Abstract
Porphyromonas gingivalis (Pg) is one of the main pathogens in chronic periodontitis (CP). Studies on the immunogenicity of its virulence factors may contribute to understanding the host response to infection. The present study aimed to use in silico analysis as a tool to identify epitopes from Lys-gingipain (Kgp) and neuraminidase virulence factors of the Pg ATCC 33277 strain. Protein sequences were obtained from the NCBI Protein Database and they were scanned for amino acid patterns indicative of MHC II binding using the MHC-II Binding Predictions tool from the Immune Epitope Database (IEDB). Peptides from different regions of the proteins were chemically synthesized and tested by the indirect ELISA method to verify IgG immunoreactivity in serum of subjects with CP and without periodontitis (WP). T cell epitope prediction resulted in 16 peptide sequences from Kgp and 18 peptide sequences from neuraminidase. All tested Kgp peptides exhibited IgG immunoreactivity whereas tested neuraminidase peptides presented low IgG immunoreactivity. Thus, the IgG reactivity to Kgp protein could be reaffirmed and the low IgG reactivity to Pg neuraminidase could be suggested. The novel peptide epitopes from Pg were useful to evaluate its immunoreactivity based on the IgG-mediated host response. In silico analysis was useful for preselecting epitopes for immune response studies in CP.
Similar content being viewed by others
Introduction
Chronic periodontitis is a multifactorial and polymicrobial disease, which may negatively influence systemic diseases (Hajishengallis 2015). Its pathogenesis is related to host immune inflammatory factors and to a synergistic and dysbiotic oral microbiome (Hajishengallis et al. 2012; Hajishengallis 2014; Hajishengallis and Lamont 2014). In light of the diversity of the human oral microbiome (Proctor et al. 2018), immunoinformatics brings tools that provide faster analysis of virulence factors, considering the polymicrobial character of chronic periodontitis, and contribute to understanding the interaction between the oral microbiome and the host.
Porphyromonas gingivalis, a keystone pathogen related to periodontal dysbiosis (Hajishengallis 2014), produces various virulence factors that can act as immunogenic molecules. The host response to infection may be better understood by progressively investigating the immunogenicity of P. gingivalis virulence factors (Trindade et al. 2008, 2012a, b, 2013; Olczak et al. 2010; Bengtsson et al. 2015; Carvalho-Filho et al. 2016; Tada et al. 2016; Dashper et al. 2017).
Gingipains (cysteine proteases) are the main proteases of P. gingivalis (Guo et al. 2010), one of their main functions being heme acquisition (Smalley and Olczak 2017). These proteases are also immunogenic proteins, contributing to the pathogen’s ability to induce chronic periodontitis, since they can elicit a humoral immune response in humans, inducing higher serum levels of specific IgG in individuals with chronic periodontitis (O’Brien-Simpson et al. 2000; Inagaki et al. 2003; Nguyen et al. 2004).
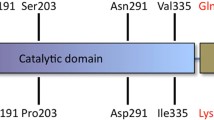
Lys-gingipain (Kgp) is considered a major virulence factor of P. gingivalis (de Diego et al. 2014) and is also involved in the bacteria-host interaction through the production of cytokines, such as IL-17A and INF-γ (Bittner-Eddy et al. 2013). Kgp (1723 aa/187 kDa) is cleaved into four chains: the catalytic subunit and three adhesion domains (UniProt B2RLK2).
Although not as well studied as Kgp, neuraminidase (sialidase) of P. gingivalis is secreted by the microorganism for neuraminic acid (sialic acid) acquisition from sialoglycoconjugates of the host. Sialic acids are incorporated into the microorganism structures, thus mimicking the host cell and confounding the immune response, mainly under stressful microenvironmental conditions (Li et al. 2012; Xu et al. 2017). Sialidase deficiency in P. gingivalis increases sensitivity to hydrogen peroxide, decreases resistance to the action of complement and reduces virulence after subcutaneous injection in mice, likely by influencing capsule biosynthesis (Moncla et al. 1990; Aruni et al. 2011; Li et al. 2012; Xu et al. 2017).
Moreover, the sialidase activity may be involved in the production, maturation and secretion of gingipains and other virulence factors of P. gingivalis, probably due to their sialylation (glycosylation) (Aruni et al. 2011; Xu et al. 2017). Importantly, inhibitors of these two virulence factors studied herein could be promising for use in the treatment of chronic periodontitis and associated systemic diseases (Cueno et al. 2014; Olsen and Potempa 2014; Inaba et al. 2016; Xu et al. 2017).
The present study aimed to use in silico analysis as a strategy to identify potential immunogenic peptides from those P. gingivalis relevant proteins. Selected epitopes were evaluated concerning their immunoreactivity based on the IgG-mediated host response in order to contribute to immunogenicity studies of this keystone pathogen.
Materials and methods
Selection of subjects for immunoreactivity test of peptides
For sample size calculation, considering the human IgG levels against P. gingivalis extract (Trindade et al. 2008), the absorbance value of 133 was estimated as a relevant difference to be detected, with a standard deviation of 126. Therefore, 21 participants were estimated in each group, considering the significance level of 5%, the test power of 90%, and 10% increase to predict losses.
Participants were selected considering exclusion criteria: age less than 18 years, number of teeth less than 10, history of systemic diseases, current pregnancy, periodontal treatment performed up to 1 year before oral examination, current or former cigarette smoking habit, alcoholism, use of antibiotics and anti-inflammatories, respectively, at 6 and 2 months before the selection.
A structured questionnaire was applied to obtain information about individuals’ health conditions, and then periodontal clinical examination and collection of peripheral blood (5 mL) were performed. Chronic periodontitis was diagnosis followed the consensus of the American Academy of Periodontology (Armitage 1999; Lindhe et al. 1999; Caton et al. 2018) and subjects were classified according to the periodontal criteria proposed by Gomes-Filho et al. (2007). Participants were separated into two groups according to the periodontal diagnosis: a group with chronic periodontitis (CP) and a group without periodontitis (WP).
Production of P. gingivalis extract
After anaerobic culture of bacteria, the immunogenic extract of P. gingivalis ATCC 33277 strain (NCBI Taxonomy ID: 431947) was produced according to the standardized protocol (Trindade et al. 2008). The total protein was measured, and the sonicated extract was stored at − 20 °C.
Sera pools for immunoreactivity test of peptides
The indirect ELISA method was performed for IgG detection in each serum sample of selected individuals, and 5 µg/mL of P. gingivalis (Pg) extract was used as the antigen (Trindade et al. 2008). Two sera pools were obtained for use in the immunoreactivity test. The CP pool comprised serum samples from individuals with chronic periodontitis with the highest levels of anti-Pg IgG obtained among samples. The WP pool comprised serum samples from individuals without periodontitis with the lowest levels of anti-Pg IgG obtained among samples. 200 µL of each sample were pooled into CP and WP pools, homogenized and stored at − 20 °C.
Peptide prediction
This in silico analysis assumed that CD4+ T cells mainly recognize short linear peptides from proteins produced by extracellular pathogens, such as P. gingivalis, presented by MHC class II molecules (Vyas et al. 2008; Rocha and Neefjes 2008). Protein sequences (YP_001929844/BAG34127.1) were obtained from the Protein Database of the National Center for Biotechnology Information (NCBI), USA, and they were scanned for amino acid patterns indicative of MHC II binding using the MHC II Binding Prediction tool (http://tools.immuneepitope.org/mhcii/) from the Immune Epitope Database and Analysis Resource (IEDB), which is a previously validated method. This tool employs different methods to predict MHC class II epitopes, including a consensus approach (default) used herein (Wang et al. 2008, 2010; Vita et al. 2010).
This tool requires specification of the HLA allele/haplotype to make binding predictions; therefore the analysis considered 09 HLA alleles (loci DQ and DR), which were observed in the previous study involving subjects with chronic periodontitis from Salvador, Bahia, and Brazil (Monteiro et al. 2017). The same HLA alleles were used to predict Kgp and neuraminidase immunogenic peptides (Additional file 1: Table S1).
Post-prediction analysis
Epitope Cluster Analysis (http://tools.iedb.org/cluster/) (Kim et al. 2012) was performed to group peptide sequences into clusters. Peptide sequences were compared to those in the IEDB by searching for similarity, using the Basic Local Alignment Search Tool (BLAST) (Altschul et al. 1990). Finally, the peptide sequences obtained were compared to current data published by the NCBI Protein Database (YP_001929844/BAG34127.1) to identify protein regions.
Peptide synthesis
Peptides from different regions of the proteins were chemically synthesized by AminoTech Pesquisa e Desenvolvimento LTDA, Diadema, SP, Brazil, using the Fmoc strategy. Purification (95%) was achieved by reverse-phase high-performance liquid chromatography (HPLC) and the peptides were characterized by mass spectrometry by AminoTech. Then, freeze-dried peptides were solubilized in 0.5 M carbonate-bicarbonate buffer (pH 9.6) and stored at − 20 °C.
IgG immunoreactivity test of peptides
Synthetic peptides were tested by the indirect ELISA method (Trindade et al. 2008) to verify the levels of IgG in CP and WP sera pools. 10 µg/mL of each peptide was used as an antigen and 5 µg/mL of P. gingivalis extract was used as a positive control. A checkerboard ELISA was performed to obtain the appropriate conditions for the equivalence zone in the antigen–antibody reaction, analyzing the best concentrations of antigen, serum and conjugate. For each combination (antigen, serum and conjugate), the coefficients of O.D. (optical density) between the CP pool and the WP pool were determined for each analyzed peptide. The coefficient expresses the difference of the mean IgG levels between the CP and the WP sera pools.
Submission to the Immune Epitope Database
The tested peptides were submitted to the Immune Epitope Database and Analysis Resource (IEDB) through the data submission tool (DST)/Wizard submission method and they can be accessed at submission ID 1000760 to Kgp peptides and at ID 1000766 to neuraminidase peptides.
Analysis of population coverage
A coverage analysis of published peptides was performed using the Population Coverage tool from IEDB “Class II separate” (http://tools.iedb.org/population/) (Bui et al. 2006), which uses the HLA allele genotypic frequencies of the Allele Frequency Net Database. At present, according to the Population Coverage Tutorial, the Allele Frequency Database provides allele frequencies for 115 countries and 21 different ethnicities grouped into 16 different geographical areas (http://tools.iedb.org/population/help/#population_info. Accessed 15 August 2018).
Peptide presentation by protein modeling
The published peptides were schematically presented in their protein region after protein structure prediction. I-TASSER on-line server (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) (Roy et al. 2010) and PyMOL 1.7.4.4 Edu software were used for modeling analysis. The sequence of each Kgp protein region and neuraminidase was obtained from the NCBI Protein Database annotation (YP_001929844 and BAG34127.1, respectively).
Statistical analysis
Descriptive analysis was performed for the characterization of the groups. To compare the groups, the Mann–Whitney U test was used, based on the distribution assessed by the Kolmogorov–Smirnov test. Data related to the dichotomous variable were tested with Fisher’s exact test. For all statistical procedures a significance level of 5% was applied (P ≤ 0.05).
For screening of peptides, a checkerboard evaluation was performed and the coefficients of absorbance between CP and WP pools were determined (difference of the O.D. value between the sera pools). The nature of the data does not allow statistical analysis between the coefficients.
Results
Selection of subjects for immunoreactivity test of peptides
Forty-one participants who attended the School of Dentistry of the Feira de Santana State University, Bahia, Brazil, were enrolled. They were clinically classified into the CP group (20 subjects) and the WP group (21 subjects) according to periodontal parameters. The groups were homogeneous with respect to age, the proportion between female and male subjects, and teeth number (Table 1).
Sera pools for immunoreactivity test
Forty-one serum samples were tested (anti-Pg IgG): 20 samples of the CP group and 21 samples of the WP group. Six samples were selected and pooled into the CP pool (O.D.: 0.53–1.10) and five samples were selected and pooled into the WP pool (O.D.: 0.22–0.38).
Peptide prediction and post-prediction analysis
T-cell epitope prediction resulted in 16 peptide sequences from Kgp and 18 peptide sequences from neuraminidase: two predicted epitopes for each tested HLA allele, which had presented a lower percentile rank and had been located in two different regions of the analyzed protein (Tables 2, 3).
There was no sequence cluster formation (identity threshold 90%) for 16 peptide sequences from Kgp and for 18 peptide sequences from neuraminidase, which indicates that all sequences obtained are different from each other.
There was also no exact similarity (exact matches—100%) when comparing peptide sequences with peptides published in IEDB, which indicated that those sequences had not been published yet by another research group.
The peptide sequences were compared to current data published by the NCBI Protein Database (YP_001929844/BAG34127.1) to identify protein regions (Additional file 1: Tables S2, S3).
In addition, Kgp14 was not synthesized due to its position in the protein (1–15), which in principle should no longer be present in the mature protein.
IgG immunoreactivity of peptides
Nine predicted peptides from Kgp (Kgp1, 6, 11, 12, 15, 16, 17, 18 and 20) and eight peptides from neuraminidase (N5, 6, 10, 11, 15, 16, 17 and 18) were selected for synthesis and immunoreactivity analysis.
Kgp synthetic peptides presented immunoreactivity to IgG. Kgp12, Kgp18 and Kgp17, respectively, represented a higher coefficient between CP and WP sera pools, so they were selected for subsequent assays. A greater difference between the absorbance values of the CP and WP sera pools was observed when the Kgp12 was tested in the following conditions: 10 µg/mL peptide concentration, 1:250 sera pool concentration and 1:25,000 anti-human IgG peroxidase conjugate concentration (Checkerboard ELISA best acquired condition) (Fig. 1).
Coefficients of absorbance between CP sera pool and WP sera pool after checkerboard ELISA analysis of Kgp synthetic peptides. Brazil, 2018. CP: chronic periodontitis; WP: without periodontitis. The coefficient is the difference of the O.D. value between the sera pools, and it expresses the difference of the mean IgG levels between the CP and the WP sera pools. P. gingivalis (Pg) extract and peptides were used as the antigen in the indirect ELISA test. 1:100 and 1:250 serum concentrations are represented. Kgp12 presented a higher coefficient between CP and WP sera pools in 1:250 serum concentration. Checkerboard ELISA best acquired condition: 5 µg/mL Pg extract concentration, 10 µg/mL peptide concentration, 1:250 sera pool concentration and 1:25,000 anti-human IgG peroxidase conjugate concentration. The nature of the data does not allow statistical analysis between the coefficients
Low levels of IgG were observed for each neuraminidase synthetic peptides tested. The IgG reactivity of the CP sera pool was similar to the WP sera pool for all of the peptides and it could not be compared to the immunoreactivity observed when the P. gingivalis extract was used as an antigen (Additional file 1: Figure S1).
In this immunoreactivity analysis, all HLA alleles tested with neuraminidase peptides were also tested with Kgp peptides. DQB1*03:01 and DRB1*13:02 were tested only with Kgp peptides (Additional file 1: Table S4).
Submission to the Immune Epitope Database
Tested peptides were deposited in the IEDB and they are available for public visualization: Kgp reference ID 1032999 and neuraminidase reference ID 1033135 (Table 4).
Population coverage
The result of the population coverage analysis is shown in Additional file 1: Tables S5, S6.
Peptide presentation by protein modeling
The published peptides were presented in 3D models shown in surface mode (Figs. 2, 3) and in cartoon mode (Fig. 4). It was not possible to present the entire Kgp protein (1723 aa) in cartoon mode because the tool used for modeling allows one to model up to 1500 amino acid residues.
Peptides from different regions of the proteins were chemically synthesized, tested and published. Figure 4 shows the relationship between neuraminidase peptides. It was not possible to present the entire Kgp protein, but we can provide an overview of Kgp peptides within the Kgp protein in Additional file 1: Table S2.
Discussion
It is known that in silico models are used for understanding biological systems as well as to select, to complement, and to inspire the required laboratory experiments (Kollmann and Sourjik 2007; Setty 2014; Brodland 2015). In this context, immunoinformatics brings advances in immunology and can contribute to understanding the immune response (Lefranc 2014; Qiu et al. 2018). In the present study, the in silico analysis enabled the prediction and selection of immunoreactive peptides of P. gingivalis before being synthesized. Two virulence factors of P. gingivalis were analyzed: Kgp, which is widely studied, and neuraminidase, which is still being evaluated in a few studies.
The same HLA alleles were used to predict immunogenic peptides of virulence factors. The HLA alleles tested with neuraminidase peptides were also tested with Kgp peptides and the same pools of sera were used for the immunoreactivity test of the synthetic peptides. However, all tested Kgp peptides presented immunoreactivity to IgG, whereas neuraminidase peptides presented low immunoreactivity to IgG.
Besides that, Kgp12 distinguished between CP and WP pools. The coefficient obtained from the fraction CP pool/WP pool indicated that the absorbance of the CP pool was twice as high as that of the WP pool. Kgp12 was tested in another study to detect specific serum IgG of 71 subjects, and it distinguished the ones with gingivitis from those with chronic periodontitis (Cardoso, unpublished data, 2017).
All tested Kgp peptides presented immunoreactivity to IgG, whereas neuraminidase peptides presented low immunoreactivity to IgG and no neuraminidase peptide presented a coefficient between CP and WP sera that could differentiate subjects with CP; thus the low IgG reactivity of those neuraminidase peptides could be suggested. However, further studies need to be conducted to better define this characteristic of neuraminidase peptides tested herein.
Porphyromonas gingivalis extract and gingipains have the capability to induce immunogenicity since they are recognized by IgG from serum of individuals with chronic periodontitis (O’Brien-Simpson et al. 2000; Inagaki et al. 2003; Nguyen et al. 2004; Franca et al. 2007; Trindade et al. 2008, 2012a). The IgG-mediated response in humans to neuraminidase (sialidase) of P. gingivalis remains, to our knowledge, unknown. However, in the present study, based on the in silico analysis of tested peptides, under the conditions studied, the IgG reactivity of Kgp could be reaffirmed and the low reactivity of neuraminidase could be speculated.
Besides being asaccharolytic, P. gingivalis probably does not use N-acetylneuraminic acid (Neu5Ac), the most studied sialic acid, as a nutrient. This deduction was made because in the culture supplemented with Neu5Ac, it did not interfere with P. gingivalis planktonic growth, and the inactivation of the neuraminidase gene did not influence its growth (Li et al. 2012). Additionally, not only P. gingivalis but also other periodontopathogens, Tannerella forsythia and Treponema denticola, perform glycosylation of their proteins to evade the immune response, persist in the host and cause periodontal destruction (Stafford et al. 2012; Kurniyati et al. 2013; Settem et al. 2013). This is one reason why the low immunogenicity of its neuraminidase should be a favorable characteristic to P. gingivalis, since this virulence factor may be used to evade immune system inflammatory responses.
We also need to consider that, despite being primarily an extracellular pathogen, P. gingivalis has the ability to internalize non-phagocytic cells of the host (Nakagawa et al. 2006; Olsen and Progulske-Fox 2015) and to survive in a macrophage (Gmiterek et al. 2016; Yang et al. 2018) in order to evade the immune system. As in the case of other pathogens (Shtyrya et al. 2009; Banerjee et al. 2010; Freire-de-Lima et al. 2015), sialidase may be used by P. gingivalis as one of the cellular invasion mechanisms.
The ATCC 33277 strain of P. gingivalis (NCBI Taxonomy ID: 431947) is used for pathophysiological characterization of the microorganism, and it is considered a less virulent strain (Naito et al. 2008). Its genome was published by Naito et al. (2008), and the sequences of its proteins and its peptide epitopes have been deposited in online public databases. Epitopes from other antigens of this strain had already been tested and deposited at the IEDB. For example, Kgp peptide DKYFLAIGNCC (Epitope detailed search: Epitope ID 190728) induced IL-17A e IFN-γ production in Mus musculus C57BL/6 (Bittner-Eddy et al. 2013). On the other hand, in the IEDB, epitopes related to neuraminidase (sialidase) of P. gingivalis were not identified until our deposition. In the present study, eight neuraminidase peptides of P. gingivalis were originally deposited in the IEDB, as well as nine novel Kgp peptides, and they are available for public visualization and for use in other assays.
A limitation of the study is that binding to MHC is necessary but not sufficient for epitope recognition by T cells. However, the use of in silico analysis for prediction of immunogenic peptides allowed their selection for chemical synthesis and immunogenicity tests.
Porphyromonas gingivalis manipulates the host immune response using the diversity of its virulence factors (Hajishengallis and Lamont 2014; Dashper et al. 2017). The peptides tested herein could be useful for application in immunogenic studies of virulence factors, since it is more economical to use synthetic peptides than to use recombinant proteins. In addition, specific peptides decrease the risk of cross reactivity in the opposite way of the total extract of the microorganism.
The strategy used herein reduced the total analysis cost and the research expended time in the search for promising results. It could be applicable to a global understanding of P. gingivalis pathogenicity (as well as other periodontal pathogens) in an efficient and rapid manner using chimeric peptides as well. Thus, in silico tools provide comprehensive facilities for designing in vitro and in vivo immunology experiments. This can be a useful strategy in the study of the etiology and pathogenesis of human diseases, especially periodontitis.
In conclusion, here we report the identification of P. gingivalis epitopes. In silico analysis represented a viable strategy to obtain candidate epitopes with human IgG reactivity to study P. gingivalis virulence factors, because the IgG reactivity of Kgp could be reaffirmed and the low reactivity of neuraminidase could be suggested. Kgp12, Kgp17 and Kgp18 peptides were selected for subsequent assays. Besides that, the present study has provided immunogenic peptide sequences from P. gingivalis virulence factors, which may be tested by several different assays in order to contribute to the understanding of the host response to infection and to differentiate subjects with chronic periodontitis.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Armitage G (1999) Development of a classification system for periodontal diseases conditions. Ann Periodontal 4:1–6
Aruni W, Vanterpool E, Osbourne D, Roy F, Muthiah A, Dou Y, Fletcher HM (2011) Sialidase and sialoglycoproteases can modulate virulence in Porphyromonas gingivalis. Infect Immun 79(7):2779–2791
Banerjee A, van Sorge NM, Sheen TR, Uchiyama S, Mitchell TJ, Doran KS (2010) Activation of brain endothelium by pneumococcal neuraminidase NanA promotes bacterial internalization. Cell Microbiol 12(11):1576–1588
Bengtsson T, Khalaf A, Khalaf H (2015) Secreted gingipains from Porphyromonas gingivalis colonies exert potent immunomodulatory effects on human gingival fibroblasts. Microbiol Res 178:18–26
Bittner-Eddy PD, Fischer LA, Costalonga M (2013) Identification of gingipain-specific IAb-restricted CD4+ T cells following mucosal colonization with Porphyromonas gingivalis in C57BL/6 mice. Mol Oral Microbiol 28:452–466
Brodland GW (2015) How computational models can help unlock biological systems. Semin Cell Dev Biol 47–48:62–73
Bui HH, Sidney J, Dinh K, Southwood S, Newman MJ, Sette A (2006) Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinform 17:153
Carvalho-Filho PC, Gomes-Filho IS, Meyer R, Olczak T, Xavier MT, Trindade SC (2016) Role of Porphyromonas gingivalis HmuY in immunopathogenesis of chronic periodontitis. Mediat Inflamm. https://doi.org/10.1155/2016/7465852
Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS (2018) A new classification scheme for periodontal and peri-implant diseases and conditions—introduction and key changes from the 1999 classification. J Clin Periodontol 45(Suppl 20):S1–S8
Cueno ME, Kamio N, Imai K, Ohya M, Tamura M, Ochiai K (2014) Structural significance of the β1K396 residue found in the Porphyromonas gingivalis sialidase β-propeller domain: a computational study with implications for novel therapeutics against periodontal disease. OMICS 18(9):591–599
Dashper SG, Mitchell HL, Seers CA, Gladman SL, Seemann T, Bulach DM, Chandry PS, Cross KJ, Cleal SM, Reynolds EC (2017) Porphyromonas gingivalis uses specific domain rearrangements and allelic exchange to generate diversity in surface virulence factors. Front Microbiol 8:48
de Diego I, Veillard F, Sztukowska MN, Guevara T, Potempa B, Pomowski A, Huntington JA, Potempa J, Gomis-Rüth FX (2014) Structure and mechanism of cysteine peptidase gingipain K (Kgp), a major virulence factor of Porphyromonas gingivalis in periodontitis. J Biol Chem 289(46):32291–32302
Franca M, Moura-Costa L, Meyer RJ, Trindade SC, Tunes UR, Freire SM (2007) Humoral immune response to antigens of Porphyromonas gingivalis ATCC 33277 in chronic periodontitis. J Appl Oral Sci 15(3):213–219
Freire-de-Lima L, Fonseca LM, Oeltmann T, Mendonça-Previato L, Previato JO (2015) The trans-sialidase, the major Trypanosoma cruzi virulence factor: three decades of studies. Glycobiology 25(11):1142–1149
Gmiterek A, Kłopot A, Wójtowicz H, Trindade SC, Olczak M, Olczak T (2016) Immune response of macrophages induced by Porphyromonas gingivalis requires HmuY protein. Immunobiology 221(12):1382–1394
Gomes-Filho IS, Cruz SS, Rezende EJ, dos Santos CA, Soledade KR, Magalhães MA, de Azevedo AC, Trindade SC, Vianna MI, Passos JDS, Cerqueira EM (2007) Exposure measurement in the association between periodontal disease and prematurity/low birth weight. J Clin Periodontol 34(11):95763
Guo Y, Nguyen KA, Potempa J (2010) Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeons knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000 54:15–44
Hajishengallis G (2014) Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35(1):3–11
Hajishengallis G (2015) Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 15:30–44
Hajishengallis G, Lamont RJ (2014) Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol 44:328–338
Hajishengallis G, Darveau RP, Curtis MA (2012) The keystone pathogen hypothesis. Nat Rev Microbiol 10(10):717–725
Inaba H, Tagashira M, Kanda T, Murakami Y, Amano A, Matsumoto-Nakano M (2016) Apple- and hop-polyphenols inhibit Porphyromonas gingivalis-mediated precursor of matrix metalloproteinase-9 activation and invasion of oral squamous cell carcinoma cells. J Periodontol 87(9):1103–1111
Inagaki S, Ishihara K, Yasaki Y, Yamada S, Okuda K (2003) Antibody responses of periodontitis patients to gingipains of Porphyromonas gingivalis. J Periodontol 74(10):1432–1439
Kim Y, Ponomarenko J, Zhu Z, Tamang D, Wang P, Greenbaum J, Lundegaard C, Sette A, Lund O, Bourne PE, Nielsen M, Peters B (2012) Immune epitope database analysis resource. Nucleic Acids Res 40(Web Server issue):W525–W530
Kollmann M, Sourjik V (2007) In silico biology: from simulation to understanding. Curr Biol 17(4):R132–R134
Kurniyati K, Zhang W, Zhang K, Li C (2013) A surface-exposed neuraminidase affects complement resistance and virulence of the oral spirochaete Treponema denticola. Mol Microbiol 89(5):842–856
Lefranc MP (2014) Immunoglobulin and T cell receptor genes: IMGT® and the birth and rise of immunoinformatics. Front Immunol 5:22
Li C, Kurniyati HuB, Bian J, Sun J, Zhang W, Liu J, Pan Y, Lia C (2012) Abrogation of neuraminidase reduces biofilm formation, capsule biosynthesis, and virulence of Porphyromonas gingivalis. Infect Immun 80(1):3–13
Lindhe J, Ranney R, Lamster I, Charles A, Chung CP, Flemmig T, Kinane D, Listgarten M, Löe H, Schoor R, Seymour G, Somerman M (1999) Consensus report: chronic periodontitis. Ann Periodontol 4(1):38
Moncla BJ, Braham P, Hillier SL (1990) Sialidase (neuraminidase) activity among gram-negative anaerobic and capnophilic bacteria. J Clin Microbiol 28(3):422–425
Monteiro AMA, Freire SM, Bendicho MT, Meyer R, Gomes-Filho IS, Tunes UR, Pinheiro CS, Trindade SC, Lemaire DC (2017) Higher prevalence of HLA-DQB1*0301 in non-diabetic mestee subjects with chronic periodontitis. EC Dent Sci 13(6):252–260
Naito M, Hirakawa H, Yamashita A, Ohara N, Shoji M, Yukitake H, Nakayama K, Toh H, Yoshimura F, Kuhara S, Hattori M, Hayashi T, Nakayama K (2008) Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res 15(4):215–225
Nakagawa I, Inaba H, Yamamura T, Kato T, Kawai S, Ooshima T, Amano A (2006) Invasion of epithelial cells and proteolysis of cellular focal adhesion components by distinct types of Porphyromonas gingivalis fimbriae. Infect Immun 74(7):3773–3782
Nguyen KA, DeCarlo AA, Paramaesvaran M, Collyer CA, Langley DB, Hunter N (2004) Humoral responses to Porphyromonas gingivalis gingipain adhesin domains in subjects with chronic periodontitis. Infect Immun 72(3):1374–1382
O’Brien-Simpson NM, Black CL, Bhogal PS, Cleal SM, Slakeski N, Higgins TJ, Reynolds EC (2000) Serum immunoglobulin G (IgG) and IgG subclass responses to the RgpA-Kgp proteinase-adhesin complex of Porphyromonas gingivalis in adult periodontitis. Infect Immun 68(5):2704–2712
Olczak T, Wójtowicz H, Ciuraszkiewicz J, Olczak M (2010) Species specificity, surface exposure, protein expression, immunogenicity, and participation in biofilm formation of Porphyromonas gingivalis HmuY. BMC Microbiol 10:134
Olsen I, Potempa J (2014) Strategies for the inhibition of gingipains for the potential treatment of periodontitis and associated systemic diseases. J Oral Microbiol 6:24800
Olsen I, Progulske-Fox A (2015) Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol 7:28788
Proctor DM, Fukuyama JA, Loomer PM, Armitage GC, Lee SA, Davis NM, Ryder MI, Holmes SP, Relman DA (2018) A spatial gradient of bacterial diversity in the human oral cavity shaped by salivary flow. Nat Commun 9(1):681
Qiu T, Yang Y, Qiu J, Huang Y, Xu T, Xiao H, Wu D, Zhang Q, Zhou C, Zhang X, Tang K, Xu J, Cao Z (2018) CE-BLAST makes it possible to compute antigenic similarity for newly emerging pathogens. Nat Commun 9(1):1772
Rocha N, Neefjes J (2008) MHC class II molecules on the move for successful antigen presentation. EMBO J 27(1):1–5
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738
Settem RP, Honma K, Stafford GP, Sharma A (2013) Protein-linked glycans in periodontal bacteria: prevalence and role at the immune interface. Front Microbiol 4(310):1–6
Setty Y (2014) In-silico models of stem cell and developmental systems. Theor Biol Med Model 11:1
Shtyrya YA, Mochalova LV, Bovin NV (2009) Influenza virus neuraminidase: structure and function. Acta Naturae 1(2):26–32
Smalley JW, Olczak T (2017) Heme acquisition mechanisms of Porphyromonas gingivalis—strategies used in a polymicrobial community in a heme-limited host environment. Mol Oral Microbiol 32(1):1–23
Stafford G, Roy S, Honma K, Sharma A (2012) Sialic acid, periodontal pathogens and Tannerella forsythia: stick around and enjoy the feast! Mol Oral Microbiol 27(1):11–22
Tada H, Matsuyama T, Nishioka T, Hagiwara M, Kiyoura Y, Shimauchi H, Matsushita K (2016) Porphyromonas gingivalis gingipain-dependently enhances IL-33 production in human gingival epithelial cells. PLoS ONE 11(4):e0152794
Trindade SC, Gomes-Filho IS, Meyer RJ, Vale VC, Pugliese L, Freire SM (2008) Serum antibody levels against Porphyromonas gingivalis extract and its chromatographic fraction in chronic and aggressive periodontitis. J Int Acad Periodontol 10(2):50–58
Trindade SC, Olczak T, Gomes-Filho IS, Moura-Costa LF, Cerqueira EMM, Galdino-Neto M, Alves H, Carvalho-Filho PC, Xavier MT, Meyer R (2012a) Induction of interleukin (IL)-1b, IL-10, IL-8 and immunoglobulin G by Porphyromonas gingivalis HmuY in humans. J Periodont Res 47:27–32
Trindade SC, Olczak T, Gomes-Filho IS, Moura-Costa LF, Vale VL, Galdino-Neto M, Alves H, Carvalho-Filho PC, Sampaio GP, Xavier MT, Sarmento VA, Meyer R (2012b) Porphyromonas gingivalis antigens differently participate in the proliferation and cell death of human PBMC. Arch Oral Biol 57:314–320
Trindade SC, Olczak T, Gomes-Filho IS, Moura-Costa LF, Vale VC, Galdino-Neto M, Santos HA, Carvalho Filho PC, Stocker A, Bendicho MT, Xavier MT, Cerqueira EMM, Meyer R (2013) Porphyromonas gingivalis HmuY-induced production of interleukin-6 and IL-6 polymorphism in chronic periodontitis. J Periodontol 84(5):650–655
Vita R, Zarebski L, Greenbaum JA, Emami H, Hoof I, Salimi N, Damle R, Sette A, Peters B (2010) The immune epitope database 2.0. Nucleic Acids Res 38(Database issue):D854–D862
Vyas JM, Van der Veen AG, Ploegh HL (2008) The known unknowns of antigen processing and presentation. Nat Rev Immunol 8(8):607–618
Wang P, Sidney J, Dow C, Mothé B, Sette A, Peters B (2008) A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol 4(4):e1000048
Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, Peters B (2010) Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinform 11:568
Xu X, Tong T, Yang X, Pan Y, Lin L, Li C (2017) Differences in survival, virulence and biofilm formation between sialidase-deficient and W83 wild-type Porphyromonas gingivalis strains under stressful environmental conditions. BMC Microbiol 17(1):178
Yang X, Pan Y, Xu X, Tong T, Yu S, Zhao Y, Lin L, Liu J, Zhang D, Li C (2018) Sialidase deficiency in Porphyromonas gingivalis increases IL-12 secretion in stimulated macrophages through regulation of CR3, lncRNA GAS5 and miR-21. Front Cell Infect Microbiol 8:100
Authors’ contributions
EKNSL, MTX and SCT designed the study. EKNSL, KAPAC, ACMP, YAO and PCCF carried out sample collection and diagnostics. EKNSL, PCCF and LFMC carried out the microbiological experiments. EKNSL, MTX and SCT carried out the in silico analysis. EKNSL, KAPAC, PMM and PCCF carried out the immunoassays. EKNSL, KAPAC and SCT performed the statistical analysis. ISGF, RMN, MTX and SCT coordinated the work. EKNSL wrote de manuscript. MTX, SCT and TO critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are sincerely grateful to the technical staff of the Laboratory of Immunology and Molecular Biology (LABIMUNO), of the Health Sciences Institute of the Federal University of Bahia and of the Laboratory of Microbiology of the Health Sciences Institute of the Federal University of Bahia, Brazil. They are also grateful to the Postgraduate Program in Immunology and to the Postgraduate Program in Biotechnology of the Federal University of Bahia, Brazil. Professor Mario Julio Avila-Campos (Department of Microbiology, Institute of Biomedical Sciences, University of São Paulo, SP, Brazil) is acknowledged for microbiology support. Professor Michelle Miranda Lopes Falcão (Department of Health, Feira de Santana State University, BA, Brazil) is acknowledged for scientific support. Richard Ashcroft is acknowledged for English correction.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Please contact the author for data requests.
Consent to publish
Not applicable.
Ethics approval and consent to participate
This work has been approved by the ethics committee of Feira de Santana State University (Feira de Santana, Bahia, Brazil) and of the Federal University of Bahia (Salvador, Bahia, Brazil) through Plataforma Brasil (CAAE number: 32535914.4.0000.0053).
All participants signed the consent to participate form.
Funding
This work was supported by the Research Support Foundation of the State of Bahia (FAPESB); by the Laboratory of Immunology and Molecular Biology (LABIMUNO), Health Sciences Institute, Federal University of Bahia; and by the Foundation for Research and Extension Support (FAPEX), Brazil.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1.
Additional Figure and Tables.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
dos Santos-Lima, E.K.N., Cardoso, K.A.P.A., de Miranda, P.M. et al. In silico analysis as a strategy to identify candidate epitopes with human IgG reactivity to study Porphyromonas gingivalis virulence factors. AMB Expr 9, 35 (2019). https://doi.org/10.1186/s13568-019-0757-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-019-0757-x