Abstract
Phasins are amphiphilic proteins involved in the regulation of the number and size of polyhydroxybutyrate (PHB) granules. The plant growth promoting bacterium Azospirillum brasilense Sp7 accumulates high quantities of bioplastic PHB as carbon and energy source. By analyzing the genome, we identified six genes that code for proteins with a Phasin_2 domain. To understand the role of A. brasilense Sp7 PhaP1 (PhaP1Abs) on PHB synthesis, the phaP1 gene (AMK58_RS17065) was deleted. The morphology of the PHB granules was analyzed by transmission electron microscopy (TEM) and the PHB produced was quantified under three different C:N ratios in cultures subjected to null or low-oxygen transfer. The results showed that PhaP1Abs is involved in PHB granules morphology and in controlling early biopolymer accumulation. Using RT-PCR it was found that phasin genes, except phaP4, are transcribed in accordance with the C:N ratio used for the growth of A. brasilense. phaP1, phaP2 and phaP3 genes were able to respond to the growth conditions tested. This study reports the first analysis of a phasin protein in A. brasilense Sp7.
Similar content being viewed by others
Introduction
Since it was reported for the first time in 1926 (Lemoigne 1926), polyhydroxybutyrate (PHB) has been the best studied bacterial biopolymer. PHB exhibits similar characteristics with respect to petrochemical plastics in addition to its biodegradability and biocompatibility properties. These characteristics enable PHB convenient to reduce the use of petrochemical plastic derivatives (Madison and Huisman 1999; Anjum et al. 2016). Besides the significant advantages of PHB, it is currently not used in large quantities because of the high production costs (US$ 5.5/kg) (Brigham and Riedel 2018; Pavan et al. 2019), mainly caused by the expensive carbon sources and the ineffective recovery process. A solution for improve the large-scale production of PHB could be the use of economic carbon substrates for PHB production such as food wastes (Brigham and Riedel 2018). Nonetheless, usage of cheaper carbon source combined with optimized processes for PHA accumulation and extraction could reduce the cost of biopolymer production and this could allow to address the large-scale production challenge. Since the demand in the health industry, the making of renewable materials and the advances in technologies, will make the market increase at a rate of 6.3%, reaching 119.15 million dollars by 2025 (Global Polyhydroxyalkanoate (PHA) Market Analysis & Trends—Industry Forecast to 2025).
More than 300 microorganisms have been reported to be PHB-producers (Koller et al. 2010; Alarfaj et al. 2015). PHB-producing microorganisms synthesize PHB by three enzymatic reactions. β-ketothiolase (PhaA) condenses two acetyl-CoA into acetoacetyl-CoA, then acetoacetyl-CoA is reduced to 3-hydroxybutyril-CoA by an acetoacetyl-CoA reductase (PhaB) and finally, 3-hydroxybutyril-CoA monomers are polymerized to PHB by a PHB synthase (PhaC) (Madison and Huisman 1999; Sagong et al. 2018).
Once synthesized, PHB is packaged into granules named carbonosomes which contain PHB covered by granule associated proteins (GAP’s) (Steinbüchel et al. 1995; Jendrossek 2009; Pfeiffer and Jendrossek 2012). Four main GAP’s have been reported: PHB synthases (PhaC), PHB depolymerases (PhaZ), regulators (PhaR or PhaM) and phasins (PhaP) (Steinbüchel et al. 1995; Tirapelle et al. 2013). Synthases and depolymerases are involved in PHB accumulation and utilization, respectively (Juengert et al. 2018; Mezzolla et al. 2018). Phasins are small amphiphilic proteins that coat and stabilize PHB in the cytoplasm (Sznajder et al. 2015) and therefore, phasins are involved in regulate the number and size of carbonosomes (Pfeiffer and Jendrossek 2012; Hauf et al. 2015). Regulator proteins (PhaR) control phasin synthesis (Maehara et al. 2002; Pötter et al. 2002; York et al. 2002) and possibly granule localization (PhaM) (Pfeiffer et al. 2011; Pfeiffer and Jendrossek 2014).
It has been demonstrated that phasins are the main GAP’s (Steinbüchel et al. 1995: Sznajder et al. 2015). Studies on phasins have suggested possible interactions with PHB polymerases and PHB depolymerases depending on the PHB-producing microorganism (York et al. 2001; Fukui et al. 2001; Handrick et al. 2004a, b; Tian et al. 2005; Ushimaru et al. 2014). In Aeromonas hydrophila the PHB content increased twofold when the only PhaP was overexpressed (Ushimaru et al. 2014), also changes in the monomer composition of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(HB-co-HHx)] copolyester were documented (Tian et al. 2005). It has also been shown that PHB is easily degraded in the presence of PhaP in Rhodospirillum rubrum (Handrick et al. 2004a, b; Kuchta et al. 2007).
PHB synthesis has been poorly studied in the α-proteobacterium A. brasilense Sp7. This bacterium accumulates up to 70% of its dry-cell weight (DCW) as PHB. The PHB accumulated energizes metabolic processes that favor bacterial survival under stressful environments (Tal and Okon 1985; Itzigsohn et al. 1995). PHB accumulation in A. brasilense Sp7 requires an excess in carbon and limited nitrogen sources (Tal and Okon 1985; Itzigsohn et al. 1995). Malic acid and ammonium chloride are the best carbon and nitrogen sources to improve PHB accumulation in A. brasilense Sp7 (Itzigsohn et al. 1995). Biochemical analyses of the PHB synthase and PHB depolymerase in A. brasilense have been carried out (Tal et al. 1990; Kadouri et al. 2002, 2003). However, there are no reports dealing with phasin proteins.
Our goal in this study was to analyze the function of the PhaP1Abs on the morphology of PHB granules and PHB accumulation using carbon:nitrogen (C:N) ratios of 30:1, 60:1 and 90:1, in cultures subjected to null or low-oxygen transfer. By means of bioinformatics analyses we found six genes that code for proteins containing a Phasin_2 domain (PF09361). In this work we deleted phaP1 gene and the resulting mutant was characterized. The results showed that PhaP1Abs controls the morphology of the PHB granules and is involved in the control of early PHB synthesis in some of the growth conditions evaluated.
Materials and methods
Bacterial strains, plasmids and growth conditions
Bacteria used in this study are listed in Table 1. Escherichia coli strains were grown in Luria Bertani (LB) broth supplemented with the appropriate antibiotics at 37 °C and 150 rpm. LB composition per liter: yeast extract 5 g, casein peptone 10 g and NaCl 10 g. For A. brasilense, a minimal medium supplemented with malic acid and ammonium chloride as carbon and nitrogen sources, was used. Minimal medium composition per liter: Malic acid 4.355 g, NH4Cl 0.2 g, K2HPO4 1.67 g, KH2PO4 0.87 g, MgSO4 0.29 g, NaCl 0.48 g, CaCl2 0.07 g, FeCl3·6H2O 0.01 g, Na2MoO4·2H2O 0.025 g and 10 mL of trace element solution (containing MnSO4·H2O 250 mg, ZnSO4·7H2O 70 mg, CoSO4·7H2O 14 mg, CuSO4·5H2O 12.5 mg and H3BO3 3 mg per liter). A. brasilense Sp7 strains were cultured, with the appropriated antibiotics, at 32 °C and 110 rpm. All incubations were carried out in a 25 mm amplitude orbital shaker (INO-650M SEV®).
Bioinformatics analyses
Proteins containing a Phasin_2 domain (PF09361) in the A. brasilense Sp7 genome were searched on National Center for Biotechnology Information (NCBI) (Geer et al. 2009), Simple Modular Architecture Research Tool (SMART) (Letunic and Bork 2017) and PFAM databases (Finn et al. 2015). Secondary structures were predicted using the PSI-blast based secondary structure PREDiction (PSIPRED) software (Buchan et al. 2013) and Protein Homology/AnalogY Recognition Engine (Phyre2) server (Kelley et al. 2015). Three-dimensional structures of putative phasins were predicted by using the Iterative Threading ASSEmbly Refinement (I-TASSER) (Roy et al. 2010; Yang and Zhang 2015; Zhang et al. 2017) and SWISS-MODEL (Waterhouse et al. 2018) servers. The obtained three-dimensional structures were evaluated by Qualitative Model Energy ANalysis (QMEAN) (Benkert et al. 2011) and Ramachandran plots at SWISS-MODEL Structure Assessment server. Alignments were done using Clustal X software (Larkin et al. 2007). Identity and similarity percentages were calculated on the Sequence Manipulation Suite (Stothard 2000). For the phylogenetic analyses, 18 amino acid sequences of phasins from main PHB-producing microorganisms were aligned using Clustal X software (Larkin et al. 2007). The phylogenetic tree was inferred using the Neighbor-Joining method and the evolutionary distances were computed using the Poisson correction method at the Molecular Evolutionary Genetics Analysis software version 7.0 (MEGA 7.0) (Kumar et al. 2016). The number of replicates were 500. Amino acid sequences of Ralstonia eutropha phasins were used as outgroup for the tree.
DNA manipulation
DNA was obtained by the phenol:chloroform method as previously reported (Cheng and Jiang 2006). Briefly, bacterial cells were harvested by centrifugation (8000×g for 5 min), the supernatant was removed, and the pellet washed in Sodium chloride–Tris–EDTA (STE) buffer twice. Cells were recovered and resuspended in Tris–EDTA (TE) buffer. Tris-saturated phenol (pH 8) was added, and the samples were mixed by vortexing and centrifuged as above. Phenol traces and debris were cleaned by three chloroform washes and the aqueous phase containing nucleic acids was recovered. RNA was eliminated by adding RNase H (10 mg/mL) to the aqueous phase and incubating for 10 min at 37 °C followed by purification with phenol:chloroform (Invitrogen). Pure DNA was contained in the aqueous phase. Plasmid DNA was isolated by the miniprep technique (Sambrook et al. 1989). Cloning procedures such as PCR amplifications, digestions and ligations were performed according to the instructions provided by the manufacturer (ThermoScientific).
In frame deletion of the phaP1 gene in A. brasilense Sp7
Upstream and downstream regions of the phaP1 gene (AMK58_RS17065) were PCR amplified using the primers phaP1FAPstI/phaP1RAXmaI (upstream) and phaP1FBXmaI/phaP1RBEcoRI (downstream) (Table 2). Primers were designed to contain PstI/XmaI and XmaI/EcoRI restriction sites. PCR conditions consisted of an initial denaturalization step at 94 °C for 5 min, followed by 30 cycles of denaturing at 94 °C for 45 s, annealing at 58 °C for 45 s and extension at 72 °C for 1 min as suggested by the manufacturer (ThermoScientific). Finally, an additional extension of 5 min at 72 °C was carried out. Both PCR fragments were digested with the specified endonucleases and cloned into PstI/EcoRI sites of the suicide vector pSUP202 generating the plasmid pSUPAB. Then, kanamycin resistance gene from pCR2.1TOPO vector was cloned into XmaI sites of pSUPAB. The resulting suicide vector, pSUPAKB, was mobilized into A. brasilense Sp7 using E. coli S17-1 as a donor strain as previously reported (Mishra et al. 2011). The A. brasilense phaP1 deleted mutant strain (named A. brasilense ΔphaP1) was selected on minimal medium supplemented with kanamycin (50 µg/mL) and ampicillin (100 μg/mL).
Complementation of the A. brasilense ΔphaP1 strain
The complementation of the A. brasilense ΔphaP1 was done in two steps. First, the pMBA-2 vector was generated by cloning of the arabinose promoter and the araC gene from pGLO into ApaI/EcoRI sites of the expression vector pMMB206. Second, the phaP1 gene was amplified by PCR using the primers phaP1-FMfeI/phaP1-RSmaI and wild type total DNA as a template. The PCR product was digested and ligated into EcoRI/SmaI restriction sites of pMBA-2. The resulting pMBA-12 plasmid was mobilized into A. brasilense ΔphaP1 using E. coli S17-1 as a donor strain. The complemented mutant (named A. brasilense PhaP1+++) was selected on minimal medium supplemented with chloramphenicol (10 µg/mL), kanamycin (50 µg/mL) and ampicillin (100 µg/mL). Induction of the arabinose promoter for PhaP1Abs expression in pMBA-12 vector was carried out with 0.1% arabinose (Sigma-Aldrich).
Growth curves of A. brasilense strains
Flasks containing 50 mL of minimal medium supplemented with malic acid and ammonium chloride at C:N ratios of 30:1, 60:1 and 90:1 were inoculated with the A. brasilense strains to an optical density at 600 nm (OD600) of 0.01, then the cultures were grown at 32 °C at 110 rpm. The OD600 was determined every 3 h during 24 h.
PHB quantitation
Strains of A. brasilense were grown on minimal medium supplemented with malic acid and ammonium chloride at C:N ratios of 30:1, 60:1 and 90:1 in flasks subjected to null or low-oxygen transfer. To generate null-oxygen transfer condition, unbaffled Erlenmeyer flasks of 125 mL of capacity were filled with 25 mL of minimal medium and capped with a number 5 rubber stopper, which keep off from the atmospheric oxygen transfer throughout the assay. To generate low-oxygen transfer condition, unbaffled Erlenmeyer flasks of 125 mL of capacity were filled with 25 mL of minimal medium and capped with a 5 g cotton stopper, which keep off from the atmospheric oxygen transfer throughout the assay. The flasks containing the minimal medium were inoculated with an overnight culture to an OD600 of 0.01 and grown at 32 °C and 110 rpm. For each strain, four flasks with 25 mL of minimal medium were inoculated with the appropriated strain. One flask was uncovered at 24 h to determine PHB production, another flask was uncovered at 48 h and so on. This led us to maintain the oxygen conditions throughout the study. Samples of A. brasilense strains (10 mL) were taken at 24, 48, 72 and 96 h and harvested by centrifugation (6000×g for 5 min). Cells were washed with TE buffer twice, weighed and dried at 60 °C until weight was constant. PHB was extracted as reported elsewhere (Hahn et al. 1994) and quantified spectrophotometrically (Law and Slepecky 1961). Data were correlated with a commercial standard of PHB (Sigma-Aldrich). Determinations were carried out in triplicate. Results are shown as mean ± standard deviation of % PHB/DCW. Statistical analysis was performed in Statgraphics Centurion 18 software using One-way ANalysis Of VAriance (ANOVA) at 95% confidence level.
Transmission electron microscopy (TEM)
Samples of A. brasilense strains were grown at C:N ratios of 30:1, and 90:1, harvested (6000×g for 5 min) and washed with TE buffer twice. Cells were fixed in 2.5% (v/v) glutaraldehyde for 3 h, postfixed in 1% (w/v) OsO4 for 2 h and rinsed 3 times in 50 mM of sodium cacodylate buffer (pH 7.2). Bacteria were dehydrated with a graded ethanol series and embedded in a low-viscosity epoxy resin that was subsequently dried at 60 °C for 24 h. Ultra-thin sections were stained with uranyl acetate and examined on a Jeol JEM-1200 EX II transmission electron microscope.
RT-PCR analysis
RNA was extracted from cultures of A. brasilense strains (wild type, mutant and complemented) grown on minimal medium at C:N ratios of 30:1 and 90:1. The extraction was performed using the Trizol reagent method according to the instructions provided (Sigma-Aldrich). A mix consisting of: 1 μg of pure RNA, phaP1 to phaP6 gene-specific primers (RT-phaP1-R, RT-phaP2-R, RT-phaP3-R, RT-phaP4-R, RT-phaP5-R and RT-phaP6-R) (Table 2), dNTP mix, RNase inhibitor, RevertAid reverse transcriptase and the RevertAid reverse transcriptase buffer, was used to produce the cDNA according to the recommendations of the manufacturer (ThermoScientific). For the PCR amplification of each putative phasin gene (internal region of ~ 120 nucleotides), a mix consisting of: cDNA (1 μL), dNTPs, gene specified pair of primers (RT-phaP1-F/RT-phaP1-R, RT-phaP2-F/RT-phaP2-R, RT-phaP3-F/RT-phaP3-R, RT-phaP4-F/RT-phaP4-R, RT-phaP5-F/RT-phaP5-R or RT-phaP6-F/RT-phaP6-R) (Table 2), Taq polymerase recombinant and Taq polymerase recombinant buffer, was utilized. PCR conditions consisted of an initial denaturalization step at 94 °C for 5 min, followed by 30 cycles of denaturing at 94 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 1 min as suggested by the manufacturer (ThermoScientific). Finally, an additional extension of 5 min at 72 °C was carried out. 16S rRNA gene (AMK58_09830) was used as control. PCR products were analyzed by gel electrophoresis on 3% agarose gels and subsequent ethidium bromide staining. RT-PCR assays were repeated three times.
Results
A. brasilense Sp7 contains six putative phaP genes
In silico analyses of the A. brasilense Sp7 genome revealed six genes that code for proteins containing a Phasin_2 domain (PF09361). Two putative phasin genes are in plasmid 1: AMK58_RS17065 (Gene ID: 36110503) and AMK58_RS20955 (Gene ID: 36111302) and the other putative phasin genes are in the chromosome: AMK58_RS04265 (Gene ID: 36107916), AMK58_RS04270 (Gene ID: 36107917), AMK58_RS07520 (Gene ID: 36108566) and AMK58_RS13850 (Gene ID: 36109844) (Additional file 1). The putative phasin proteins were referred as PhaP1Abs to PhaP6Abs according to the e-value (Table 3) presented with respect to the Phasin_2 domain. The size of the PhaP proteins ranges from 15 to 29 kDa. Analysis of the predicted secondary structure showed that phasins PhaP1Abs to PhaP5Abs possess a high α-helix content (85 to 95%) in contrast to PhaP6Abs which shows a low percentage (14%) (Table 3).
The phasin amino acid sequences from other PHB-producing microorganisms [R. eutropha (PhaP1 to PhaP7 and PhaM), Pseudomonas putida (PhaI and PhaF), Aeromonas hydrophila (PhaP), Herbaspirillum seropedicae (PhaP1 and PhaP2) and Azotobacter FA8 (PhaP)] were used for protein alignment with the A. brasilense Sp7 phasins (Fig. 1a). The results obtained showed that some A. brasilense Sp7 phasins are moderately conserved among phasins previously reported, with identities ranging from 3 to 33.7% and similarities from 6 to 55.8% (Additional file 2). The low identity and similarity percentages between the analyzed phasins, occurs by the diversity of aligned amino acid sequences of phasins belonging to the α, β, and γ-proteobacteria classes and does not pretend to identify a phasin domain. Moreover, the entire amino acid phasin sequences from the above-mentioned microorganisms were used to make a Neighbor-Joining phylogenetic tree (Fig. 1b). The putative PhaP2Abs, PhaP3Abs, PhaP4Abs and PhaP5Abs were clustered together on the 500 bootstrap tree. PhaP1Abs maybe share a common ancestor with this clade. The putative PhaP6Abs was clustered alone. A gene duplication event is suggested for PhaP2Abs and PhaP3Abs. It is important to mention that was not possible enroot the phylogenetic tree due to the diversity of amino acid sequences of phasins used for tree construction.
Phylogeny of A. brasilense Sp7 phasin proteins. a The amino acids sequences of phasin proteins of PHB-producing microorganisms were aligned to A. brasilense Sp7 proteins containing a phasin domain (PF09361). The conserved residues are highlighted. b A phylogenetic tree based on amino acid sequences of phasins was inferred using the Neighbor-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. The analysis involved 18 amino acid sequences. All the positions containing gaps and missing data were eliminated. PhaP1AbSp7 to PhaP6AbSp7 denotes the A. brasilense Sp7 PhaP1 to PhaP6. PhaP1Reu to PhaP7Reu indicates the phasins PhaP1 to PhaP7 of R. eutropha. PhaIPpu and PhaFPpu are the phasins PhaI and PhaF, respectively, of P. putida. PhaP1Hse and PhaP2Hse are the H. seropedicae PhaP1 and PhaP2. PhaPAhy denotes the A. hydrophila PhaP
Because of the non-existence of genetic, biochemical and structural information about phasin proteins of the most-well studied PHB-producing microorganisms, it was decided to analyze whether the putative A. brasilense Sp7 phasins share any structural homology with the crystal structure of PhaPAhy (Aeromonas hydrophila) (PDB number 5IP0) (Zhao et al. 2016). The PhaPAhy was used as template to predict the three-dimensional structures of the A. brasilense Sp7 phasins by the I-TASSER and SWISS-MODEL servers (Fig. 2 and Additional file 3). The results showed a good structural similarity for PhaP1Abs to PhaP4Abs models (QMEAN ranging from − 1.03 to − 3.60).
Predicted three-dimensional structures of A. brasilense Sp7 phasins. Structures were predicted using the I-TASSER and SWISS-MODEL servers. The crystal structure of PhaP from A. hydrophila (PDB number 5IP0) was used as template. For each A. brasilense Sp7 phasin, the QMEAN value was calculated and the Ramachandran plots were obtained (see Additional file 3). a PhaP1Abs, b PhaP2Abs, c PhaP3Abs, d PhaP4Abs, e PhaP5Abs and f PhaP6Abs
Due to the similarity between PhaP1Abs and PhaP5Reu (R. eutropha PhaP5) (55.8%) and based on the data that we obtained it was decided to focus on the analyses of the function of PhaP1Abs on PHB granule morphology and PHB accumulation.
PhaP1 does not affect growth of A. brasilense
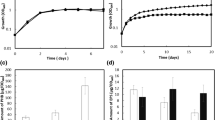
Growth curves of A. brasilense strains were carried out using minimal medium containing malic acid and ammonium chloride at C:N ratios of 30:1, 60:1 and 90:1 (Fig. 3). At all evaluated C:N ratios, the exponential growth phase was reached at 15 h of culturing, whereas the stationary phase occurred at 21 h. As was expected, the strains of study grew better when a C:N ratio of 30:1 was used. By comparing the growth curves of the mutant (A. brasilense ΔphaP1) and complemented (A. brasilense PhaP1+++) strains with respect to the wild type (A. brasilense Sp7) strain was shown that PhaP1Abs is not important for the growth of A. brasilense.
Growth kinetic behavior of A. brasilense strains. A. brasilense strains were inoculated in flasks containing minimal medium supplemented with malic acid and ammonium chloride, at C:N ratios of 30:1, 60:1 and 90:1, to an OD600 of 0.01. Samples were taken every 3 h for 24 h and measured spectrophotometrically at an OD600. Growth of A. brasilense Sp7 (WT) (circles), A. brasilense ΔphaP1 (ΔphaP1) (squares) and A. brasilense PhaP1+++ (PhaP1+++) (diamonds). Dark spots indicate a C:N ratio of 30:1, gray spots for a C:N ratio of 60:1 and light gray spots denotes a C:N ratio of 90:1
Deletion of phaP1 gene affects the morphology of the PHB granules
Wild type, mutant and complemented strains were cultivated for 15 h in minimal medium at C:N ratios of 30:1 and 90:1. Ultra-thin sections of the strains were analyzed by TEM (Fig. 4). At a C:N ratio of 30:1, several PHB granules were observed in the wild type strain. These granules are distributed throughout the cytoplasm (Fig. 4a). On the contrary, the mutant strain produced few PHB granules that appear to be larger and are located either at the center or at the pole of the cell (Fig. 4b). By restoring the PhaP1Abs function in the complemented strain, the number and appearance of the PHB granules was restored (Fig. 4c). This suggests that at a C:N ratio of 30:1, PhaP1Abs is involved in the control of the number and size of the PHB granules. However, when the bacteria were grown at a C:N ratio of 90:1, no differences in the size or distribution of the PHB granules could be observed (Fig. 4d–f). These results could suggest the participation of other PhaP proteins that contribute in the maintenance of the architecture of the PHB granules at the evaluated conditions.
Transmission electron microscopy of ultra-thin sections of A. brasilense. Strains were grown on minimal medium containing malic acid and ammonium chloride at C:N ratios of 30:1 (a–c) and 90:1 (e–g). Strains were incubated at 32 °C and 110 rpm for 24 h. PHB granules of wild type (A. brasilense Sp7) (a and e), mutant (A. brasilense ΔphaP1) (b and f) and complemented (A. brasilense PhaP1+++) (c and g) strains are shown as intracellular granules. Label Scale bar = 1 µm
The deletion of the phaP1 gene affects early (24 h) PHB accumulation depending on the C:N ratio used
PHB accumulation was quantified to evaluate the possible involvement of PhaP1Abs on biopolymer metabolism. Malic acid and ammonium chloride were used at C:N ratios of 30:1, 60:1 and 90:1 in cultures subjected to low or null oxygen transfer (as described in materials and methods). General comparison of the growth conditions evaluated for PHB accumulation (C:N ratio with low or null-oxygen transfer) is observed that the oxygen transfer limits PHB accumulation by approximately 50% (Fig. 5).
PHB/DCW content in A. brasilense strains. For PHB quantitation, flasks containing minimal medium supplemented with malic acid and ammonium chloride at C:N ratios of 30:1 (a and b), 60:1 (c and d), and 90:1 (e and f) were inoculated with the A. brasilense strains in flasks subjected to null (a, c and e) or low (b, d and f) oxygen transfer. Samples of cultures of A. brasilense were taken every 24 h for 96 h. Cells were harvested and dried, then PHB was extracted and quantified. PHB produced by A. brasilense Sp7 (WT), A. brasilense ΔphaP1 (ΔphaP1) and A. brasilense PhaP1+++ (PhaP1+++) are shown. Data are presented as mean ± standard deviation of three independent experiments. Letters in each graphic denote significant differences (p > 0.05) according to a one-way ANOVA test
Moreover, by analyzing the PHB accumulation at a C:N ratio of 30:1 in cultures with null-oxygen transfer (Fig. 5a) it can be observed that the PHB is accumulated at 24 h of growth and subsequently degraded in wild type and mutant strain. However, it is observed that the mutant strain accumulates 17% PHB/DCW with respect to 9.5% PHB/DCW that was accumulated by the wild type strain. In addition, the PHB produced by the mutant is easily degraded to similar levels as wild type strain. This suggests that PhaP1Abs somehow regulates the early stage of PHB accumulation. Moreover, the restoration of PhaP1Abs function in the complemented strain results in an increased PHB content to reach 17.5% PHB/DCW, it is about 90% more PHB with respect to the PHB accumulated by wild type strain. It should be noted that there was no signal observed for PHB degradation, since the PHB content decreased with time in the wild type and mutant strains but it was increased in the complemented strain. We also quantified the PHB accumulation in A. brasilense strains cultured at a C:N ratio of 30:1 but subjected to low-oxygen transfer (Fig. 5b). Both, wild type and mutant strains reported a similar behavior in PHB accumulation. In these strains, PHB content reach the maximum level at 24 h (~ 5% PHB/DCW) and it was subsequently decreased to minimum levels (~ 1% PHB/DCW) at the end of bacterial culturing time (96 h). Taken together data obtained is suggested that PhaP1Abs could be involved in the control of PHB synthesis or utilization at a C:N ratio of 30:1 in cultures subjected to null-oxygen transfer when early PHB synthesis occurs (24 h) (Fig. 5a). It should be noted that under low-oxygen transfer this does not occur (Fig. 5b).
To analyze whether the behavior observed is constant at other C:N ratios, it was decided to quantify PHB accumulation by increasing the C:N ratio to 60:1 and 90:1 under low or null-oxygen transfer (Fig. 5c–f). The data obtained at a C:N ratio of 60:1 in cultures with null-oxygen transfer showed a similar behavior by wild type, mutant and complemented strains from 72 to 96 h. However, at 24 h it appears that PHB synthesis in the mutant and complemented strains is delayed but at 48 h, they accumulated more PHB than the wild type strain (Fig. 5c). The observed phenotype may be due to the presence of another phasin protein that support PHB accumulation when a C:N ratio of 60:1 is combined with null-oxygen transfer. The above mentioned does not occur when PHB is measured in cultures subjected to low-oxygen transfer as can be observed in Fig. 5d.
Furthermore, when the PHB was quantified in cultures grown at a C:N ratio of 90:1 was observed that PHB is rapidly synthesized by the mutant strain when it is grown in cultures subjected to a null-oxygen transfer. At 24 h, the mutant strain accumulates approximately 50% PHB/DCW, whereas the wild type strain accumulates 35% PHB/DCW (Fig. 5e). It should be noted that the excess of PHB accumulated by the mutant strain is degraded after 48 h and remains constant up to 96 h of culture. Whereas, the PHB produced by the wild type strain increases over time. The complemented strain does not recover the wild type phenotype in PHB accumulation (Fig. 5e). Moreover, when cultures were grown at a C:N ratio of 90:1 and subjected to a low-oxygen transfer (Fig. 5f), it was found that, between 24 and 48 h of culture, the mutant strain increases the PHB content from 50 to 60% with respect to the wild type strain. PHB is accumulated rapidly by the mutant strain in contrast to a slow accumulation of PHB by the wild type.
Differential transcription of putative phasin genes under three different C:N ratios
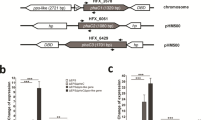
TEM images obtained from ultra-thin sections of A. brasilense strains grown at a C:N ratio of 90:1 showed no differences in the size and number of PHB granules by both, mutant and complemented strains, compared to the wild type strain (Fig. 4d–f). Additionally, the data obtained from the PHB quantification at C:N ratios of 60:1 and 90:1 showed an unclear phenotype. This suggests the possible involvement of other phasin proteins for the PHB accumulation and the maintenance of the structure of the PHB granules. To address this possibility, the transcripts of the phaP genes of wild type and mutant strains were evaluated by RT-PCR (Fig. 6). The results show that, phaP1 and phaP2 are the main genes transcribed in the wild type strain when cultures are grown at a C:N ratio of 30:1 (Fig. 6a). However, in the mutant, the phaP2 transcript was the most prominent of all phasins and the phaP3 transcript was partially increased (Fig. 6a). Nevertheless, it is still possible that the phaP2 product could compensate for the lack of PhaP1Abs in the granule structure as well as the PHB content in the mutant strain. Transcripts of the phaP4 and phaP6 were not observed either in wild type and mutant strains and the transcript of phaP5 was slightly detected at a C:N ratio of 30:1 (Fig. 6a). However, when A. brasilense was grown at a C:N ratio of 90:1 (Fig. 6b), phaP3 transcript increased to levels similar to phaP2 in the wild type strain. A similar behavior was observed for the mutant strain (Fig. 6b). It should be mentioned that the transcript of the phaP4 gene was not observed in both, wild type and mutant strains, whereas the phaP5 and phaP6 transcripts were present at low levels (Fig. 6b). Our results suggest that the C:N ratio has important implications on phasin genes transcription in this bacterium.
Analysis of the transcription of A. brasilense Sp7 phasin genes. RT-PCR assays of the internal region of putative phasin genes were conducted using cDNA of wild type (A. brasilense Sp7) and mutant (A. brasilense ΔphaP1) strains. RNA was extracted from cultures grown on minimal medium containing malic acid and ammonium chloride at C:N ratios of 30:1 and 90:1. Transcripts were visualized on a 3% agarose gel electrophoresis. a Transcription analyses of putative phasin genes of wild type and mutant strains grown at a C:N ratio of 30:1. For wild type: phaP1 (lane 1), phaP2 (lane 2), phaP3 (lane 3), phaP4 (lane 4), phaP5 (lane 5), phaP6 (lane 6). GeneRuler 1 Kb DNA ladder ThermoScientific (lane 7). For mutant strain: phaP2 (lane 8), phaP3 (lane 9), phaP4 (lane 10), phaP5 (lane 11), phaP6 (lane 12). 16S rRNA was used as positive control (lane 13). Negative control using cDNA of mutant strain with gene-specified primers for phaP1 transcript was done (lane 14). b Transcription analyses of putative phasin genes in wild type and mutant strains grown at a C:N ratio of 90:1. For wild type strain: phaP1 (lane 1), phaP2 (lane 2), phaP3 (lane 3), phaP4 (lane 4), phaP5 (lane 5), phaP6 (lane 6). GeneRuler 1 Kb DNA ladder ThermoScientific (lane 7). For mutant strain: phaP2 (lane 8), phaP3 (lane 9), phaP4 (lane 10), phaP5 (lane 11), phaP6 (lane 12). 16S rRNA was used as positive control (lane 13). Negative control using cDNA of mutant strain with gene-specified primers for phaP1 transcript was done (lane 14)
Discussion
The function of PhaP1Abs on PHB synthesis and PHB granule structure in A. brasilense was analyzed. Phasin proteins have been mainly implicated in PHB granule stabilization which occurs due to their amphiphilic properties (Pötter and Steinbüchel 2005) that avoid the coalescence of the PHB granules (Wieczorek et al. 1995; Pfeiffer et al. 2011). Every PHB-producing microorganism has at least one phasin, however there are reports of bacteria with several phasin proteins such as R. eutropha with seven PhaP proteins (Wieczorek et al. 1995; Pötter et al. 2004; Pfeiffer and Jendrossek 2011, 2012). Despite the existence of multiple homologues in some bacterial species and the fact that most of them can be bound to the PHB granule, only one or two are covering the PHB granules (Pötter et al. 2004; Yoshida et al. 2013). In this study, bioinformatic analyses of the A. brasilense Sp7 genome revealed six putative phasin genes, referred as phaP1 to phaP6. The amino acid sequences of A. brasilense phasins are not highly conserved however similar results have been reported for phasins of R. eutropha (Pötter et al. 2004; Pfeiffer and Jendrossek 2012), H. seropedicae (Alves et al. 2016), Bradyrhizobium japonicum (Yoshida et al. 2013) and Haloferax mediterranei (Cai et al. 2012) among others. The predicted secondary structures of PhaP1Abs to PhaP5Abs reveals a high percentage of α-helix content as has been reported for this type of proteins (Mezzina and Pettinari 2016; Zhao et al. 2016).
The deletion of the main phasin that covers the PHB granules results in a single enlarged granule rather than five to ten granules that are observed in the wild type strains (Steinbüchel et al. 1995; Jurasek and Marchessault 2002; Cai et al. 2012). To analyze the role of the PhaP1Abs in the PHB granule morphology, the phaP1 gene was deleted. TEM images of the carbonosomes from the mutant strain showed fewer PHB granules that were larger than those observed in the wild type strain. This strongly suggests that PhaP1Abs controls to a certain extent the number and size of the PHB granules.
Among other functions attributable to phasins are the regulation of synthesis and degradation of PHB (York et al. 2001; Handrick et al. 2004a, b; Cai et al. 2012) and the distribution of the PHB granules during cell division (Galán et al. 2011; Pfeiffer and Jendrossek 2011; Wahl et al. 2012; Bresan and Jendrossek 2017). To analyze the possible involvement of PhaP1Abs on PHB synthesis and degradation in A. brasilense Sp7, a phaP1 mutant strain was constructed and PHB accumulation was determined. For that, minimal medium supplemented with malic acid and ammonium chloride at different C:N ratios in addition to either low or null-oxygen transfer conditions was used. The results showed that PhaP1Abs controls early PHB synthesis at a C:N ratio of 30:1 when the oxygen transfer is null. The overexpression of PhaP1Abs in the complemented strain resulted in the increase of PHB over time, suggesting a possible increment in the PHB synthesis or a decrement in the PHB utilization. However, when the accumulation of PHB in A. brasilense was quantified in cultures grown in minimal medium at C:N ratios of 60:1 and 90:1 and subjected to low or null-oxygen transfer, no clear effect caused by the lack or overexpression of PhaP1Abs was observed. This suggest a possible participation of other phasin proteins to maintain PHB accumulation.
By analyzing the behavior of phasins in R. eutropha, it was found that the phaP1 mutant strain accumulates approximately 40% PHB/DCW less than the wild type strain (Pötter et al. 2005). However, the deletion of phaP2, phaP3 or phaP4 did not affect PHB accumulation (Pötter et al. 2005). In H. seropedicae SmR1, the deletion of phaP1 reduced PHB accumulation by 50% PHB/DCW but the phaP2 deleted strain produced 30% PHB/DCW more than the wild type strain when grown on glucose as a carbon source, suggesting that PhaP2Hse (Herbaspirillum seropedicae PhaP2) compensates for the lack of PhaP1Hse (Alves et al. 2016). Similar results have been obtained for Methylobacterium extorquens AM1 (Korotkova et al. 2002). The deletion of phaP1 and phaP2 in H. seropedicae and phaP1 to phaP4 in R. eutropha H16 abolished PHB accumulation (Pötter et al. 2005; Alves et al. 2016). These results, in addition to the changes observed in polymer accumulation allow to infer a possible interaction between phasin proteins and the PHB polymerase, which has been corroborated for R. eutropha (York et al. 2001), Aeromonas caviae (Fukui et al. 2001; Ushimaru et al. 2014) A. hydrophila (Tian et al. 2005), and, in this work, is suggested for A. brasilense Sp7, however the mechanism by which the interactions take place has not been elucidated.
Furthermore, a strong interaction between PhaP5Reu and the PHB depolymerase or the regulator protein (PhaR) has been proven (Pötter et al. 2005; Pfeiffer and Jendrossek 2011). Studies of PhaP2Reu and PhaP4Reu on PHB degradation conclude that both phasins, limit PHB degradation by interacting with the depolymerases PhaZ2, PhaZ3 and PhaZ7. Moreover, the expression of PhaP1Reu reduced the PHB content in E. coli when expressing the phaCAB operon of R. eutropha (Eggers and Steinbüchel 2014).
In conclusion, in this study we demonstrated that early PHB synthesis and the morphology of the PHB granules are influenced by PhaP1Abs when A. brasilense Sp7 is grown in a medium with a C:N ratio of 30:1. Nevertheless, when C:N ratios of 60:1 and 90:1 were used, PHB synthesis and the morphology of the PHB granules seem to be influenced by the coordinated action of several phasin proteins.
Availability of data and materials
Please contact to the authors for all request.
Abbreviations
- PHB:
-
polyhydroxybutyrate
- [P(HB-co-HHx)]:
-
poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)
- GAP’s:
-
granule associated proteins
- C:N:
-
carbon:nitrogen ratio
- LB:
-
Luria–Bertani
- h:
-
hours
- min:
-
minutes
- s:
-
seconds
- Kg:
-
kilograms
- g:
-
grams
- g :
-
gravity
- OD600:
-
optical density at 600 nm
- DCW:
-
dry cell weight
- TEM:
-
transmission electron microscopy
References
Alarfaj AA, Arshad M, Sholkamy EN, Munusamy MA (2015) Extraction and characterization of polyhydroxybutyrates (PHB) from Bacillus thuringiensis KSADL127 isolated from Mangrove environments of Saudi Arabia. Braz Arch Biol Technol 58(5):781–788
Alves LPS, Teixeira CS, Tirapelle EF, Donatti L, Tadra-Sfeir MZ, Steffens MBR, De Souza EM, De Oliveira PF, Chubatsu LS, Müller-Santos M (2016) Backup expression of the PhaP2 phasin compensates for phaP1 deletion in Herbaspirillum seropedicae, maintaining fitness and PHB accumulation. Front Microbiol 7:739
Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S (2016) Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int J Biol Macromol 89:161–174
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350
Bresan S, Jendrossek D (2017) New insights into PhaM-PhaC-mediated localization of polyhydroxybutyrate granules in Ralstonia eutropha H16. Appl Environ Microbiol 83(12):e00505-17
Brigham CJ, Riedel Sl (2018) The potential of polyhydroxyalkanoate production from food wastes. Appl Food Biotecnol. 6(1):7–18
Buchan DWA, Minneci F, Nugent TCO, Bryson K, Jones DT (2013) Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res 41(W1):W340–W348
Cai S, Cai L, Liu H, Liu X, Han J, Zhou J, Xiang H (2012) Identification of the haloarchaeal type phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl Environ Microbiol. https://doi.org/10.1128/AEM.07114-11
Cheng HR, Jiang N (2006) Extremely rapid extraction of DNA from bacteria and yeasts. Biotechnol Lett 28(1):55–59
Eggers J, Steinbüchel A (2014) Impact of Ralstonia eutropha’s poly (3-Hydroxybutyrate) (PHB) depolymerases and phasins on PHB storage in recombinant Escherichia coli. Appl Environ Microbiol 80(24):7702–7709
Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A (2015) The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44(D1):D279–D285
Fukui T, Kichise T, Iwata T, Doi Y (2001) Characterization of 13 kDa granule-associated protein in Aeromonas caviae and biosynthesis of Polyhydroxyalkanoates with altered molar composition by recombinant bacteria. Biomacromolecules 2(1):148–153
Galán B, Dinjaski N, Maestro B, De Eugenio LI, Escapa IF, Sanz JM, García JL, Prieto MA (2011) Nucleoid-associated PhaF phasin drives intracellular location and segregation of polyhydroxyalkanoate granules in Pseudomonas putida KT2442. Mol Microbiol 79(2):402–418
Geer LY, Marchler-Bauer A, Geer RC, Han L, He J, He S, Liu C, Shi W, Bryant SH (2009) The NCBI biosystems database. Nucleic Acids Res 38:D492–D496
Hahn SK, Chang YK, Kim BS, Chang HN (1994) Optimization of microbial poly (3-ß-hydroxybutyrate) recover using dispersions of sodium hypochlorite solution and chloroform. Biotechnol Bioengin 44(2):256–261
Handrick R, Reinhardt S, Schultheiss D, Reichart T, Schüler D, Jendrossek V, Jendrossek D (2004a) Unraveling the function of the Rhodospirillum rubrum activator of polyhydroxybutyrate (PHB) degradation: the activator is a PHB-granule-bound protein (phasin). J Bacteriol 186(8):2466–2475
Handrick R, Technow U, Reichart T, Reinhardt S, Sander T, Jendrossek D (2004b) The activator of the Rhodospirillum rubrum PHB depolymerase is a polypeptide that is extremely resistant to high temperature (121 C) and other physical or chemical stresses. FEMS Microbiol Lett 230(2):265–274
Hauf W, Watzer B, Roos N, Klotz A, Forchhammer K (2015) Photoautotrophic polyhydroxybutyrate granule formation is regulated by cyanobacterial phasin PhaP in Synechocystis sp. strain PCC 6803. Appl Environ Microbiol 81(13):4411–4422
Itzigsohn R, Yarden O, Okon Y (1995) Polyhydroxyalkanoate analysis in Azospirillum brasilense. Can J Microbiol 41(13):73–76
Jendrossek D (2009) Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes). J Bacteriol 191(10):3195–3202
Juengert JR, Petterson C, Jendrossek D (2018) Poly (3-hydroxybutyrate) (PHB) polymerase PhaC1 and PHB depolymerase PhaZa1 of Ralstonia eutropha are phosphorylated in vivo. Appl Environ Microbiol 84(13):e00604-18
Jurasek L, Marchessault RH (2002) The role of phasins in the morphogenesis of poly (3-hydroxybutyrate) granules. Biomacromolecules 3(2):256–261
Kadouri D, Burdman S, Jurkevitch E, Okon Y (2002) Identification and isolation of genes involved in poly (β-hydroxybutyrate) biosynthesis in Azospirillum brasilense and characterization of a phbC mutant. Appl Environ Microbiol 68(6):2943–2949
Kadouri D, Jurkevitch E, Okon Y (2003) Poly β-hydroxybutyrate depolymerase (PhaZ) in Azospirillum brasilense and characterization of a phaZ mutant. Arch Microbiol 180(5):309–318
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10(6):845
Koller M, Salerno A, Dias M, Reiterer A, Braunegg G (2010) Modern biotechnological polymer synthesis: a review. Food Technol Biotechnol 48(3):255–269
Korotkova N, Chistoserdova L, Lidstrom ME (2002) Poly-β-hydroxybutyrate biosynthesis in the facultative methylotroph Methylobacterium extorquens AM1: identification and mutation of gap11, gap20, and phaR. J Bacteriol 184(22):6174–6181
Kuchta K, Chi L, Fuchs H, Pötter M, Steinbüchel A (2007) Studies on the influence of phasins on accumulation and degradation of PHB and nanostructure of PHB granules in Ralstonia eutropha H16. Biomacromol 8(2):657–662
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Law JH, Slepecky RA (1961) Assay of poly-β-hydroxybutyric acid. J Bacteriol 82(1):33–36
Lemoigne M (1926) Produits de dehydration et de polymerisation de l’acide ß-oxobutyrique. Bull Soc Chim Biol 8:770–773
Letunic I, Bork P (2017) 20 years of the SMART protein domain annotation resource. Nucleic Acids Res 46(D1):D493–D496
Madison LL, Huisman GW (1999) Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63(1):21–53
Maehara A, Taguchi S, Nishiyama T, Yamane T, Doi Y (2002) A repressor protein, PhaR, regulates polyhydroxyalkanoate (PHA) synthesis via its direct interaction with PHA. J Bacteriol 184(14):3992–4002
Mezzina MP, Pettinari MJ (2016) Phasins, multifaceted polyhydroxyalkanoate granule-associated proteins. Appl Environ Microbiol 82(17):5060–5067
Mezzolla V, DÚrso O, Poltronieri P (2018) Role of PhaC type I and type II enzymes during PHA biosynthesis. Polymers 10(8):910
Mishra MN, Kumar S, Gupta N, Kaur S, Gupta A, Tripathi AK (2011) An extracytoplasmic function sigma factor cotranscribed with its cognate anti-sigma factor confers tolerance to NaCl, ethanol and methylene blue in Azospirillum brasilense Sp7. Microbiology 157(4):988–999
Morales VM, Bäckman A, Bagdasarian M (1991) A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97(1):39–47
Pavan FA, Junqueira TL, Watanabe MD, Bonomi A, Quines LK, Schmidell W, de Aragao GM (2019) Economic analysis of polyhydroxybutyrate production by Cupriavidus necator using different routes for product recovery. Biochem Eng J 146:97–104
Pfeiffer D, Jendrossek D (2011) Interaction between poly (3-hydroxybutyrate) granule-associated proteins as revealed by two-hybrid analysis and identification of a new phasin in Ralstonia eutropha H16. Microbiology 157(10):2795–2807
Pfeiffer D, Jendrossek D (2012) Localization of PHB granule associated proteins during PHB granule formation and identification of two new phasins, PhaP6 and PhaP7, in Ralstonia eutropha H16. J Bacteriol. https://doi.org/10.1128/JB.00779-12
Pfeiffer D, Jendrossek D (2014) PhaM is the physiological activator of poly (3-hydroxybutyrate) (PHB) synthase (PhaC1) in Ralstonia eutropha. Appl Environ Microbiol 80(2):555–563
Pfeiffer D, Wahl A, Jendrossek D (2011) Identification of a multifunctional protein, PhaM, that determines number, surface to volume ratio, subcellular localization and distribution to daughter cells of poly (3-ß-hydroxybutyrate), PHB, granules in Ralstonia eutropha H16. Mol Microbiol 82(4):936–951
Pötter M, Steinbüchel A (2005) Poly (3-hydroxybutyrate) granule-associated proteins: impacts on poly (3-hydroxybutyrate) synthesis and degradation. Biomacromolecules 6(2):552–560
Pötter M, Madkour MH, Mayer F, Steinbüchel A (2002) Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 148(8):2413–2426
Pötter M, Müller H, Reinecke F, Wieczorek R, Fricke F, Bowien B, Friedrich B, Steinbüchel A (2004) The complex structure of polyhydroxybutyrate (PHB) granules: four orthologous and paralogous phasins occur in Ralstonia eutropha. Microbiology 150(7):2301–2311
Pötter M, Müller H, Steinbüchel A (2005) Influence of homologous phasins (PhaP) on PHA accumulation and regulation of their expression by the transcriptional repressor PhaR in Ralstonia eutropha H16. Microbiology 151(3):825–833
Roschanski N, Strauch E (2011) Assessment of the mobilizable vector plasmids pSUP202 and pSUP404. 2 as genetic tools for the predatory bacterium Bdellovibrio bacteriovorus. Curr Microbiol 62(2):589–596
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738
Sagong HY, Son HF, Choi SY, Lee SY, Kim KJ (2018) Structural insights into polyhydroxyalkanoates biosynthesis. Trends Biochem Sci 43:790–805
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold spring harbor laboratory press, Cold Spring Harbor
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1(9):784
Steinbüchel A, Aerts K, Liebergesell M, Wieczorek R, Babel W, Föllner C, Hussien MM, Mayer F, Pieper-Fürst U, Pries A, Valentin HE (1995) Considerations on the structure and biochemistry of bacterial polyhydroxyalkanoic acid inclusions. Can J Microbiol 41(13):94–105
Stothard P (2000) The Sequence Manipulation Suite: javaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 28:1102–1104
Sznajder A, Pfeiffer D, Jendrossek D (2015) Comparative proteome analysis reveals four novel polyhydroxybutyrate (PHB) granule-associated proteins in Ralstonia eutropha H16. Appl Environ Microbiol 81(5):1847–1858
Tal S, Okon Y (1985) Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can J Microbiol 31(7):608–613
Tal S, Smirnoff P, Okon Y (1990) The regulation of poly-β-hydroxybutyrate metabolism in Azospirillum brasilense during balanced growth and starvation. Microbiology 136(7):1191–1196
Tarrand JJ, Krieg NR, Döbereiner J (1978) A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol 24(8):967–980
Tian SJ, Lai WJ, Zheng Z, Wang HX, Chen GQ (2005) Effect of over-expression of phasin gene from Aeromonas hydrophila on biosynthesis of copolyesters of 3-hydroxybutyrate and 3-hydroxyhexanoate. FEMS Microbiol Lett 244(1):19–25
Tirapelle EF, Müller-Santos M, Tadra-Sfeir MZ, Kadowaki MAS, Steffens MBR, Monteiro RA, Souza EM, Pedrosa FO, Chubatsu LS (2013) Identification of proteins associated with polyhydroxybutyrate granules from Herbaspirillum seropedicae SmR1-old partners, new players. PLoS ONE 8(9):e75066
Ushimaru K, Motoda Y, Numata K, Tsuge T (2014) Phasin proteins activate Aeromonas caviae polyhydroxyalkanoate (PHA) synthase but not Ralstonia eutropha PHA synthase. Appl Environ Microbiol 80(9):2867–2873
Wahl A, Schuth N, Pfeiffer D, Nussberger S, Jendrossek D (2012) PHB granules are attached to the nucleoid via PhaM in Ralstonia eutropha. BMC Microbiol 12(1):262
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303
Wieczorek R, Pries A, Steinbüchel A, Mayer F (1995) Analysis of a 24-kilodalton protein associated with the polyhydroxyalkanoic acid granules in Alcaligenes eutrophus. J Bacteriol 177(9):2425–2435
Yang J, Zhang Y (2015) I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res 43:W174–W181
York GM, Stubbe J, Sinskey AJ (2001) New insight into the role of the PhaP phasin of Ralstonia eutropha in promoting synthesis of polyhydroxybutyrate. J Bacteriol 183(7):2394–2397
York GM, Stubbe J, Sinskey AJ (2002) The Ralstonia eutropha PhaR protein couples synthesis of the PhaP phasin to the presence of polyhydroxybutyrate in cells and promotes polyhydroxybutyrate production. J Bacteriol 184(1):59–66
Yoshida KI, Takemoto Y, Sotsuka T, Tanaka K, Takenaka S (2013) PhaP phasins play a principal role in poly-β-hydroxybutyrate accumulation in free-living Bradyrhizobium japonicum. BMC Microbiol 13(1):290
Zhang C, Freddolino PL, Zhang Y (2017) COFACTOR: improved protein function prediction by combining structure, sequence and protein–protein interaction information. Nucleic Acids Res 45:W291–W299
Zhao H, Wei H, Liu X, Yao Z, Xu M, Wei D, Wang J, Wang X, Chen G (2016) Structural insights on PHA binding protein PhaP from Aeromonas hydrophila. Sci Rep 6:39424
Acknowledgements
The Unidad de Imagenología of the Instituto de Fisiología Celular, UNAM.
Teresa Ballado, Javier de la Mora and Norma Espinosa for technical assistance.
Consejo Nacional de Ciencia y Tecnología (CONACyT) for the scholarship (474387) for MAMM.
Funding
This work was supported in part by grants VIEP-BUAP; ID: 00249 to LJMM and ID: 00122 to LSU).
Author information
Authors and Affiliations
Contributions
LJMM and MAMM designed this study. MAMM performed the experiments. MAMM, LSU and LJMM interpreted the results. BGP and GD supported the project by critical evaluation of experimental results, TEM experiments and providing facilities required. MAMM, BGP, GD, LSU and LJMM participated substantially in writing the manuscript, critical evaluation and modifications. LJMM supervised the study. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Putative phasin genes localization.
Additional file 2.
Identity and similarity percentages between the entire amino acids sequences of phasins from PHB-producing microorganisms.
Additional file 3.
Ramachandran plots of predicted three-dimensional structures of putative A. brasilense Sp7 phasins.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Martínez-Martínez, M.d., González-Pedrajo, B., Dreyfus, G. et al. Phasin PhaP1 is involved in polyhydroxybutyrate granules morphology and in controlling early biopolymer accumulation in Azospirillum brasilense Sp7. AMB Expr 9, 155 (2019). https://doi.org/10.1186/s13568-019-0876-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-019-0876-4