Abstract
Background
Somatosensory (SSEP) and brainstem auditory (BAEP) evoked potentials are neurophysiological tools which, respectively, explore the intracranial conduction time (ICCT) and the intrapontine conduction time (IPCT). The prognostic values of prolonged cerebral conduction times in deeply sedated patients have never been assessed. Sedated patients are at risk of developing new neurological complications, undetected. In this prospective observational bi-center pilot study, we investigated whether early impairment of SSEP’s ICCT and/or BAEP’s IPCT could predict in-ICU mortality or altered mental status (AMS), in deeply sedated critically ill patients.
Methods
SSEP by stimulation of the median nerve and BAEP were assessed in critically ill patients receiving deep sedation on day 3 following ICU admission. Deep sedation was defined by a Richmond Assessment sedation Scale (RASS) <−3. Mean left- and right-side ICCT and IPCT were measured for each patient. Primary and secondary outcomes were, respectively, in-ICU mortality and AMS defined as the occurrence of delirium and/or delayed awakening after discontinuation of sedation.
Results
Eighty-six patients were studied of which 49 (57%) were non-brain-injured and 37 (43%) were brain-injured. Impaired ICCT was a predictor of in-ICU mortality after adjustment on the global Sequential Organ Failure Assessment score (SOFA) [OR (95% CI) = 2.69 (1.05–6.85); p = 0.039] and on the non-neurological SOFA components [2.67 (1.05–6.81); p = 0.040]. IPCT was more frequently delayed in the subgroup of patients who developed post-sedation AMS (24%) compared those without AMS (0%). However, this difference did not reach statistical significance (p = 0.053). Impairment rates of ICCT and IPCT were not found to be significantly different between non-brain- and brain-injured subgroups of patients.
Conclusion
In critically ill patients receiving deep sedation, early ICCT impairment was associated with mortality. Somatosensory and brainstem auditory evoked potentials may be useful early warning indicators of brain dysfunction as well as prognostic markers in deeply sedated critically ill patients.
Similar content being viewed by others
Background
While current guidelines advocate discontinuing sedation as soon as possible [1, 2], 30–70% of all ICU patients are, at one time, deeply sedated [3, 4]. Indeed, deep sedation, usually defined by a Richmond Assessment Sedation Scale (RASS) beneath −3, may be required in several conditions, including severe respiratory failure, septic shock, or controlling intracranial hypertension in severely brain-injured patients. However, this practice raises major concerns since the use of deep sedation has been incriminated in brain dysfunction [4, 5] and may contribute to increase the prevalence of delirium [6,7,8] and mortality [9, 10]. Indeed, more than half of all critically ill patients develop delirium, which is associated with a greater risk of death and long-term cognitive dysfunction [11,12,13,14,15]. Furthermore, severe brain injury may cause long-term disability or even be life-threatening [16]. Therefore, assessing and monitoring brain dysfunction in deeply sedated patient and determining its impact on outcome are major issues in the daily management of critically ill patients [17,18,19,20,21]. Neurophysiological testing enables assessment of brain dysfunction at the bedside [22]. Somatosensory evoked potentials (SSEP) and brainstem auditory evoked potentials (BAEP) explore the brainstem, as well as cortical and subcortical regions of the brain and are little influenced by the administration of sedatives [23,24,25]. More specifically, inter-peak latencies (IPL) of the components of the SSEP and BAEP are determined by conduction times between neuroanatomical regions or structures [26] and provide information about the functional state of the given brain portion [24, 27]. The prognostic values of SSEP and BAEP have been explored in various causes of coma but never in a cohort of deeply sedated critically ill patients [17, 19,20,21]. We hypothesized that the occurrence of brain dysfunction was associated with impaired intracranial (ICCT) and intrapontine (IPCT) conduction times assessed, respectively, by SSEP and BAEP. This bi-center prospective pilot cohort study was designed to determine whether early impairment of ICCT and IPCT could predict in-ICU mortality and the occurrence of post-sedation altered mental status (delirium or delayed awakening) in deeply sedated critically ill patients.
Methods
Study design and setting
This prospective observational study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [28]. The current pilot study was preparatory to the design of a larger prospective multicenter study assessing the prognostic value of brainstem dysfunction in sedated critically ill patients (ClinicalTrials.gov number: NCT02395861). Subjects were enrolled between January 2012 and January 2015. Participating centers were a medical (center 1) and a surgical (center 2) ICU in two French teaching hospitals. The former unit specializes in the management of medical critically ill patients, while the latter specializes in the management of subjects suffering from traumatic brain injuries.
Characteristics of participants
Consecutive critically ill patients deeply sedated on day 3 following admission were enrolled into the study, irrespective of the existence or not of a primary brain injury and underwent electrophysiological explorations. Deep sedation was defined as a Richmond Assessment Sedation Scale (RASS) <−3 [29]. All included patients were mechanically ventilated. Post-cardiac arrest and moribund patients, patients in whom cerebral death was suspected or for whom withdrawal of life-sustaining therapies had been decided, and patients suffering from preexisting of acquired neuropathies were not included. Hypothermia may influence evoked potentials’ conduction times [30,31,32,33]. To avoid any confounding effect related to temperature, no evoked potential was recorded while body temperature was below 35 °C.
Baseline clinical data collection
Demographic characteristics (i.e., age, sex) as well as body weight, date and time of ICU admission, category of admission (medical or surgical), co-morbidities, preexisting risk factors for delirium, main cause of critical illness and brain injury, and the date and cause of initiation of mechanical ventilation were collected. Baseline data collection was performed following a previously described method [34, 35].
Sedation and analgesia
The decision to initiate deep sedation and the subsequent management thereof were overseen by the physicians in charge of the patient, following recent guidelines [1, 2]. Sedation was administered through a continuous infusion of midazolam and/or propofol, in combination with sufentanil. Total cumulative doses of administered drugs at the time of neurophysiological examination were collected. In both centers, the depth of sedation was monitored using the RASS [29], recorded every 4 h until awakening. Sedation was interrupted daily in center 1 (in which non-brain-injured patients were referred) and administrated as a titration aiming at obtaining the desired RASS in center 2 (in which severe traumatic brain-injured patients were cared for) [29]. The time of onset, the reason for administration, and duration of deep sedation, as well as the time of awakening, defined by the occurrence of spontaneous opening of the eyes with RASS >−1, were collected.
Neurological examination
At the time of inclusion, the Glasgow Coma Scale (GCS), the Full Outline of Unresponsiveness (FOUR) score [36], and the Richmond Agitation-Sedation Scale (RASS) and the cough reflex were assessed. RASS is a simple and reliable tool that is intuitive, easy to use, and informs on both agitation and sedation. The RASS has been validated in mechanically ventilated and sedated patients [29, 37].
Evoked potential (EP) assessment and analysis
SSEP after stimulation of the median nerve and BAEP were recorded at the bedside by an experimented neurophysiologist (EA) for all studied patients. EPs were recorded following the guidelines of the International Federation of Clinical Neurophysiology [24, 38]. A Natus France, Dantec™ KEYPOINT® G4 EMG/NCS/EP Workstation was used for data acquisition and processing. All recordings were separately interpreted by two experienced neurophysiologists (EA and RMK). Any series of SSEP or of BAEP recording contaminated by noise was rejected, and recording was repeated following the administration of a neuromuscular blocking agent in order to eliminate muscular artifacts [24, 38].
Somatosensory evoked potentials (SSEP)
SSEP were recorded following stimulation of the median nerve at the wrist using repeated square-wave pulses lasting 0.2 ms, at 4.7 Hz, with stimulus intensity sufficient to produce a twitching of the thumb (motor threshold). Recording of the proximal peripheral nerve (brachial plexus) response N9 was performed through an electrode placed at the ipsilateral Erb’s point, while the reference electrode was situated over the centro-frontal region (Fz). Recording and reference electrodes were placed at Cv7 (7th cervical vertebra)—Fz for the N13 cervical spinal cord response and Cz—cSh (contralateral shoulder) for the subcortical far-field potential: P14. The cortical components, N20 and P25, were recorded at the contralateral C3′ or C4′ positions (2 cm behind C3 or C4) according to the international 10–20 system. Impedance was kept below 5 kOhms. The filter pass band ranged from 30 to 1500 Hz. Two sets of 500 sweeps were averaged. Figure 2—Appendix shows a schematic representation of median nerve’s SSEP responses localizations on a brain MRI (A) and typical examples of their normal waveforms as well as recording electrodes montages (B). Peak latencies (PL) of N9, N13, P14, and N20 responses, as well as IPL N9–N13 and P14–N20 IPLs, i.e., conduction times, were measured. N9–N13 IPL represents a proximal peripheral nerve conduction time, and P14–N20 IPL, the intracranial conduction time (ICCT). The absolute amplitude of the peak of N20 was measured. Each parameter was measured on both right and left median nerves for each patient. N20 component was considered absent (abolished) when its absolute amplitude did not exceed 0.1 µV.
Brainstem auditory evoked potentials (BAEP)
BAEP were recorded following auditory stimulation by a 100-µs 80-dB click applied to one ear, with a (−20 dB) contralateral masking using “white noise.” The recurrence frequency was 19.3 Hz (bandpass, 150–1500 Hz; sweep time, 10 ms). Two sets of 2000 sweeps were averaged. BAEP were picked up in Fz. BAEP recording in the Fz location of the electrode is more convenient when patients are supine since this location is easier to access than the vertex (Cz). The reference electrode was placed at the earlobe ipsilateral to the stimulated ear. Figure 3—Appendix shows a schematic representation of BAEP responses localizations on a brain MRI (A) and typical examples of their normal waveforms (B). PL of waves I, III, and V, as well as I–III, III–V, and I–V IPL, were measured. The I–III IPL represents the peripheral conduction time of the auditory pathway, while the III–V IPL represents the intrapontine conduction time (IPCT). Recordings of both right and left ears were made for all the patients.
Evoked potentials data analysis
For each SSEP or BAEP parameter, the mean value of the left and right side was used for analysis. PL or IPL was considered delayed when they exceeded the mean value +2.5 SD obtained from a control group of 20 healthy subjects in our laboratory. Table 1 provides median nerve SSEP and BAEP’s PL and IPL data obtained our control group.
Method for bias and confounding factors assessment
Confounding factors which may influence neurological examination, neurophysiological tests, mortality, and the occurrence of delirium were assessed. Neurological examination was performed by a specifically trained senior ICU physician. It has previously been shown that inter-observer agreement for such an examination was satisfactory (kappa scores ranged from 0.62 to 1) [35]. Neurophysiological tests were performed and interpreted in a standard manner following international guidelines [24, 38]. Each evoked potential recording was independently analyzed offline by two senior neurophysiologists (EA and RMK) who were blinded to clinical data. We assessed inter-observer rate of concordance for the studied evoked potential parameters using the kappa analysis. The physician in charge of the patient was not informed of the results of the neurophysiological test and the neurophysiologist was blinded to the clinical status and outcome of patients. Management of sedation was assessed by recording the indication of initiation, the daily RASS, modality of discontinuation (daily interruption versus titration) and duration. The cause of death and its main risk factors were also assessed, using the SAPS II and SOFA scores as well as the cause of critical illness. We were therefore able not only to compare subgroups but also to ensure that the management of sedation was appropriate and that the studied population was representative of French ICUs patients.
Outcome assessment
The primary outcome was mortality in the ICU. We collected both the date and cause of death. Secondary outcome was the occurrence of altered mental status defined as either delirium or delayed awakening after discontinuation of sedation. Delayed awakening and the occurrence of delirium following discontinuation of sedation were assessed daily using the RASS and the Confusion Assessment Method in ICU (CAM-ICU), respectively [39]. Delayed awakening was defined by absence of spontaneous eye opening with RASS ≤−1 more than 3 days after discontinuation of sedation. Finally, the duration of mechanical ventilation and length of stay in the ICU were recorded.
Statistical analysis
No published data enabled us to calculate a number of subject to be recruited, since the predictive values of SSEP and BAEP’s components IPL in deeply sedated critically ill patients have never been previously assessed. The present study was a pilot study for the design of a prospective multicenter study on the prognostic value of SSEP and BAEP in the ICU (ClinicalTrials.gov number: NCT02395861). We estimated that 80–100 included patients would be sufficient to test predictors of in-ICU mortality (primary objective). Data are reported as numbers (percentage), mean (standard deviation), or median (inter-quartile range). Groups were compared using the Mann–Whitney rank-sum test. Multivariable logistic regression was used to explore associations between impaired conduction times and in-ICU mortality adjusted to the global SOFA score and the non-neurological SOFA score. p values <0.05 were considered as statistically significant.
Results
Patients’ characteristics
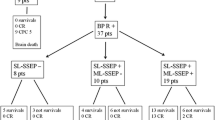
Between January 2012 and January 2015, 95 consecutive sedated critically ill patients were eligible for inclusion (Fig. 1). Nine patients were not included: Two patients exhibited clinical features of brain death, and for three patients, sedation had been discontinued prior to inclusion. In addition, we did not include four patients with severe encephalopathies related to either liver failure (2 cases) or status epilepticus (2 cases). Overall, 86 patients were enrolled; their baseline characteristics are presented in Table 2. Cause of ICU admission was neurological in 37 (43%) patients, mainly traumatic brain injury (TBI) 54%. Non-neurological critical illness related to sepsis was the cause of admission in 49 (57%) patients. Deep sedation was administrated at the time of inclusion for synchronization with the ventilator in 44 (51%) cases, for control of severe intracranial hypertension in 16 (19%) cases and for other vital causes in 26 (30%) cases.
Clinical and neurophysiological characteristics of the patients at inclusion
At the time of inclusion (i.e., time of EP recording), the median RASS was −5 [−5 to −4] (Table 2). Temperature values ranged from 35.1 to 39.3 °C with a mean (±SD) of 36.6 (±1.0) °C. Administered hypnotics were midazolam in 79 (92%) cases and propofol in 9 (10%) cases; 2 (2%) patients received both drugs simultaneously. Sufentanil was administered to 79 (92%) patients, while neuromuscular blocking agents were administered to 13 (15%) patients. Main neurological and neurophysiological features are presented in Table 3. Recordings from all included patients were deemed suitable for analysis. Inter-observer agreement for interpreting EP findings was excellent. Observed agreement was 100% (kappa = 1) for peak detection and placement of markers. The prevalence of delayed IPL of SSEP’s components increased from the periphery to the cortical level: proximal peripheral nerve conduction time (i.e., N9–N13 IPL) was prolonged in 14 (16%) of cases whereas the intracranial conduction time—ICCT (i.e., P14–N20 IPL) was prolonged in 39 (45%) of cases. I–III and I–V IPL were prolonged in 17 (20%) and the intrapontine conduction time—IPCT (i.e., III–V IPL) in 15 (17%) of cases (Tables 1, 3). SSEP N20 was unilaterally abolished in 3 patients. P14–N20 IPL was consequently considered as delayed for these 3 patients with abolished N20. No case with bilaterally abolished N20 was found. Brain imaging was performed in 59 (69%) patients using conventional CT or MRI in, respectively, 36 (61%) and 23 (39%) patients. Brain imaging was normal in 25 (42%) patients. Among the 34 (58%) patients with abnormal imaging, no brainstem injury was reported.
Comparisons of clinical and neurophysiological findings between the two subgroups of patients: brain-injured versus non-brain-injured group
Prevalence of sepsis, day 3 global SOFA, renal SOFA scores, and proportion of patients sedated with midazolam were significantly greater in non-brain-injured patients (Table 2). The median GCS, the FOUR scores, and the median RASS were not significantly different between the two groups (Table 3). The two groups did not differ in terms of abolition of cough reflex and conduction times (Table 3). Prevalence of delayed conduction times did not differ between subgroups of patients with and without abnormal brain imaging. Conduction times were neither statistically correlated with cumulative doses of midazolam, propofol, or sufentanil at inclusion, and no significant difference exists between temperature values of patients with versus without delayed conduction times (Table 5).
Correlations between clinical, neurophysiological features, and in-ICU mortality
Thirty-seven patients (43%) died in the ICU, of which 24 (65%) were non-brain-injured patients. Causes of death were refractory hypotension in 28 (76%) patients, respiratory failure in five cases (13%), and brain death in four (11%) patients. Most non-brain-injured patients died of refractory hypotension 18 (75%), and most brain-injured patients died of refractory intracranial hypertension 4 (31%). Withdrawal of life-sustaining therapies was decided in 6 (7%) patients and was never decided based on the results of evoked potential recordings. Comparisons of clinical and neurophysiological features between survivors and non-survivors appear in Table 4. The SOFA score was significantly higher in non-survivor (p = 0.004). The RASS and FOUR scores were significantly lower in non-survivors (p = 0.001 and p = 0.026, respectively). Impaired ICCT (i.e., delayed SSEP’s P14–N20 IPL) and abolition of cough reflex, were significantly associated to in-ICU mortality (p = 0.029, p = 0.003, respectively). On multivariate analysis, impaired ICCT was independently associated with in-ICU mortality after adjustment to the total SOFA score [OR (95% CI) = 2.69 (1.05–6.85) p = 0.039] and after adjustment to non-neurological SOFA score [OR (95% CI) = 2.67 (1.05–6.81) p = 0.040].
Correlations between clinical, neurophysiological features, and the occurrence of an altered mental status (AMS)
Median duration of sedation after inclusion was 7 days [4–14]. Twenty-three (27%) patients died before discontinuation of sedation. Among the 63 (73%) remaining patients, 49 (78%) patients developed an altered mental status, with delirium occurring in 35 (57%) cases and delayed awakening in 23 (63%) cases. Delayed awakening, but not delirium was significantly more frequent among brain-injured patients (p = 0.02) (Table 4). Median duration of delirium was 5 days [3–17]. No patient evolved toward a vegetative or minimally conscious state. There was no difference between patients with and without altered mental status in terms of demography, cause and severity of critical illness, brainstem reflexes or sedation (Table 4). Impaired IPCT (i.e., delayed BAEP’s III-V IPL) was more frequent among the subgroup of patients who developed AMS compared with the one without AMS (24 vs. 0%); however, this association did not reach statistical significance (p = 0.053).
Discussion
In the present study, we found that in critically ill patients receiving deep sedation, early impairment of ICCT (i.e., delayed SSEP’s P14–N20 IPL) adjusted to patient severity (day 3 SOFA score), predicted in-ICU mortality, and that impairment of IPCT (i.e., delayed III–V IPL) tended to be associated with the occurrence of an altered mental status. We also found an impaired conduction time at the peripheral level, suggesting that neurological dysfunction of these patients affects both the central and the peripheral compartments. These findings support the fact that ICCT and IPCT are useful early warning indicators of brain dysfunction and prognosis markers in deeply sedated critically ill patients in ICU.
Neurophysiological assessment of comatose critically ill patients using evoked potentials have multiple advantages; being noninvasive, available at the bedside, capable of detecting subclinical injuries or providing objective measures when information derived from the clinical examination is poor [22]. Evoked potentials have already been extensively studied when seeking to determine a prognosis following coma from various origins [18, 19, 26, 40,41,42,43,44]; however, little is known regarding critically ill patients receiving deep sedation, which may be required in severe critically ill patients who are at high risk of developing acute brain dysfunction.
Sedative drugs, at least those used during our study, mainly act on cortical receptors [24, 45]. Deep sedation has been reported to be associated with increased ICU mortality [9, 10, 37, 46]. Critically, short-latency evoked potentials used in this study are largely unaffected by commonly used sedative drugs [24, 45]. Indeed, we did not find a correlation between conduction times and sedation doses. To ensure that deep sedation was not involved in the delay of ICCT and IPCT, it would have been necessary to modify the infusion rate of sedative agents. However, such an intervention was prohibited as our study was strictly observational and since sedation was managed by the physician in charge of the patient. The observed delay of intracranial conduction time therefore probably resulted from subcortical demyelinating [47, 48] and/or axonal insults [49, 50]. These lesions have already been described in neuropathological or neuroradiological studies of various acute brain dysfunctions, notably traumatic brain injury [16, 17] and critical illness [51,52,53].
Our findings provide valuable information on the incidence of secondary brain insults occurring in the ICU, in both primary and non-primary brain-injured patients. While delayed intracranial conduction time was expected in the group of brain-injured patients, it was found with a similar incidence in non-primary brain-injured patients. This finding suggests that secondary insults occur in both primary brain-injured and non-brain-injured patients, homogenizing these two subgroups in terms of central conduction times. Both primary brain-injured and non-brain-injured patients are liable to secondary insults, including ischemic, metabolic, toxic or inflammatory factors [54]. Therefore, this finding would also indicate that medical critically ill patients should potentially be considered brain injured. Brain injury and insults have been documented in septic patients. Therefore, one may argue that the dichotomy between brain injured and non-brain injured is inaccurate and that a dichotomy between primary versus secondary brain insult should be preferred (Table 5).
While delayed intracranial conduction time is probably caused by secondary brain insults and to a lesser extent, by sedatives, its association with mortality may be explained by the extent of cerebral suffering, including involvement of critical areas implicated in preserving vital functions such as the brain stem [34, 35, 55]. Delirium, characterized by impaired cognition, conscience and arousal, is frequent in the ICU [6,7,8]. Impairment of the ascending reticular activation system (ARAS) located in the upper part of the brainstem may also be implicated in the genesis of delirium, notably impairment of arousal. Interestingly, increased IPCT may reflect a dysfunction at the level of pons or midbrain, which includes the ARAS [56]. Finally, impaired intracranial conduction times have long been evidenced in various primary brain insults; that are also associated with delirium [26, 44].
Study limitations
Our study has several limitations. First, since our aim was to assess the usefulness of evoked potential in patients requiring deep sedation, we studied both primarily brain-injured and non-brain-injured patients. Identifying neurophysiological differences between brain-injured and non-brain-injured patients and between septic and non-septic patients might be hampered by a lack of power. Due to the observational nature of the study, we are unable to determine whether the observed impaired consciousness results from brain insult, sedation or both. Finally, since we did not adjust statistical tests for multiple comparisons, our results should be viewed as exploratory. This limitation is mitigated by the fact that we tested a small number of scientific hypotheses—those pertaining to the association of EPs with the outcomes.
Overall, in deeply sedated critically ill patients, early impairment of ICCT was associated with in-ICU mortality while early impairment of IPCT tended to be associated with the occurrence of altered mental status. Confirmation of these results is currently under investigation in a larger multicenter prospective cohort study (ClinicalTrials.gov number: NCT02395861).
Abbreviations
- AMS:
-
altered mental status
- BAEP:
-
brainstem auditory evoked potentials
- CAM-ICU:
-
Confusion Assessment Method in ICU
- EEG:
-
electroencephalogram
- EP:
-
evoked potential
- FOUR:
-
the Full Outline of Unresponsiveness score
- GCS:
-
the Glasgow Coma Scale
- ICCT:
-
intracranial conduction time
- ICU:
-
intensive care unit
- IPCT:
-
intrapontine conduction time
- IPL:
-
inter-peak latency
- MCA:
-
multiple correspondence analysis
- PL:
-
peak latency
- RASS:
-
Richmond Assessment Sedation Scale
- SAPS II:
-
Simplified Acute Physiology Score
- SIRS:
-
Systemic Inflammatory Response Syndrome
- SOFA:
-
Sequential Organ Failure Assessment score
- SSEP:
-
somatosensory evoked potentials
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- TBI:
-
traumatic brain injury
References
Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306.
Shehabi Y, Bellomo R, Mehta S, Riker R, Takala J. Intensive care sedation: the past, present and the future. Crit Care. 2013;17:322.
Grap MJ, Munro CL, Wetzel PA, Best AM, Ketchum JM, et al. Sedation in adults receiving mechanical ventilation: physiological and comfort outcomes. Am J Crit Care. 2012;21:e53–e63; quiz e64.
Shehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39:910–8.
Lee CM, Mehta S. Early sedation use in critically ill mechanically ventilated patients: when less is really more. Crit Care. 2014;18:600.
Longrois D, Conti G, Mantz J, Faltlhauser A, Aantaa R, et al. Sedation in non-invasive ventilation: do we know what to do (and why)? Multidiscip Respir Med. 2014;9:56.
Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28:3–6.
Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, et al. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37:177–83.
Balzer F, Weiss B, Kumpf O, Treskatsch S, Spies C, et al. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit Care. 2015;19:197.
Tanaka LM, Azevedo LC, Park M, Schettino G, Nassar AP, et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Crit Care. 2014;18:R156.
Annane D, Sharshar T. Cognitive decline after sepsis. Lancet Respir Med. 2015;3:61–9.
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62.
Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med. 2014;370:185–6.
Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38.
Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538.
Zammit C, Knight WA. Severe traumatic brain injury in adults. Emerg Med Pract. 2013;15:1–28.
Amantini A, Grippo A, Fossi S, Cesaretti C, Piccioli A, et al. Prediction of ‘awakening’ and outcome in prolonged acute coma from severe traumatic brain injury: evidence for validity of short latency SEPs. Clin Neurophysiol. 2005;116:229–35.
Fossi S, Amantini A, Grippo A, Innocenti P, Amadori A, et al. Continuous EEG-SEP monitoring of severely brain injured patients in NICU: methods and feasibility. Neurophysiol Clin. 2006;36:195–205.
Haupt WF, Hojer C, Pawlik G. Prognostic value of evoked potentials and clinical grading in primary subarachnoid haemorrhage. Acta Neurochir (Wien). 1995;137:146–150, discussion 150.
Logi F, Fischer C, Murri L, Mauguiere F. The prognostic value of evoked responses from primary somatosensory and auditory cortex in comatose patients. Clin Neurophysiol. 2003;114:1615–27.
Zhang Y, Su YY, Haupt WF, Zhao JW, Xiao SY, et al. Application of electrophysiologic techniques in poor outcome prediction among patients with severe focal and diffuse ischemic brain injury. J Clin Neurophysiol. 2011;28:497–503.
Azabou E, Fischer C, Guerit JM, Annane D, Mauguiere F, et al. Neurophysiological assessment ofbrain dysfunction in critically ill patients: an update. Neurol Sci. 2017;38(5):715–26.
Boisseau N, Madany M, Staccini P, Armando G, Martin F, et al. Comparison of the effects of sevoflurane and propofol on cortical somatosensory evoked potentials. Br J Anaesth. 2002;88:785–9.
Guerit JM, Amantini A, Amodio P, Andersen KV, Butler S, et al. Consensus on the use of neurophysiological tests in the intensive care unit (ICU): electroencephalogram (EEG), evoked potentials (EP), and electroneuromyography (ENMG). Neurophysiol Clin. 2009;39:71–83.
Liu EH, Wong HK, Chia CP, Lim HJ, Chen ZY, et al. Effects of isoflurane and propofol on cortical somatosensory evoked potentials during comparable depth of anaesthesia as guided by bispectral index. Br J Anaesth. 2005;94:193–7.
Zentner J, Ebner A. Prognostic value of somatosensory- and motor-evoked potentials in patients with a non-traumatic coma. Eur Arch Psychiatry Neurol Sci. 1988;237(3):184–7.
Recommendations for the practice of clinical neurophysiology: guidelines of the International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1999;Suppl 52:1–304.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44.
Bouwes A, Doesborg PG, Laman DM, Koelman JH, Imanse JG, et al. Hypothermia after CPR prolongs conduction times of somatosensory evoked potentials. Neurocrit Care. 2013;19:25–30.
Guerit JM, Fischer C, Facco E, Tinuper P, Murri L, et al. Standards of clinical practice of EEG and EPs in comatose and other unresponsive states. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:117–31.
Lang M, Welte M, Syben R, Hansen D. Effects of hypothermia on median nerve somatosensory evoked potentials during spontaneous circulation. J Neurosurg Anesthesiol. 2002;14:141–5.
Zanatta P, Bosco E, Comin A, Mazzarolo AP, Di Pasquale P, et al. Effect of mild hypothermic cardiopulmonary bypass on the amplitude of somatosensory-evoked potentials. J Neurosurg Anesthesiol. 2014;26:161–6.
Azabou E, Magalhaes E, Braconnier A, Yahiaoui L, Moneger G, et al. Early standard electroencephalogram abnormalities predict mortality in septic intensive care unit patients. PLoS ONE. 2015;10:e0139969.
Sharshar T, Porcher R, Siami S, Rohaut B, Bailly-Salin J, et al. Brainstem responses can predict death and delirium in sedated patients in intensive care unit. Crit Care Med. 2011;39:1960–7.
Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58:585–93.
Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289:2983–91.
Koenig MA, Kaplan PW. Clinical Applications for EPs in the ICU. J Clin Neurophysiol. 2015;32:472–80.
Frenette AJ, Bebawi ER, Deslauriers LC, Tessier AA, Perreault MM, et al. Validation and comparison of CAM-ICU and ICDSC in mild and moderate traumatic brain injury patients. Intensive Care Med. 2016;42(1):122–3.
Facco E, Munari M, Baratto F, Behr AU, Giron GP. Multimodality evoked potentials (auditory, somatosensory and motor) in coma. Neurophysiol Clin. 1993;23:237–58.
Fischer C, Luaute J, Nemoz C, Morlet D, Kirkorian G, et al. Improved prediction of awakening or nonawakening from severe anoxic coma using tree-based classification analysis. Crit Care Med. 2006;34:1520–4.
Guerit JM. Prognostic contribution for potentials evoked in unit of intensive care. Ann Fr Anesth Reanim. 2004;23:99–101.
Guerit JM, de Tourtchaninoff M, Soveges L, Mahieu P. The prognostic value of three-modality evoked potentials (TMEPs) in anoxic and traumatic comas. Neurophysiol Clin. 1993;23:209–26.
Zauner C, Gendo A, Kramer L, Funk GC, Bauer E, et al. Impaired subcortical and cortical sensory evoked potential pathways in septic patients. Crit Care Med. 2002;30:1136–9.
Amantini A, Amadori A, Fossi S. Evoked potentials in the ICU. Eur J Anaesthesiol Suppl. 2008;42:196–202.
Constantin JM, Momon A, Mantz J, Payen JF, De Jonghe B, et al. Efficacy and safety of sedation with dexmedetomidine in critical care patients: a meta-analysis of randomized controlled trials. Anaesth Crit Care Pain Med. 2016;35:7–15.
Parry GJ, Aminoff MJ. Somatosensory evoked potentials in chronic acquired demyelinating peripheral neuropathy. Neurology. 1987;37:313–6.
Rattay F, Potrusil T, Wenger C, Wise AK, Glueckert R, et al. Impact of morphometry, myelinization and synaptic current strength on spike conduction in human and cat spiral ganglion neurons. PLoS ONE. 2013;8:e79256.
Klistorner A, Garrick R, Barnett MH, Graham SL, Arvind H, et al. Axonal loss in non-optic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology. 2013;80:242–5.
Walsh JC, Yiannikas C, McLeod JG. Abnormalities of proximal conduction in acute idiopathic polyneuritis: comparison of short latency evoked potentials and F-waves. J Neurol Neurosurg Psychiatry. 1984;47:197–200.
Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806.
Sharshar T, Gray F, Poron F, Raphael JC, Gajdos P, et al. Multifocal necrotizing leukoencephalopathy in septic shock. Crit Care Med. 2002;30:2371–5.
Zauner C, Gendo A, Kramer L, Kranz A, Grimm G, et al. Metabolic encephalopathy in critically ill patients suffering from septic or nonseptic multiple organ failure. Crit Care Med. 2000;28:1310–5.
Polito A, Eischwald F, Maho AL, Azabou E, Annane D, et al. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013;17:R204.
Rohaut B, Porcher R, Hissem T, Heming N, Chillet P, et al. Brainstem response patterns in deeply-sedated critically-ill patients predict 28-day mortality. PLoS ONE. 2017;12:e0176012.
Fischer C, Bognar L, Turjman F, Villanyi E, Lapras C. Auditory early- and middle-latency evoked potentials in patients with quadrigeminal plate tumors. Neurosurgery. 1994;35:45–51.
Authors’ contributions
EA, EM, BR, RP, FC, FL, JM, and TS contributed to the conception and design of the study. EA, BR, EM, AP, SK, JA, RM-K, GM, VM, and NH performed data acquisition. EA, BR, RP, SK, JA, RM-K, GM, NH, VM, AP, EM, FC, DA, FL, JM, and TS contributed to interpretation and analysis of the data; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data are available upon request by contacting the study supervisor Prof. Tarek Sharshar: tarek.sharshar@aphp.fr.
Consent for publication
Written consent for publication of the results of this study was obtained from the patients’ legal representatives together with the informed consent for their participation to this study.
Funding
EM was supported by a “Master 2 Bourse” Grant allocated by French Intensive Care Society (FICS)/Société de Réanimation de Langue Française (SRLF) in 2012.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
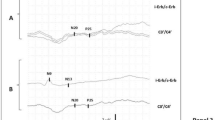
Schematic representation of median nerve’s somatosensory evoked potentials (SSEP) responses localizations on brain MRI (a) and typical examples of their normal wave forms as well as recording electrodes montages (b). SSEP elicited by electric stimulation (15 mA) of median nerve at the wrist: N9, N13 and P14, respectively, the brachial plexus, cervical spinal cord, and cervico-medullary (subcortical) responses. N20 and P25 are responses of the primary sensory cortex. N9–N13 inter-peak latencies (IPL) represent a proximal peripheral nerve conduction time, and P14–N20 IPL the intracranial conduction time (ICCT). Recording and reference electrodes were placed at Cv7 (7th cervical vertebra)—Fz: for the N13 cervical spinal cord response and Cz-cSh (contralateral shoulder): for the subcortical far-field potential: P14. The cortical components, N20 and P25 were recorded at the contralateral C3′ or C4′ positions (2 cm behind C3 or C4) according to the international 10–20 system. Two sets of 500 sweeps were averaged
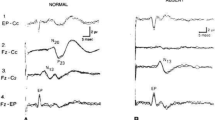
Schematic representation of brainstem auditory evoked potentials (BAEP) responses localizations on a brain MRI (a) and typical examples of their normal wave forms as well as recording montage (b). BAEP elicited by auditory click (100-µs 80-dB) applied to one ear, with a (−20 dB) masking of the contralateral ear by “white noise.” The recurrence frequency was 19.3 Hz (bandpass, 150–1500 Hz; sweep time, 10 ms). BAEP were picked up in Fz. with the reference electrode placed at the earlobe ipsilateral to the stimulated ear (Fz—A1 or A2). Two sets of 2000 sweeps were averaged. The I–III IPL represents the peripheral conduction time of the auditory pathway, when the III–V IPL represents the intrapontine conduction time (IPCT)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Azabou, E., Rohaut, B., Heming, N. et al. Early impairment of intracranial conduction time predicts mortality in deeply sedated critically ill patients: a prospective observational pilot study. Ann. Intensive Care 7, 63 (2017). https://doi.org/10.1186/s13613-017-0290-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-017-0290-5