.
Maas JJ, Geerts BF, Jansen JR. Evaluation of mean systemic filling pressure from pulse contour cardiac output and central venous pressure. J Clin Monit Comput. 2011;25:193–201.
Maas JJ, Pinsky MR, Aarts LP, Jansen JR. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth Analg. 2012;115:880–7.
Fessler HE, Brower RG, Wise RA, Permutt S. Effects of positive end-expiratory pressure on the gradient for venous return. Am Rev Respir Dis. 1991;143:19–24.
Hedenstierna G. Pulmonary perfusion during anesthesia and mechanical ventilation. Minerva Anestesiol. 2005;71:319–24.
Jellinek H, Krenn H, Oczenski W, Veit F, Schwarz S, Fitzgerald RD. Influence of positive airway pressure on the pressure gradient for venous return in humans. J Appl Physiol. 2000;88:926–32.
Berger D, Moller PW, Weber A, Bloch A, Bloechlinger S, Haenggi M, et al. Effect of PEEP, blood volume and inspiratory hold maneuvers on venous return. Am J Physiol Heart Circ Physiol. 2016;311:H794–806.
Borst C, Karemaker JM. Time delays in the human baroreceptor reflex. J Auton Nerv Syst. 1983;9:399–409.
Peters JK, Lister G, Nadel ER, Mack GW. Venous and arterial reflex responses to positive-pressure breathing and lower body negative pressure. J Appl Physiol. 1997;82(6):1889–96.
Sato M, Tanaka M, Umehara S, Nishikawa T. Baroreflex control of heart rate during and after propofol infusion in humans. Br J Anaesth. 2005;94:577–81.
Ebert TJ. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology. 2005;103:20–4.
Lennander O, Henriksson BA, Martner J, Biber B. Effects of fentanyl, nitrous oxide, or both, on baroreceptor reflex regulation in the cat. Br J Anaesth. 1996;77:399–403.
Anderson RM. Appendix: clinical determination of mean cardiovascular pressure. In: Anderson RM, editor. The gross physiology of the cardiovascular system. 2nd ed. Tucson: Racquet Press; 1993. p. 61–2.
Aya HD, Rhodes A, Fletcher N, Grounds RM, Cecconi M. Transient stop-flow arm arterial-venous equilibrium pressure measurement: determination of precision of the technique. J Clin Monit Comput. 2016;30:55–61.
Aya HD, Rhodes A, Chis Ster I, Fletcher N, Grounds RM, Cecconi M. Hemodynamic effect of different doses of fluids for a fluid challenge: a quasi-randomized controlled study. Crit Care Med. 2017;45:e161–8.
Geerts BF, Maas J, de Wilde RB, Aarts LP, Jansen JR. Arm occlusion pressure is a useful predictor of an increase in cardiac output after fluid loading following cardiac surgery. Eur J Anaesthesiol. 2011;28:802–6.
Kawalilak CE, Johnston JD, Olszynski WP, Kontulainen SA. Least significant changes and monitoring time intervals for high-resolution pQCT-derived bone outcomes in postmenopausal women. J Musculoskelet Neuronal Interact. 2015;15:190–6.
Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol. 2004;286:H4428.
Hatib F, Jansen JR, Pinsky MR. Peripheral vascular decoupling in porcine endotoxic shock. J Appl Physiol. 2011;111:853–60.
Stern DH, Gerson JI, Allen FB, Parker FB. Can we trust the direct radial artery pressure immediately following cardiopulmonary bypass? Anesthesiology. 1985;62:557–61.
Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;39:1299–305.
Parkin G, Wright C, Bellomo R, Boyce N. Use of a mean systemic filling pressure analogue during the closed-loop control of fluid replacement in continuous hemodiafiltration. J Crit Care. 1994;9:124–33.
Crozier TM, Wallace EM, Parkin WG. Haemodynamic assessment in pregnancy and pre-eclampsia: a Guytonian approach. Pregnancy Hypertens. 2015;5:177–81.
Aya HD, Ster IC, Fletcher N, Grounds RM, Rhodes A, Cecconi M. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44:880–91.
Brengelmann GL. A critical analysis of the view that right atrial pressure determines venous return. J Appl Physiol. 2003;94:849–59.
Repessé X, Charron C, Fink J, Beauchet A, Deleu F, Slama M, et al. Value and determinants of the mean systemic filling pressure in critically ill patients’. Am J Physiol Heart Circ Physiol. 2015;309:H1003–7.
Brengelmann GL. Letter to the editor: comments on “Value and determinants of the mean systemic filling pressure in critically ill patients”. Am J Physiol Heart Circ Physiol. 2015;309:H1370–1.
Repessé X, Vieillard-Baron A. Reply to Letter to the editor: comments on ‘Value and determinants of the mean systemic filling pressure in critically ill patients’. Am J Physiol Heart Circ Physiol. 2015;309:H1372–3.
Beard DA, Feigl EO. Understanding Guyton’s venous return curves. Am J Physiol Heart Circ Physiol. 2011;301:H629–33.
Teboul JL. Mean systemic filling pressure: we can now estimate it, but for what? Intensive Care Med. 2013;39:1487–8.
Parkin G. Re: mean systemic filling pressure: we can now estimate it but for what? Intensive Care Med. 2014;40:139.
Teboul JL. Mean systemic filling pressure: we can now estimate it, but for what? Response to comment by Parkin. Intensive Care Med. 2014;40:140.
Authors’ contributions
All authors contributed to the manuscript. MW, DV and BG designed the study. MW performed a systematic search of the literature. MW and DS independently screened articles for relevance and subsequently performed data extraction into predefined forms. Quality assessment of the included articles was also independently performed by MW and DS. MW, DV, BG and MP wrote the manuscript. JJ, EO and AV critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
None of the authors have relevant conflict of interest present for any aspect of the submitted work. Denise Veelo performed consultancy work for Edwards Lifesciences, Hemologic and Merck outside the submitted work. Bart Geerts performed consultancy work for Edwards Lifesciences and Philips outside the submitted work. Michael Pinsky is a consultant for Cheetah Medical, Edwards Lifesciences, Exotstat Medical, LiDCO Ltd and Cyberonics outside the submitted work.
Ethics approval and consent to participate
Not applicable.
Funding
None.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1.
I: Search in EMBASE, MEDLINE and Cochrane Library: Description of the used search terms per database. II: Quality assessment according to a modified version of the Newcastle–Ottawa scale for cohort studies: Including representativeness, ascertainment, demonstration, comparability and outcome. III: PRISMA Flowchart: Description of results of systematic literature search, reasons for excluding studies and the amount of included studies. IV: Expanded baseline characteristics for included studies: Authors, described Pmcf measurement method, patient population, exclusion criteria, age and sex of included patients, type of cardiac output measurement, used vasopressors, sedation and anesthesia techniques and timeframes of Pmcf measurements. V: PRISMA 2009 Checklist: an evidence-based minimum set of items for reporting in systematic reviews and meta-analysis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wijnberge, M., Sindhunata, D.P., Pinsky, M.R. et al. Estimating mean circulatory filling pressure in clinical practice: a systematic review comparing three bedside methods in the critically ill. Ann. Intensive Care 8, 73 (2018). https://doi.org/10.1186/s13613-018-0418-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-018-0418-2