Abstract
Engineered Salmonella typhimurium (S.t-ΔpGlux/pT-ClyA) and attenuated Salmonella typhimurium (SL: Salmonella typhimurium with a defect in the synthesis of guanine 5′-diphosphate-3′-diphosphate) exhibit similar tumor targeting capabilities (Kim et al. in Theranostics 5:1328–1342, 2015; Jiang et al. in Mol Ther 18:635–642, 2013), but S.t-ΔpGlux/pT-ClyA exerts superior tumor suppressive effects. The aim of this study was to investigate whether S.t-ΔpGlux/pT-ClyA inhibits colon cancer growth and recurrence by promoting increased IL-1β production. The CT26 tumor mouse model was used, and mice were treated in the following ways: PBS, S.t-ΔpGlux/pT-ClyA(+) + IL-1βAb, SL, S.t-ΔpGlux/pT-ClyA(−), and S.t-ΔpGlux/pT-ClyA(+). Dynamic evaluation of the efficacy of S.t-ΔpGlux/pT-ClyA in the treatment of colon cancer was assessed by MRI. Western blot, immunofluorescence and flow cytometry analysis were used to investigate IL-1β-derived cells and IL-1β expression on tumor cells and immune cells to analyze the regulatory mechanism. IL-1β levels in tumors colonized by S.t-ΔpGlux/pT-ClyA were significantly increased and maintained at high levels compared to control treatments. This increase caused tumors to subside without recurrence. We examined the immune cells mediating S.t-ΔpGlux/pT-ClyA-induced tumor suppression and examined the major cell types producing IL-1β. We found that macrophages and dendritic cells were the primary IL-1β producers. Inhibition of IL-1β in mice treated with S.t-ΔpGlux/pT-ClyA using an IL-1β antibody caused tumor growth to resume. This suggests that IL-1β plays an important role in the treatment of cancer by S.t-ΔpGlux/pT-ClyA. We found that in St-ΔpGlux/pT-ClyA-treated tumors, expression of molecules involved in signaling pathways, such as NLRP3, ASC, Caspase1, TLR4, MyD88, NF-kB and IL-1β, were upregulated, while in ΔppGpp S. typhimurium treated animals, TLR4, MyD88, NF-kB and IL-1β were upregulated with NLRP3, ASC, and Caspase1 being rarely expressed or not expressed at all. Using S.t-ΔpGlux/pT-ClyA may simultaneously activate TLR4 and NLRP3 signaling pathways, which increase IL-1β expression and enhance inhibition of colon cancer growth without tumor recurrence. This study provides a novel platform for treating colon cancer.
Similar content being viewed by others
Introduction
Colon cancer is one of the most common malignant tumors in the world. With changing lifestyles and increasing lifespans, the incidence of colon cancer is increasing by the year. In 2018, the estimated number of new cases of colon cancer in the United States was 97,220, and the number of deaths was estimated at 50,630 (Siegel et al. 2018). Bacterial-mediated cancer therapy (BCT) was originally used to treat solid tumors (Nguyen and Min 2017). A variety of bacteria have been found to be useful in anti-tumor therapy, such as Bifidobacterium (Luo et al. 2016), Escherichia (Ninomiya et al. 2014), Clostridium (Luo et al. 2016), Salmonella (Park et al. 2016; Nguyen and Min 2017) and Listeria (Loessner and Weiss 2004; van Pijkeren et al. 2010). Compared to peripheral tumor proliferative tissues, facultative anaerobic bacteria, such as attenuated Salmonella and Escherichia coli, are more likely to colonize hypoxic and necrotic areas by accurately targeting the hypoxic regions of solid tumors (Xie et al. 2018; Frahm 2015; Bolhassani and Zahedifard 2012). As conventional therapies fail to provide sustainable remission for most cancer patients, the emerging and unique immune therapeutic approach of bacteria-mediated cancer therapy (BCT) is marching towards a feasible solution.
Attenuated S. typhimurium that is defective in ppGpp (ΔppGpp S. typhimurium) synthesis has tumor-targeting ability and significantly suppresses tumor growth (Cao et al. 2016; Yoon et al. 2018; Vola et al. 2018). Colonization and subsequent proliferation of ΔppGpp S. typhimurium within tumor tissues induces infiltration of immune cells, such as neutrophils, macrophages, and dendritic cells, which then secrete pro-inflammatory cytokines, such as IL-1β, which contribute to anti-cancer efficacy (Yu 2018; Qu et al. 2012; Palsson-McDermott et al. 2015; Kim et al. 2015). The latest literature reports that ΔppGpp S. typhimurium exerts anti-cancer effects by promoting secretion of IL-1β from macrophages or dendritic cells. However, this method is typified by tumor recurrence after treatment, which is related with decreased IL-1β levels (Kim et al. 2015). Some studies have shown that low concentrations of IL-1β promote the secretion of IL-17 from CD4+ T cells, inhibiting the body’s anti-tumor mechanisms. High concentrations of IL-1β activate CD8+ T cells, which promotes anti-tumor effects (Ghiringhelli et al. 2009; Bruchard et al. 2013). IL-1β production is closely related to the toll receptor 4 (TLR4) signaling and NOD-like receptor NLRP3 signaling pathways (Jimenez-Dalmaroni et al. 2016; Kim et al. 2015). Lipopolysaccharide (LPS) (Mariathasan et al. 2004) and cytolysin (ClyA) (Wallace et al. 2000) are important ligands for the activation of TLR4 and NLRP3 signaling pathways, respectively. LPS is an endotoxin of gram-negative bacteria (Kahler et al. 2018). The ligand bacterial toxin of NLRP3 can be activated from formed plasma membrane pores, thereby activating NLRP3. However, the pores formed on the cell membrane are rapidly closed after the external stimulus is cleared, so it is difficult to maintain sustained release of IL-1β (Jia et al. 2018). Of note, cytolysin A (ClyA) can act on the mammalian cell membrane to form a persistent pore, resulting in a significant outflow of K+ from the cell (Jia et al. 2018), thereby activating the NLRP3 inflammatory body pathway (Gupta et al. 2014). In addition, the combination of attenuated Salmonella and ClyA genes through bioengineering technology may offer the possibility sustained, increased release of IL-1β. This may overcome the phenomenon of tumor recurrence caused by downregulation of IL-1β in the tumor microenvironment during the late stage of colon cancer treatment by a single attenuated Salmonella.
Previous studies have successfully transfected the ClyA gene into ΔppGpp S. typhimurium using genetic engineering technology to form an engineered Salmonella: S.t-ΔpGlux/pT-ClyA, which was confirmed to significantly inhibit subcutaneous inoculation of tumors in CT26 colon cancer (Jiang et al. 2013). To investigate whether St-ΔpGlux/pT-ClyA inhibits colon cancer growth by promoting increased production of IL-1β, we dynamically evaluated the efficacy of S.t-ΔpGlux/pT-ClyA for colon cancer treatment by MRI and explored the cellular origin of IL-1β in colon cancer treatment. In addition, we assessed the effect of IL-1β on tumors and analyzed the regulatory mechanisms involved using molecular biology techniques.
Materials and methods
Tumor cell line and animal model
The murine CT26 colon adenocarcinoma cell line was obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). Cells were grown at 37 °C and 5% CO2 in Dulbecco’s Modified Eagle’s Medium (Gibco®, Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (HyClone Laboratories, Inc., Logan, UT, USA).
Male BALB/c mice (4–5 weeks old) were purchased from the Experimental Animal Center of Central South University and were housed individually at 22 °C to 25 °C under a 12 h light/dark cycle with free access to food and water. To generate in situ colorectal cancer in mice, CT26 cells were injected into the right side of the mouse through a 1 ml syringe to achieve subcutaneous tumor formation. After the tumor body grew to a certain size, subcutaneous tumor-forming mice were euthanized, and the tumor mass was cut into 1–2 mm pieces. The tumor block was then transplanted into the cecum of a normal mouse to establish an orthotopic colon cancer model, as previously described (Rajput et al. 2008). When tumor volume exceeded 2000 mm3, animals were euthanized and excluded from the experiment. All animal experimental procedures used in this study were approved by the Animal Ethics Committee of Central South University and conducted in accordance with the Guideline of the Care and Use of Laboratory Animals in Central South University.
Animal experiments
Tumor-forming mice were treated with PBS, SL, S.t-ΔpGlux/pT-ClyA(+/−), or S.t-ΔpGlux/pT-ClyA + IL-1βAb. Experimental groups included a placebo (Intravenous injection of PBS, 100 µl), SL treatment (Intravenous injection of 3 × 107 CFU ΔppGpp S. typhimurium suspension, 100 µl), and engineered Salmonella St-ΔpGlux/pT-ClyA treatment group (Intravenous injection of 3 × 107 CFU S.t-ΔpGlux/pT-ClyA suspension, 100 µl). Mice were fed daily doxycycline (17 mg/kg/day) to induce ClyA protein expression (previous studies have achieved relevant results) (Chen et al. 2015; Jiang et al. 2010, 2013). We injected 5 µg of IL-1β antibody (AF401-NA, R&D Systems) into tumor-bearing mice via the tail vein 1 day before and during treatment (two times a week). The mice were euthanized, and tumors were isolated at the end of the experiment. The tumor size of each group was measured using MRI. Tumor volumes (mm3) were estimated using the formula (L × H×W)/2 where L is the length, W is the width, and H is the height of the tumor in millimeters (Yu et al. 2004).
Preparation of engineered S. typhimurium
The primers, plasmids, and bacterial strains (S. typhimurium ΔppGpp/lux) used in this study were kindly provided by Shengnan Jian (Chonnam National University, South Korea). S. typhimurium ΔppGpp/lux-pTet/ClyA(S.t-ΔpGlux/pT-ClyA) were constructed as described previously (Jiang et al. 2013; Williams et al. 2010). We constructed the plasmid pJL43 based on the plasmid pJL39. The antitumor gene (ClyA) was placed under the control of the TetR system in the pJL43 plasmid and was then transformed into the S. typhimurium ΔppGpp/lux strains. All the primers and constructs used in this study are listed in Tables 1 and 2.
MRI and optical bioluminescence imaging
Magnetic resonance imaging (MRI) was performed using a 3.0 T MRI system (Signa HDxt; GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) with a small animal head coil (Shanghai Chenguang Medical Technologies Co. Ltd, Shanghai, People’s Republic of China). MRI sequences and parameters were as follows: T2-weighted sequence (echo train length = 4; repetition time = 3000 ms; echo time = 120 ms; number of averages = 1) and T1-weighted sequence (repetition time = 350 ms; echo time = minimum value; number of average = 2) before and after injection of 0.2 mL contrast medium (gadodiamide, Omniscan; Amersham Health, Princeton, NJ, USA). Images were acquired for eight slices in the axial plane (slice thickness = 2.0 mm; matrix = 256 × 192). Mice were anesthetized with isoflurane (initial dose 2.5%, maintenance 1.6%). Bioluminescence imaging was performed with the IVIS 100 system (Caliper Life, Sciences, Hopkinton, MA, USA) (Jiang et al. 2013; Nguyen et al. 2010; Massoud and Gambhir 2003). ImageJ was used to measure tumor volume and necrotic volume.
Western blot analysis, immunofluorescence and antibodies
The third day after bacterial treatment, tumor tissue was taken. After resecting tumors from rats, tumors were homogenized on ice in RIPA buffer (Sigma-Aldrich Co.) containing protease inhibitor cocktail (Sigma-Aldrich Co.). After 120 min of incubation and 30 min of centrifugation (12,000 rpm at 4 °C), the supernatant was then pipetted off and kept on ice. Protein concentrations were measured using the bicinchoninic acid assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Protein samples were subjected to SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad). Membranes were probed with polyclonal goat anti-mouse antibodies followed by horseradish peroxidase-conjugated donkey anti-goat IgG (sc-2020, Santa Cruz Biotechnology; 1:2000 dilution). Protein levels were determined using enhanced chemiluminescence plus (Amersham, Buckinghamshire, UK) and Image Reader (LAS-3000 Imaging System; Fuji Photo Film, Tokyo, Japan). Details concerning protein extraction and western blot (WB) can be found in our previous study (Chen et al. 2015).
Immunol Fluorescence was performed using the Immunol Fluorescence Staining Kit with kFluor555-labeled goat anti-rabbit IgG (Keygenbiotech) according to the manufacturer’s instructions. For immunofluorescence staining, tissue sections were permeabilized and blocked in TBS containing 0.1% Tween 20, 0.3% Triton X-100, and 5% BSA. Sections were then incubated with antibodies overnight at 4 °C followed by addition of secondary antibodies (all from Invitrogen). All antibodies were diluted 1:200 in TBS containing 0.1% Tween 20, 1% BSA, and 0.1% Triton X-100. Samples were mounted using Pro-long® Gold antifade reagent (P36930, Invitrogen) and analyzed under a Fluoview-1000 (FV-1000) laser scanning confocal microscope (Olympus). The antibodies used in this article are as follows: NLRP3 (1:1000), ASC (1:1000), Caspase-1 (1:500), IL-1β (1:300), TLR4 (1:1000), MyD88 (1;500), NF-κB (1;300), GAPDH (1:500). Antibodies for NLRP3, ASC and caspase-1 were from Adipogen International (San Diego, CA, USA). Antibody for IL-1β, TLR4 and MyD88 was purchased from R&D Systems (Minneapolis, MN, USA). NF-κB were from Cell Signaling Technology (Beverly, MA, USA). Antibody for GAPDH was from Sigma-Aldrich (St. Louis, MO, USA). Secondary antibodies for western blot were from Sigma-Aldrich (St. Louis, MO, USA). Secondary antibodies for immunofluorescence were from Jackson Immunoresearch Laboratories (West Grove, PA, USA) and Abcam (Cambridge, MA, USA).
Measurement of IL-1β levels in tumor tissues and serum
Blood was collected by cardiac puncture at 0, 0.5, 3, 12 and 24 h, and then serum was harvested by removing the clotted blood after centrifugation. Cytokine levels were measured using individual ELISA kits (eBioscience) according to the manufacturer’s instructions. The substrate color reaction was measured at 450 nm in an ELISA plate reader (SpectraMax, Molecular Devices).
Flow cytometry analysis
Mice were euthanized, and spleen tissue was isolated and made into a single cell suspension for flow cytometry analysis. Cells were digested with 0.25% trypsin, centrifuged, and then resuspended in PBS and transferred to an EP tube. Cells were fixed with the addition of 200 µl of 1% paraformaldehyde. Cells were washed with PBS and 200 µl of 90% methanol was added, and cells were then placed at 4 °C. The primary antibody was incubated after washing with PBS. Antibodies used in these experiments include CD11c (N418), CD68 (FA-11), Ly6C (HK1.4), Ly6G (1A8), and IL-1β (NJTEN3). The secondary antibody was incubated after washing with PBS. Flow cytometry was performed using a BD LSR II and BD FACS Aria III flow cytometer (BD Bioscience), and data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Statistical analysis
GraphPad Prism 7 software was used to analyze data. Survival analysis was performed using a Kaplan–Meier curve. All data are expressed as the mean ± SD, and data were considered significant at p < 0.05.
Results
Establishment of BALB/c colon cancer model
58 BALB/c mice were subjected to orthotopic colon cancer surgery with no deaths occurring during the operation. During the recovery period of 4 days, 8 mice died. On the fifth day after surgery, mice were randomly divided into 5 groups: tumor-bearing mice treated with PBS, SL, S.t-ΔpGlux/pT-ClyA (+/−Doxycycline induction), S.t-ΔpGlux/pT-ClyA + IL-1βAb. The modeling process is shown in Fig. 1.
Lux/pT-ClyA-secreting engineered bacteria to target CT26 tumor mice
We examined the tumor-targeting activity of S.t-ΔpGlux/pT-ClyA in BALB/c mice with CT26 cecal cancer. Bacterial bioluminescence was detected in tumors of injected mice (Fig. 2). These results indicate that the genetically engineered bacteria maintained their ability to target tumors.
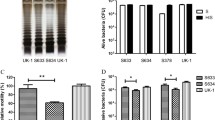
Imaging of lux/pT-ClyA-expressing S. typhimurium in a mouse model of cecal colon cancer. BALB/c mice were surgically implanted with CT26 tumors, each measuring 1–2 mm3, with fragments being transplanted onto the ceca colon. Distribution of bacteria visualized by in vivo bioluminescence imaging after injection of engineered Salmonella typhimurium secreting lux/pT-ClyA. Images show representative mice before and after euthanasia. a Bioluminescence image shows that bacteria began to colonize the tumor the third day after the injection of the engineered bacteria into the tail vein. b On the sixth day, mice were dissected. L liver, S spleen, K kidney, H heart, L lung (the above images are from the same mouse)
Tumor suppressive effects of engineered bacteria secreting lux/pT-ClyA
BALB/c mice transplanted with CT26 tumors were intravenously injected with PBS, ΔppGpp S. typhimurium (SL), or ΔppGpp S. typhimurium carrying ClyA (S.t-ΔpGlux/pT-ClyA) to evaluate the antitumor activity of engineered bacteria. Previous research reported tumor recurrence after treatment, which was related to decreased IL-1β levels (Kim et al. 2015). We examined the role of IL-1β in Salmonella-mediated cancer therapy by blocking its activity with an anti-IL-1β antibody. As such, a separate group received an intratumoral injection of ΔppGpp S. typhimurium carrying ClyA + anti-IL-1β antibody (S.t-ΔpGlux/pT-ClyA + IL-1β Ab). We discovered ΔppGpp S. typhimurium carrying lux/pT-ClyA exerted the strongest inhibitory effect on tumor growth induced by doxycycline compared to other groups. In contrast, other groups’ tumors regrew at the end of treatment. Tumors injected with PBS grew at the same rate as non-treated tumors. These results indicate that treatment with engineered Salmonella expressing lux/pT-ClyA results in significant suppression of tumor growth, and IL-1β production by S.t-ΔpGlux/pT-ClyA increases therapeutic efficacy (Fig. 3a, b). In addition, the group of animals that received engineered lux/pT-ClyA-expressing bacteria exhibited higher survival rates than other groups (Fig. 3c).
Effect of engineered lux/pT-ClyA-expressing Salmonella on growth and survival of CT26 tumors. BALB/c mice (n = 6 per group) were surgically implanted with one piece of CT26 tumor in the ceca colon. When tumors reached a volume of approximately 120–180 mm3, mice were divided into five treatment groups. Groups received intravenous (i.v.) injections of PBS, ΔppGpp S. typhimurium, or ΔppGpp S. typhimurium carrying lux/pT-ClyA either with (+) or without (−) doxycycline induction. For IL-1β depletion, mice received an i.v. injection of anti-IL-1β-specific antibody (IL-1β Ab) 1 day before engineered Salmonella typhimurium secreting lux/pT-ClyA (Day 1). The antibody was then injected twice a week for 2 weeks. Where relevant, mice received 17 mg/kg/day doxycycline. a Images of tumors from representative mice from each group. b Changes in tumor size. c Kaplan–Meier survival curves for CT26 tumor-bearing mice. Statistical significance was calculated by comparison with PBS or S.t-ΔpG lux/pT-ClyA(+)+IL-1βAb
Next, tumors were visualized after dissection. The visual map clearly shows that the tumor volume of mice treated with ΔppGpp S. typhimurium carrying ClyA (+Doxycycline induction) group was smaller compared to other groups (Fig. 4).
Secreting-lux/pT-ClyA engineered Salmonella induce elevated IL-1β serum levels
We examined serum levels of IL-1β in each group. We found that IL-1β serum levels were significantly higher in mice treated with expressing-lux/pT-ClyA S.t-ΔpGlux/pT-ClyA than in other groups treated with PBS, SL, SL plux-pT-ClyA(−), SLplux-pT-ClyA(+)+IL-1β Ab, respectively (Fig. 5a). Specifically, we analyzed serum IL-1β levels in PBS, SL, and S.t-ΔpGlux/pT-ClyA(+) groups at indicated times (Fig. 5b). Result indicated that secreting-lux/pT-ClyA engineered Salmonella increased production of IL-1β in serum.
Serum IL-1β levels in different groups at different time points. a Serum IL-1β levels were measured by ELISA. Results showed that serum IL-1β levels were significantly higher in mice treated with engineered secreting-lux/pT-ClyA salmonella compared to other groups. b Serum IL-1β levels in PBS, SL, S.t-ΔpGlux/pT-ClyA(+) at indicated times
Dendritic cells and macrophages are responsible for increased IL-1β levels in tumors colonized by engineered Salmonella (S.t-ΔpGlux/pT-ClyA)
Next, we examined immune cell infiltration into tumors colonized by bacteria. Immune cells (macrophages, dendritic cells, and neutrophils) may represent the primary sources of pro-inflammatory IL-1β. We double stained macrophages (CD68), neutrophils (Ly-6G/Ly-6C) and DCs (CD11c) for IL-1β and examined cells under an immunofluorescence (IF) microscope. First, we found that bacterial treatment resulted in increased infiltration of tumor tissues by immune cells. CT26 colon cancer mice intravenously injected with lux/pT-ClyA-secreting engineered salmonella exhibited the highest levels of invasive immune cells and IL-1β expression, consistent with previous studies (Kim et al. 2015). Second, some macrophages and DCs (CD11c) colocalized with the IL-1β signal, but neutrophils are only slightly or not colocalize with IL-1β, indicating that DCs and macrophages are the primary producers of IL-1β. Third, CT26 colon cancer mice that received an intratumoral injection of S.t-ΔpG lux/pT-ClyA(+)+IL-1β Ab had many infiltrated immune cells, but IL-1β was rarely produced (Fig. 6a–c). Flow cytometry analysis supported the IF microscopic findings, showing that treatment with engineered Salmonella led to significant increases in IL-1β expression (Fig. 6d).
Analysis of immune cell accumulation in tumor tissues and levels of IL-1β. a–c Tumor tissue was excised and fixed in 3 dpi, cryo-sectioned, and immunostained for IL-1β plus CD68 (macrophages), Ly-6G/ls), or CD11c (dendritic cells). Representative images from three independent experiments are shown. d Flow cytometry analysis was used to examine levels of IL-1β in each group in the tumor-draining spleen
Engineered Salmonella typhimurium secreting ClyA enhances cancer immunotherapy by secreting IL-1β through two pathways
To determine whether the antitumor effects of lux/pT-ClyA-secreting ΔppGpp S. typhimurium were mediated through host TLR4 and NLRP3 signaling pathways, we investigated expression levels of NLRP3, ASC, caspase1, TLR4, MyD88, and NF-kB in control and experimental group tumor tissues using western blotting (Fig. 7).
Results demonstrated that mice treated with S.t-ΔpGlux/pT-ClyA exhibited significant increases in IL-1β was notably increased, and TLR4 and NLRP3 signaling pathways were markedly activated, with their expression levels being significantly higher than other groups. Thus, in the present study, upregulation of IL-1β was observed, indicating that engineered S. typhimurium secreting lux/pT-ClyA enhances cancer immunotherapy by inducing IL-1β expression through two pathways.
Discussion
Lipopolysaccharide (LPS) and cytolysin (ClyA) are important ligands for the activation of toll-like receptor 4 (TLR4) and NOD-like receptor (NLRP3) signaling pathways, respectively (Gupta et al. 2014; Wallace et al. 2000; Mariathasan et al. 2004). IL-1β production is closely correlated with TLR4 and NLRP3 signaling pathways (Phongsisay 2016; Jimenez-Dalmaroni et al. 2016). Moreover, both innate immune cells and colon cancer cells exhibit expression of TLR4 and NLRP3 inflammatory bodies (Basith et al. 2012; Ungerback et al. 2012; Tang et al. 2010; Lee et al. 2012). Activation of the TLR4 signaling pathway leads to activation of extracellular TLR4 via the MyD88-dependent pathway, forming a TLR4 homodimer. Activated TLR4 binds to the C-terminal TIR of the intracytoplasmic junction protein MyD88 via the toll/IL-1 receptor homology region (TIR) in the cytoplasmic region and is recruited by the N-terminal death domain of MyD88 to bind to the IL-1 receptor. The kinase (IRAK) activating the TIR-MyD88/IRAK-NF-kB pathway expresses the inflammatory cytokine IL-1β (Additional file 1: Figure S1) (Shcheblyakov et al. 2010; Mariathasan et al. 2004; Lee et al. 2012). NLRP3 activation of the N-terminal thermoprotein domain (PYD) causes NLRP3 self-oligomerization, which in turn binds to apoptosis-associated microparticle proteins and recruits Pro-caspase1 to form the NLRP3 inflammatory complex (Strowig et al. 2012). Upon activation of the inflammatory complex, Pro-caspase 1 is cleaved to form caspase 1, which then promotes IL-1β release to the extracellular environment. We found that K+ efflux is a necessary signal for NLRP3 activation, and Cytolysin A (ClyA) forms a channel on the cell membrane that causes a large and sustained K+ outflow, activating the NLRP3 pathway and promoting sustained IL-1β release (Wallace et al. 2000) (Additional file 2: Figure S2). Our previous studies successfully transfected the ClyA gene into attenuated Salmonella ΔppGpp by genetic engineering technology to form engineered Salmonella St-ΔpGlux/pT-ClyA and confirmed that the construct had an obvious inhibitory effect on subcutaneous tumor inoculation of CT26 colorectal cancer (Jiang et al. 2013). We hypothesized that engineered Salmonella S.t-ΔpGlux/pT-ClyA acts by activating TLR4 and NLRP3 signaling pathways to promote increased and sustained IL-1β production. In this research, ELISA results showed that colonization of the engineered Salmonella S.t-ΔpGlux/pT-ClyA resulted in a significant increase in serum IL-1β levels compared to other groups. At the same time, we also found that in St-ΔpGlux/pT-ClyA-treated tumors, expression of involved signaling pathway molecules, such as NLRP3, ASC, Caspase1, TLR4, MyD88, NF-kB and IL-1β, were up regulated; however, TLR4, MyD88, NF-kB and IL-1β were up regulated and NLRP3, ASC, and Caspase1 were rarely expressed or not expressed in the ΔppGpp S. typhimurium group. These findings confirmed our hypothesis that engineered Salmonella S.t-ΔpGlux/pT-ClyA significantly activates TLR4 and NLRP3 signaling pathways, which promotes sustained IL-1β production through two pathways. Phan et al. showed showed highly expression of NLRP3 when treated with this bacterial strain only (Phan et al. 2015), which is different from our results and we will be further demonstrated in the next experiment.
This study demonstrated that IL-1β plays an important role in the anti-tumor process, and IL-1β source cells were primarily monocytes (Hazuda et al. 1990), macrophages (Netea et al. 2009), dendritic cells (Ghiringhelli et al. 2009) and neutrophils (Guma et al. 2009). Dendritic cell-derived IL-1β exerts anti-tumor effects by promoting CD8+ T cells to produce IFN-γ (Ghiringhelli et al. 2009). Recent studies confirmed that tumor cell-derived IL-1β increases the survival rate of EB virus-induced nasopharyngeal carcinoma via recruiting concentrated granulocytes (Chen et al. 2012). However, other studies found that low IL-1β concentrations promote CD4+ T cells to secrete IL-17, inhibiting the body’s anti-tumor effects, while high IL-1β concentrations activate CD8+ T cells and promote anti-tumor effects (Ghiringhelli et al. 2009; Bruchard et al. 2013). This suggests that the IL-1β anti-tumor activity is concentration-dependent, and increasing IL-1β concentration will increase its anti-tumor effects. The ΔppGpp S. typhimurium lipopolysaccharide (LPS) promoted mononuclear/macrophage and dendritic cells to secrete IL-1β through the TLR4 signaling pathway, exerting anti-cancer effects, but tumor recurrence occurred in late treatment stages, which might be related to reduced IL-1β levels (Kim et al. 2015). Results of this research show that blocking IL-1β activity using an IL-1β antibody abolished the anti-tumor effects of S.t-ΔpGlux/pT-ClyA. Given these results, IL-1β has potential anti-cancer activity in the context of bacterial-mediated tumor immunotherapy. Treatment with ΔppGpp S. typhimurium alone resulted in transient tumor suppression. Then, tumors began to grow again, and IL-1β levels returned to baseline. However, treatment with engineered Salmonella S.t-ΔpGlux/pT-ClyA consistently inhibited tumor growth, and IL-1β levels remained high. The survival rate of mice treated with engineered Salmonella S.t-ΔpGlux/pT-ClyA was significantly higher than other experimental groups, indicating that the attenuated Salmonella LPS and ClyA genes were combined by bioengineering technology to release high levels of IL-1β, inhibit tumor growth and improve survival rate. This overcame the tumor recurrence phenomenon caused by downregulation of IL-1β during late stages of colon cancer treated with ΔppGpp S. typhimurium alone.
Immunofluorescence results demonstrated that treatment of tumors with S.t-ΔpGlux/pT-ClyA resulted in a significant increase in tumor infiltration by macrophages (CD68), dendritic cells (CD11c), and neutrophils (Ly-6G/Ly-6C). Previous studies revealed that colonization and proliferation of engineered bacteria significantly increased the proportion of M1-like macrophages and reduced the number of Tregs in tumor tissues (Kim et al. 2015). This means that the engineered bacteria inhibited tumor growth by enhancing the anti-tumor immune response rather than by directly inhibiting growth of the tumor itself. Herein, we found that infiltrating tumor cells produced significant levels of IL-1β in response to intravenous injection of engineered bacteria, resulting in inhibition of tumor growth. In addition, we found that immune cells, particularly dendritic cells and macrophages, were the primary sources of IL-1β production in colonized tumors.
In conclusion, S.t-ΔpGlux/pT-ClyA showed significant anti-cancer effects in a mouse orthotopic colon cancer model, providing new avenues for colon cancer treatment. LPS and ClyA are important factors in activating TLR4 and NLRP3 signaling pathways, respectively. St-ΔpGlux/pT-ClyA activated TLR4-MyD88-NF-Kb-IL-1β and NLRP3-ASC-Caspase-1-IL-1β signaling pathways simultaneously, which enhanced tumor suppressive effects and inducing increased IL-1β release; these results act to overcome the tumor recurrence caused by IL-1β downregulation during late stages of colorectal cancer treatment that are a result of treatment with only ΔppGpp S. typhimurium.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. Data and materials can also be requested from the corresponding author.
References
Basith S, Manavalan B, Yoo TH, Kim SG, Choi S (2012) Roles of toll-like receptors in cancer: a double-edged sword for defense and offense. Arch Pharm Res 35:1297–1316
Bolhassani A, Zahedifard F (2012) Therapeutic live vaccines as a potential anticancer strategy. Int J Cancer 131:1733–1743
Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F (2013) Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 19:57–64
Cao H, Xiang T, Zhang C, Yang H, Jiang L, Liu S, Huang X (2016) MDA7 combined with targeted attenuated Salmonella vector SL7207/pBud-VP3 inhibited growth of gastric cancer cells. Biomed Pharmacother 83:809–815
Chen LC, Wang LJ, Tsang NM, Ojcius DM, Chen CC, Ouyang CN, Hsueh C, Liang Y, Chang KP, Chen CC, Chang YS (2012) Tumour inflammasome-derived IL-1beta recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med 4:1276–1293
Chen JQ, Zhan YF, Wang W, Jiang SN, Li XY (2015) The engineered Salmonella typhimurium inhibits tumorigenesis in advanced glioma. Onco Targets Ther 8:2555–2563
Frahm M, Felgner S, Kocijancic D, Rohde M, Hensel M, Curtiss R 3rd, Erhardt M, Weiss S (2015) Efficiency of conditionally attenuated Salmonella enterica serovar Typhimurium in bacterium-mediated tumor therapy. MBio 6:e00254-15
Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15:1170–1178
Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M (2009) Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum 60:3642–3650
Gupta R, Ghosh S, Monks B, DeOliveira RB, Tzeng TC, Kalantari P, Nandy A, Bhattacharjee B, Chan J, Ferreira F, Rathinam V, Sharma S, Lien E, Silverman N, Fitzgerald K, Firon A, Trieu-Cuot P, Henneke P, Golenbock DT (2014) RNA and beta-hemolysin of group B Streptococcus induce interleukin-1beta (IL-1beta) by activating NLRP3 inflammasomes in mouse macrophages. J Biol Chem 289:13701–13705
Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR (1990) Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem 265:6318–6322
Jia X, Liu B, Bao L, Lv Q, Li F, Li H, An Y, Zhang X, Cao B, Wang C (2018) Delayed oseltamivir plus sirolimus treatment attenuates H1N1 virus-induced severe lung injury correlated with repressed NLRP3 inflammasome activation and inflammatory cell infiltration. PLoS Pathog 14:e1007428
Jiang SN, Phan TX, Nam TK, Nguyen VH, Kim HS, Bom HS, Choy HE, Hong Y, Min JJ (2010) Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol Ther 18:635–642
Jiang SN, Park SH, Lee HJ, Zheng JH, Kim HS, Bom HS, Hong Y, Szardenings M, Shin MG, Kim SC, Ntziachristos V, Choy HE, Min JJ (2013) Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol Ther 21:1985–1995
Jimenez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE (2016) The critical role of toll-like receptors—from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev 15:1–8
Kahler CM, Nawrocki KL, Anandan A, Vrielink A, Shafer WM (2018) Structure-function relationships of the Neisserial EptA enzyme responsible for phosphoethanolamine decoration of lipid A: rationale for drug targeting. Front Microbiol 9:1922
Kim JE, Phan TX, Nguyen VH, Dinh-Vu HV, Zheng JH, Yun M, Park SG, Hong Y, Choy HE, Szardenings M, Hwang W, Park JA, Park S, Im SH, Min JJ (2015) Salmonella typhimurium suppresses tumor growth via the pro-inflammatory cytokine interleukin-1beta. Theranostics 5:1328–1342
Lee CC, Avalos AM, Ploegh HL (2012) Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol 12:168–179
Loessner H, Weiss S (2004) Bacteria-mediated DNA transfer in gene therapy and vaccination. Expert Opin Biol Ther 4:157–168
Luo CH, Huang CT, Su CH, Yeh CS (2016) Bacteria-mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett 16:3493–3499
Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218
Massoud TF, Gambhir SS (2003) Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev 17:545–580
Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA (2009) Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113:2324–2335
Nguyen VH, Min JJ (2017) Salmonella-mediated cancer therapy: roles and potential. Nucl Med Mol Imaging 51:118–126
Nguyen VH, Kim HS, Ha JM, Hong Y, Choy HE, Min JJ (2010) Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res 70:18–23
Ninomiya K, Yamada R, Meisaku H, Shimizu N (2014) Effect of ultrasound irradiation on bacterial internalization and bacteria-mediated gene transfer to cancer cells. Ultrason Sonochem 21:1187–1193
Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, van den Bosch MWM, Quinn SR, Domingo-Fernandez R, Johnston DGW, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O’Neill LAJ (2015) Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 21:347
Park SH, Zheng JH, Nguyen VH, Jiang SN, Kim DY, Szardenings M, Min JH, Hong Y, Choy HE, Min JJ (2016) RGD peptide cell-surface display enhances the targeting and therapeutic efficacy of attenuated Salmonella-mediated cancer therapy. Theranostics 6:1672–1682
Phan TX, Nguyen VH, Duong MT, Hong Y, Choy HE, Min JJ (2015) Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol Immunol 59:664–675
Phongsisay V (2016) The immunobiology of Campylobacter jejuni: innate immunity and autoimmune diseases. Immunobiology 221:535–543
Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Komuves L, Cupp JE, Arnott D, Monack D, Dixit VM (2012) Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490:539–542
Rajput A, San Martin ID, Rose R, Beko A, LeVea C, Sharratt E, Mazurchuk R, Hoffman RM, Brattain MG, Wang J (2008) Characterization of HCT116 human colon cancer cells in an orthotopic model. J Surg Res 147:276–281
Shcheblyakov DV, Logunov DY, Tukhvatulin AI, Shmarov MM, Naroditsky BS, Gintsburg AL (2010) Toll-like receptors (TLRs): the role in tumor progression. Acta Naturae 2:21–29
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30
Strowig T, Henao-Mejia J, Elinav E, Flavell R (2012) Inflammasomes in health and disease. Nature 481:278–286
Tang XY, Zhu YQ, Wei B, Wang H (2010) Expression and functional research of TLR4 in human colon carcinoma. Am J Med Sci 339:319–326
Ungerbäck J, Belenki D, Jawad ul-Hassan A, Fredrikson M, Fransén K, Elander N, Verma D, Söderkvist P (2012) Genetic variation and alterations of genes involved in NFkappaB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis 33:2126–2134
van Pijkeren JP, Morrissey D, Monk IR, Cronin M, Rajendran S, O’Sullivan GC, Gahan CG, Tangney M (2010) A novel Listeria monocytogenes-based DNA delivery system for cancer gene therapy. Hum Gene Ther 21:405–416
Vola M, Monaco A, Bascuas T, Rimsky G, Agorio CI, Chabalgoity JA, Moreno M (2018) TLR7 agonist in combination with Salmonella as an effective antimelanoma immunotherapy. Immunotherapy 10:665–679
Wallace AJ, Stillman TJ, Atkins A, Jamieson SJ, Bullough PA, Green J, Artymiuk PJ (2000) E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell 100:265–276
Williams KJ, Joyce G, Robertson BD (2010) Improved mycobacterial tetracycline inducible vectors. Plasmid 64:69–73
Xie S, Chen M, Song X, Zhang Z, Zhang Z, Chen Z, Li X (2018) Bacterial microbots for acid-labile release of hybrid micelles to promote the synergistic antitumor efficacy. Acta Biomater 78:198–210
Yoon W, Yoo Y, Chae YS, Kee SH, Kim BM (2018) Therapeutic advantage of genetically engineered Salmonella typhimurium carrying short hairpin RNA against inhibin alpha subunit in cancer treatment. Ann Oncol 29:2010–2017
Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, Goebel W, Szalay AA (2004) Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol 22:313–320
Yu X, Zhang H, Yu L, Liu M, Zuo Z, Han Q, Zhang J, Tian Z, Zhang C (2018) Intestinal lamina propria CD4(+) T cells promote bactericidal activity of macrophages via Galectin-9 and Tim-3 interaction during Salmonella enterica Serovar typhimurium infection. Infect Immun 86:e00769-17
Acknowledgements
We are grateful to Dr. Shengnan Jiang for the help in the primers, plasmids, and bacterial strains (S. typhimurium ΔppGpp/lux) provided.
Funding
This work was supported by the National Nature Science Foundation of China (No. 81760515, 81771827). This study has received funding by grants from Finance science and technology project of Hainan Province (No. ZDYF2018175, ZDYD2019164) of China, and Hainan Provincial Health and Family Planning Industry Research Project (No. 16A200107).
Author information
Authors and Affiliations
Contributions
YW, YZ and JC designed experiments, and YZ and JC directed experiments and wrote the manuscript. Yuqin Wu performed experiments. ZF, SJ and JC helped with the experimentation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

Additional file 1: Figure S1.
TLR4 signaling pathway. Activation of the TLR4 signaling pathway leads to activation of extracellular TLR4 via the MyD88-dependent pathway, forming a TLR4 homodimer. Activated TLR4 binds to the C-terminal TIR of the intracytoplasmic junction protein MyD88 via the toll/IL-1 receptor homology region (TIR) in the cytoplasmic region and is recruited by the N-terminal death domain of MyD88 to bind to the IL-1 receptor. The kinase (IRAK) activating the TIR-MyD88/IRAK-NF-kB pathway expresses the inflammatory cytokine IL-1β.

Additional file 2: Figure S2.
NLRP3 signaling pathway. NLRP3 activation of the N-terminal thermoprotein domain causes NLRP3 self-oligomerization, which in turn binds to apoptosis-associated microparticle proteins and recruits Pro-caspase1 to form the NLRP3 inflammatory complex. Upon activation of the inflammatory complex, Pro-caspase 1 is cleaved to form caspase 1, which then promotes IL-1β release to the extracellular environment. K+ efflux is a necessary signal for NLRP3 activation, and Cytolysin A forms a channel on the cell membrane that causes a large and sustained K+ outflow, activating the NLRP3 pathway and promoting sustained IL-1β release.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wu, Y., Feng, Z., Jiang, S. et al. Secreting-lux/pT-ClyA engineered bacteria suppresses tumor growth via interleukin-1β in two pathways. AMB Expr 9, 189 (2019). https://doi.org/10.1186/s13568-019-0910-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-019-0910-6