Abstract
Bovine tuberculosis is a disease caused by Mycobacterium bovis (M. bovis) that leads to great economic losses in cattle production. The discovery of a reasonable bioagent to reduce M. bovis infection risk and environment contamination becomes significant and urgent. Previous study reported that human β-defensin-3 (HBD3) participated in Mycobacterial immunity and was recognized as a suitable candidate reagent. However, its minimal inhibitory concentration to M. bovis is not yet reported. In this study, we first purified HBD3 protein by recombinant-DNA technology and prokaryotic expression system. Subsequently, anti-bacterial tests were used to evaluate the basic bioactivity of the protein. Results revealed that recombinant HBD3 (rHBD3) protein inhibits Staphylococcus multiplication but not the host Escherichia coli. The growth curve of M. bovis showed that rHBD3 protein controls the proliferation of M. bovis in 20 μg/ml concentration. In addition, rHBD3 protein-incubated M. bovis exhibited reduced infectivity to alveolar epithelial cells and macrophages. In conclusion, the expression of rHBD3 protein is a potential ideal bio-regent for reducing M. bovis infection.
Similar content being viewed by others
Introduction
Bovine tuberculosis is a chronic disease caused by Mycobacterium bovis (M. bovis) and is mainly characterized by the formation of granulomas in the lung and other organs (Muller et al. 2013). The disease causes great economic losses in cattle production every year. Killing of the suffering cattle as a common approach to reduce M. bovis prevalence results in great economic losses (Su et al. 2016). Our understanding on reducing these losses mainly focuses on tuberculosis prevention. This approach is divided into two major approaches. One of which is the vaccine method; however, the inefficient protection ability and potential virulence risk of antigen limits its application in cattle (Buddle et al. 2011). The other one is the prophylactic application of antibiotics in rational dosage. However, the ecological damage of probiotics in the animal body, and the pathogenic bacteria variation in the environment increased the cattle illness occurrence risk (Allen et al. 2010; Martinez 2008, 2009). Thus, a reasonable bio-agent is needed to reduce bovine Mycobacterial infection risk and environment contamination. Small potential human defensins that participated in the Mycobacterial immunity were recognized as suitable candidate agents that can reduce this risk (Driss et al. 2009; Rivas-Santiago et al. 2006). Among these defensins, human β-defensin 3 (HBD3) is an ideal potential one as previously reported.
As a star protein of β defensins that acts both as an antimicrobial agent and chemo-attractant molecule, HBD3 has an effective antibacterial activity for many different bacteria (Hoover et al. 2003; Maisetta et al. 2003). Moreover, the HBD3 protein exhibited low red cytotoxicity in high salt concentration relative to other proteins (Quinones-Mateu et al. 2003; Sun et al. 2005). High concentrated HBD3 protein appears in early M. bovis infection period and is reduced in the latent stage. HBD3 protein participates in Mycobacterial clearance and is also associated with long-term control of Mycobacterial proliferation (Rivas-Santiago et al. 2006). Previous study reported that His-HBD3 recombinant protein exhibited anti-Mycobacterial capacity to H37Rv strain (Corrales-Garcia et al. 2013). A recent study also confirmed that the expression of HBD3 protein in cattle evidently reduced the susceptibility to M. bovis infection (Su et al. 2016). However, the accurate inhibition concentration of this purified peptide (without His-tag) has not been determined yet.
The expression of HBD3 protein in vivo solely relies on an EGFR/MAPK/AP-1 dependent pathway, and the HBD3 protein production mainly depends on the pathogenic bacteria intensity (Steubesand et al. 2009). Hence, HBD3 protein maintaining a natural physiological concentration which is not efficiently to resist the high-density bacteria invasion within a short time. Prokaryotic expression of HBD3 fusion protein was initially designed and optimized by Huang et al. (2006, 2007). However, the existence of His tag reduced the production of HBD3. Additionally, N-terminal electric variation caused by His tag affected its biological characteristics (Hoover et al. 2003). Thus, the obtained soluble rHBD3 and its anti-M. bovis capacity analysis are critical for the tuberculosis control in cow.
In this study, highly efficient soluble rHBD3 protein was expressed by GST prokaryotic expression system. The anti-bacterial ability and anti-M. bovis capacity of rHBD3 protein were evaluated.
Materials and methods
Expression vector construction
The pGEX-5X-1 vector was purchased in Amersham Pharmacia Biotech (Piscataway, New Jersey, USA) and was then amplified in LB culture medium (tryptone 1%, yeast extract 0.5%, NaCl 1%, pH: 7.0). HBD3 sequence was obtained by mature protein sequence, and optimal sequence was synthesized in Invitrogen Company (Invitrogen, Carlsbad, CA). The optimal designed sequences are as follows: (GTGATCATTAACACTCTGCAAAAATATTACTGCCGCGTGCGTGGTGGCCGTTGTGCGGTTCTGTCCTGTCTGCCGAAAGAAGAGCAGATCGGCAAATGCTCTACCCGCGGTCGTAAATGCTGCCGTCGTAAAAAGTAATGATGAGAATTC). The vector and optimal sequence were double digested by BamHI, EcoRI and BclI, EcoRI separately and were connected by T4 ligase enzyme. Recombinant of pGEX-5X-HBD3 plasmid was identified by digestion and sequencing.
Determination of optimal induced conditions
Optimal-induced condition test was operated to obtain more soluble HBD3 protein. In the previous study, the optimal-induced temperature and IPTG concentration were tested. Hence, we selected 28 °C and 1 mM IPTG as optimized conditions, and the optimal inducement time was evaluated. Bacteria were collected at 3, 5, 7, and 9 h, and then the bacteria were cracked by lysozyme. Supernatant and sediment were separately collected after being centrifuged on 4000 rpm for 5 min. Optimal induce time was analyzed by SDS-PAGE.
Purification of GST-HBD3 fusion protein
The E. coli bacteria that containing pEGX-5X-HBD3 plasmid was amplified in LB medium (0.5% yeast extract, 1% tryptone, 1% NaCl) containing 50 μg ampicillin/ml. IPTG was then added into the medium when its OD (600) reached 0.4. The bacteria were harvested and collected by centrifuging at 4000 rpm for 20 min. The purified GST-HBD3 recombinant protein was obtained as previously reported (Huang et al. 2007).
Identification and harvest of rHBD3 protein
Purified HBD3 recombinant protein was harvested by cleavage of GST-HBD3 protein using Factor Xa (NEB) at room temperature. The enzyme and Xa buffer (20 mM Tris–HCl: pH 8.0 with 100 mM NaCl and 2 mM CaCl2) were incorporated into the GST-HBD3 protein at 23 °C for 6 h, and the total digested protein was dialyzed by binding buffer (50 mM NaH2PO4–Na2HPO4, 1 M NaCl, pH 7.4). The purified protein was obtained after flowing through Sepharose® Fast Flow system. The protein was checked by Tris-tricine-SDS-PAGE and was confirmed by western blot analysis. After being transferred on a PVDF membrane, the protein was confirmed after incubation with HBD3 antibody (Sigma, St. Louis, MO) at 4 °C overnight and with goat anti rabbit IgG (Sigma, St. Louis, MO) for 2 h.
Antimicrobial activity of purified rHBD3 on BL21 (DE3) E. coli and Staphylococcus
Antimicrobial activity tests of HBD3 protein were evaluated for its biological function by a bacteria growth curve test for BL21 host bacteria and Staphylococcus aureus (ATCC25923). Exponentially growing bacteria were re-suspended in 10 mM sodium phosphate buffer (pH 7.4) to reach a density of 5 × 107 CFU/ml. Ten microliters of each bacterial suspension was exposed for 1.5 h under the appropriate culture condition to different treatments in 100 μl of 10 mM sodium phosphate buffer (pH 7.4). The number of bacteria as directed by the optical density (OD 600) was measured every 30 min. The inhibition zone tests were performed to identify its inhibitory concentration.
Determination of minimal inhibition concentration (MIC) on M. bovis
Mycobacteria bovis virulent strain C68014 were purchased from the China Institute of Veterinary Drugs Control (Beijing, China) and cultured on Middlebrook 7H10 medium (Difco Laboratories, Detroit, MI) for 20 days. The colonies were then transferred to Middlebrook 7H9 modified medium (Difco Laboratories, Detroit, MI, USA) for 20 days. Determination of MIC was performed in M. bovis growth curve. Serial concentrated peptide dilutions in Middle Brook 7H9 broth were prepared. Subsequently, 50 μl of this suspension was mixed with 50 μl of the peptide dilution in each well. After incubation for 12, 24, 48, 60, 72, 84, and 96 h at 37 °C, the bacterial number directed by OD600 data was measured.
In vitro infection of A549 cells
Human type II alveolar pneumocytes A549 (CCL185) and RAW 264.7 cells were separately cultured in 75 cm2 culture flasks (CAS, Shanghai, China) with antibiotic-free Dulbecco’s modified Eagle’s medium (HyClone laboratories, Logan, Utah) supplemented with 10% fetal calf serum (Gibco BRL, Grand Island, NY). A549 and RAW 264.7 cells were pre-incubated in the medium for 24 h prior to M. bovis infection. The cells were infected with M. bovis at MOI 10:1 for 24 h. M. bovis was then detected by Auramine O (Sigma) methods according to the manufacturer’s instructions.
Cell apoptosis detection
Mycobacteria bovis (MOI: 10:1) and HBD3 protein were separately co-incubated with A549 and RAW 264.7 cells for 24 h. Cell apoptosis was then evaluated by DeadEnd TM Fluorometric Tunel System (Promega Corporation, Madison, WI, USA) and cell nucleus dying by PI according to the instruction book (Sigma Co, St. Louis, MO). Cell apoptosis ratio was then detected by flow cytometry.
Statistical analysis
Inhibited concentration, FCM and CFU test results were analyzed using SPASS software (SPSS, Chicago, IL, USA). The changes are presented as mean ± SEM and were compared using one-way ANOVA followed by Newman–Keuls test. P values < 0.05 were considered as statistically significant.
Results
Construction of pGEX-5X-HBD3 vector
The vector was designed as Fig. 1a, and the sequences were synthesized after the optimal design. The vectors and sequences were double digested by BamHI, EcoRI and BclI, EcoRI separately. The recombinant vector was constructed by utilizing isocaudarner and then detected by double digestion and sequencing methods. The 1st lane in Fig. 1b is the vector digested by EcoRI, and the 2nd lane is the vector double digested by BstBI and EcoRI. The sequencing data revealed that the synthesized DNA fragment was correctly inserted into the vector. The amino acid fragments labeled as red is the Factor Xa recognition site, whereas the yellow labeled is the HBD3 protein sequence (Fig. 1c).
Recombinant HBD3 protein prokaryotic expression vector construction and verification. a Designing scheme of rHBD3 prokaryotic expression vector. b Restriction enzyme analysis of the PGEX-5X-HBD3 recombinant plasmid. Lane 1 is the DNA fragment recombination plasmid digested by EcoRI. Lane 2 is the DNA fragments of recombination plasmid digested by BstBI and EcoRI. c Sequencing identification of PGEX-5X-HBD3 vector. The DNA and amino acid fragments marked by red are the Factor Xa recognition sites. The yellow one is the HBD3 protein sequences
Expression of recombinant HBD3 (rHBD3) protein
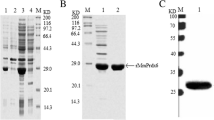
The optimal induce times were evaluated by SDS-PAGE. The data showed that 9 h is the optimal induction time to gain more soluble fusion HBD3 protein (Fig. 2a). Figure 2b shows that abundant fusion GST-HBD3 protein was purified by Sepharose® Fast Flow system. Figure 2c reveals that GST-HBD3 protein was completely cleaved by Xa factors. Western blot analysis confirmed that the purified protein was HBD3 (Fig. 2d).
Expression and identification of rHBD3 protein. a Effects of different post induction times on the expression of target fusion protein with BL21 (DE3) pGEX-5X-HBD3. Soluble and insoluble proteins were analyzed at various post induction times by using SDS-PAGE (S soluble fractions, I insoluble fractions). b SDS-PAGE analysis of collected samples of purified fusion protein. M is the protein marker. Lanes are all the purified fusion protein. c SDS-PAGE analysis of protein fragment that has been digested by Factor Xa. M is the protein marker. Lane 1 is the purified recombination HBD3, lane 2 is the digested protein fragments lane 3 is pure GST-HBD3 protein, lane 4 is all protein. d Western blot analysis of recombination HBD3. Lanes are all the purified rHBD3
rHBD3 is harmless to Escherichia coli (BL21)
The anti-E. coli activity of HBD3 protein was evaluated by bacterial growth curve and exhibition zone tests. Results showed that either GST-HBD3 or recombinant HBD3 protein is harmful to E. coli grown in any concentration (Fig. 3a, b).
Anti-bacterial capacity analysis of rHBD3 protein by E. coli and Staphylococcus. a Anti-bacterial action of different proteins (GST-HBD3, HBD-3, GST, ampicillin) treated on BL21 (DE3) strain. In this experiment, the final concentration of GST tag protein, GST-HBD3 fusion protein, and rHBD3 protein was 20 μg/ml. b Inhibition zone of different protein (GST-HBD3, HBD-3, GST, AMP) on E. coli of BL21 (DE3) stain (W water, G GST tag, GH GST-HBD3 fusion protein, H recombination HBD3 protein, A ampicillin). c Anti-bacterial action of different proteins (GST-HBD3, HBD-3, GST, and AMP) treated on Staphylococcus aureus (ATCC 25923) strain. In this experiment, the concentration of GST tag protein, GST-HBD3 fusion protein, and rHBD3 protein was 10 μg/ml. d Inhibition zone of different proteins (GST-HBD3, HBD-3, GST, and AMP) on S. aureus (ATCC 25923) strain (W water, G GST tag, GH GST-HBD3 fusion protein, H recombination HBD3 protein, A ampicillin)
rHBD3 displayed anti-Staphylococcus activity
The basic bioactivity of rHBD3 protein was reflected by the anti-Staphylococcus capacity. Results showed that rHBD3 completely inhibits the Staphylococcus proliferation in low concentration compared with other proteins obtained from purity experiments (Fig. 3c, d).
rHBD3 reduced Mycobacterial infection capacity in alveolar epithelial cells
The anti-bacterial capacity of rHBD3 to M. bovis was evaluated by its growth characteristic. Figure 4a suggests that rHBD3 protein exhibits strong anti-M. bovis capacity at 20 μg/ml relative to other concentration (Fig. 4a). When A549 cells were infected with Mycobacteria at MOI 10:1, the M. bovis bacteria were observed after staining with Auramine O (Fig. 4b). Subsequently, both rHBD3 protein (20 μg/ml), M. bovis (MOI 10:1)were mixed with A549 cells for 24 h, cells apoptosis and death ratio was evaluated by flow cytometry (FCM). The apoptosis and death ratios were evidently reduced in the rHBD3 and streptomycin-incubated group compared with those in the PBS-incubated group. However, no differences were observed in these two groups (Fig. 4c, d). Mycobacteria CFUs were separately detected in cells and cell medium; the results revealed that HBD3 treating Mycobacteria were evidently reduced both in A549 cells and its medium. However, no discrepancy was found in streptomycin- and HBD3-incubated group (Fig. 4e).
Anti-Mycobacterium bovis capacity of rHBD3 protein to A549 cells. a M. bovis growth curve after incubation with different concentrations of HBD-3. Negative control is M. bovis with Middle Brook 7H9 broth. Positive control is M. bovis with streptomycin (1000 U/ml). b Mycobacteria invasion tests. Yellow spots in the figure are Mycobacteria. c Cell apoptosis analysis of A549 cells infected by M. bovis with different treatments. d The data of cell apoptosis and death ratio analysis of A549 cells infected by M. bovis with different treatments. All the experiments were replicated three times and the changes are presented as mean ± SEM. P values < 0.05 were considered as statistically significant. e CFU tests in A549 cells and its medium (*P < 0.05)
rHBD3 reduced M. bovis infection capacity to macrophage
The anti-M. bovis tests were conducted in macrophage. The yellow spot in Fig. 5a revealed that Mycobacteria were recruited into the macrophage after dyeing with Auramine O. Subsequently, the mixture of rHBD3 (20 μg/ml) and M. bovis (MOI 10:1) was incubated with RAW264.7 cells for 24 h, and the cell apoptosis and death ratio was evaluated by flow cytometry (FCM). Apoptosis and death ratios were evidently reduced in rHBD3- and streptomycin-incubated group compared with negative control. However, no differences were observed for these two groups (Fig. 5b, c). CFU tests revealed that the Mycobacterial infection capacity was evidently reduced after treated with HBD3 (20 μg/ml) or streptomycin (1000 U) (Fig. 5d).
Mycobacterium bovis resistant capacity analysis of rHBD3 protein to macrophage. a Mycobacteria invasion tests. Yellow spots in the figure are Mycobacteria. b Cell apoptosis analysis of macrophage infected by M. bovis with different treatments. c The data of cell apoptosis and death ratio analysis of macrophages infected by M. bovis with different treatments. All the experiments were replicated three times and the changes are presented as mean ± SEM. P values < 0.05 were considered as statistically significant. d CFU tests in macrophage and its medium (a,*P < 0.05)
Discussion
Human β-defensins participate in the control of Mycobacteria multiplication in the latent infection stage (Rivas-Santiago et al. 2006). As a star molecule of human β-defensins, HBD3 participated in the in vivo killing of pathogenic microorganism that relies on its bioactivity (Auvynet and Rosenstein 2009). A recent study reported that HBD3-transgenic cattle reduces the susceptibility to M. bovis infection (Su et al. 2016). However, the expression of HBD3 in vitro and its accurate inhibition concentration are not yet determined. The results of the current study suggested that purified rHBD3 is obtained by recombinant DNA technology and prokaryotic expression system. Anti-bacterial tests revealed that rHBD3 protein maintains its basic bioactivity. Anti-Mycobacterial capacity study showed that rHBD3 protein reduces the M. bovis infectivity by killing the bacteria.
Huang et al. first purified the expression of rHBD3 recombinant protein (fused with His tag) in vitro by using optimal-induced condition (Huang et al. 2006, 2007). However, the charge diversity of His tag affected the anti-bacterial property of HBD3 protein as previously reported (Carson et al. 2007; Scudiero et al. 2010). Moreover, small quantity of soluble rHBD3 protein was produced despite using optimal induction condition because of the limited water solubility of His tag. Additionally, the potential inhibiting effects of His-HBD3 protein to host bacteria existed when the protein was produced. Thus, the GST fusion expression system is the best choice for rHBD3 protein expression. Subsequently, GST-HBD3 was expressed and proved no effects to host bacteria growth (Si et al. 2007). However, the recombinant protein was not perfectly checked by western blot analysis in their study, and instead only molecular weight comparison was operated.
The optimal induction condition was evaluated to obtain more soluble protein. In this study, the rational induced time is 9 h after adding isopropyl-β-d-thiogalactopyranoside (IPTG), which is shorter than previously reported (Huang et al. 2007). The protein was digested by Factor Xa and purified by Sepharose® Fast Flow system. Factor Xa digested temperature increased the degradation risk of GST-HBD3 protein relative to TrxA proteases (Auvynet and Rosenstein 2009). Fortunately, rHBD3 protein was not degraded for rigorous operation.
rHBD3 bioactivity was evaluated by anti-bacterial tests. The results suggested that rHBD3 protein is not harmful to E. coli, but the protein exhibits strong anti-bacterial activity to Staphylococcus, which is different from previous reports (Nuding et al. 2009). The expressed rHBD3 lost its anti-E. coli capacity in the current study compared with previously reported findings. One of the reason that causing this difference is the bacterial strain discrepancy, which is a result of different antibacterial spectrum. The previous study used ATCC 25922 E. coli strain, whereas the current study used BL21 (DE3) as a target one. The other important reason is the peptide structure differences that caused the anti-bacterial capacity discrepancy (Powers and Hancock 2003). In the current study, the peptide structure decided by GST expression system which led to antibacterial capacity discrepancy. Additionally, the anti-Staphylococcus activity of rHBD3 showed no obvious changes compared with that in the previous reports (Sass et al. 2010; Scudiero et al. 2010).
In a previous study, Bruno Rivas-Santiago et al. proved that β-defensins were important in early immune responses to Mycobacterial tuberculosis (Rivas-Santiago et al. 2005). Subsequently, they pointed out that β-defensins were expressed and play crucial roles in tuberculosis infection (Rivas-Santiago et al. 2006). The anti-Mycobacterial capacity of HBD3 was evaluated by H37Rv bacterial strain, and its MIC was 3.4 μM (Martinez 2008). However, its anti-M. bovis activity was not checked in the previous study. In the current study, rHBD3 anti-M. bovis capacity was evaluated by M. bovis growth curve and cell apoptosis tests. Results showed that rHBD3 protein suppresses the M. bovis multiplication as previously reported (Martinez 2008), and its minimal inhibitory concentration was lower than H37Rv strain. Cell apoptosis test revealed that the HBD3-treated M. bovis reduced the A549 cells and macrophage susceptibility to M. bovis, which is identical to the results of the previous report (Su et al. 2016). Additionally, no obvious discrepancy was observed between streptomycin- and HBD3-incubated group. Surprisingly, CFU assays exhibited that M. bovis counts in epithelial cells is incompletely equal to in macrophage, especially performed in cells and its medium. This discrepancy confirmed the recruitment of macrophage during M. bovis infection, which conforms to the result of previous report.
Auramine O was used for M. bovis detection; results showed that more bacteria invaded into cytoplasm, which is the same in previous reports (Alnour et al. 2012; Anthony et al. 2006). However, M. bovis that surrounding the cells were not counted because limiting of M. bovis growth characteristics. This finding exhibited that A549 cells has an important role in controlling M. bovis multiplication.
In summary, purified rHBD3 was obtained by recombinant DNA technology and prokaryotic expression system. Anti-bacterial tests revealed that rHBD3 protein inhibits the Staphylococcus multiplication rather than the host E. coli, which maintained its basic bioactivity. M. bovis inhibition test revealed that rHBD3 protein controls the M. bovis proliferation in 20 μg/ml concentration. In addition, M. bovis treated by rHBD3 protein reduced its infectivity to epithelial cells and macrophage. In conclusion, the expression of HBD3 protein inhibits M. bovis growth and thus is an ideal reagent for M. bovis prevention and therapy.
Abbreviations
- M. bovis :
-
Mycobacteria bovis
- HBD3:
-
Human bata defensin 3
References
Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J (2010) Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol 8(4):251–259. https://doi.org/10.1038/nrmicro2312
Alnour T, Hoffmann H, Thiel S (2012) Modification of auramine O fluorescence stain for differential detection of Mycobacterium tuberculosis and Mycobacteria other than tuberculosis (MOTT). Eur Respir J 40(Suppl 56):P1410
Anthony R, Kolk A, Kuijper S, Klatser P (2006) Light emitting diodes for auramine O fluorescence microscopic screening of Mycobacterium tuberculosis [Technical Note]. Int J Tuberc Lung Dis 10(9):1060–1062
Auvynet C, Rosenstein Y (2009) Multifunctional host defense peptides: antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J 276(22):6497–6508
Buddle BM, Wedlock DN, Denis M, Vordermeier HM, Hewinson RG (2011) Update on vaccination of cattle and wildlife populations against tuberculosis. Vet Microbiol 151(1–2):14–22. https://doi.org/10.1016/j.vetmic.2011.02.021
Carson M, Johnson DH, McDonald H, Brouillette C, DeLucas LJ (2007) His-tag impact on structure. Acta Crystallogr D Biol Crystallogr 63(3):295–301
Corrales-Garcia L, Ortiz E, Castañeda-Delgado J, Rivas-Santiago B, Corzo G (2013) Bacterial expression and antibiotic activities of recombinant variants of human β-defensins on pathogenic bacteria and M. tuberculosis. Protein Express Purif 89(1):33–43
Driss V, Legrand F, Hermann E, Loiseau S, Guerardel Y, Kremer L, Adam E, Woerly G, Dombrowicz D, Capron M (2009) TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood 113(14):3235–3244. https://doi.org/10.1182/blood-2008-07-166595
Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J (2003) Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob Agents Chemother 47(9):2804–2809
Huang L, Wang J, Zhong Z, Peng L, Chen H, Xu Z, Cen P (2006) Production of bioactive human β-defensin-3 in Escherichia coli by soluble fusion expression. Biotechnol Lett 28(9):627–632
Huang L, Xu Z, Zhong Z, Peng L, Chen H, Cen P (2007) Enhanced expression and primary purification of soluble HBD3 fusion protein in Escherichia coli. Appl Biochem Biotechnol 142(2):139–147
Maisetta G, Batoni G, Esin S, Luperini F, Pardini M, Bottai D, Florio W, Giuca MR, Gabriele M, Campa M (2003) Activity of human beta-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob Agents Chemother 47(10):3349–3351
Martinez JL (2008) Antibiotics and antibiotic resistance genes in natural environments. Science 321(5887):365–367. https://doi.org/10.1126/science.1159483
Martinez JL (2009) Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut 157(11):2893–2902. https://doi.org/10.1016/j.envpol.2009.05.051
Muller B, Durr S, Alonso S, Hattendorf J, Laisse CJ, Parsons SD, van Helden PD, Zinsstag J (2013) Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg Infect Dis 19(6):899–908. https://doi.org/10.3201/eid1906.120543
Nuding S, Zabel LT, Enders C, Porter E, Fellermann K, Wehkamp J, Mueller HA, Stange EF (2009) Antibacterial activity of human defensins on anaerobic intestinal bacterial species: a major role of HBD-3. Microbes Infect 11(3):384–393
Powers J-PS, Hancock RE (2003) The relationship between peptide structure and antibacterial activity. Peptides 24(11):1681–1691
Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A (2003) Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. Aids 17(16):F39–F48. https://doi.org/10.1097/01.aids.0000096878.73209.4f
Rivas-Santiago B, Schwander SK, Sarabia C, Diamond G, Klein-Patel ME, Hernandez-Pando R, Ellner JJ, Sada E (2005) Human β-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun 73(8):4505–4511
Rivas-Santiago B, Sada E, Tsutsumi V, Aguilar-León D, Contreras JL, Hernández-Pando R (2006) β-Defensin gene expression during the course of experimental tuberculosis infection. J Infect Dis 194(5):697–701
Sass V, Schneider T, Wilmes M, Körner C, Tossi A, Novikova N, Shamova O, Sahl H-G (2010) Human β-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect Immun 78(6):2793–2800
Scudiero O, Galdiero S, Cantisani M, Di Noto R, Vitiello M, Galdiero M, Naclerio G, Cassiman J-J, Pedone C, Castaldo G (2010) Novel synthetic, salt-resistant analogs of human beta-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob Agents Chemother 54(6):2312–2322
Si L-G, Liu X-C, Lu Y-Y, Wang G-Y, Li W-M (2007) Soluble expression of active human beta-defensin-3 in Escherichia coli and its effects on the growth of host cells. Chin Med J-Peking 120(8):708–713
Steubesand N, Kiehne K, Brunke G, Pahl R, Reiss K, Herzig K-H, Schubert S, Schreiber S, Fölsch UR, Rosenstiel P (2009) The expression of the β-defensins hBD-2 and hBD-3 is differentially regulated by NF-κB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. Bmc Immunol 10(1):1
Su F, Wang Y, Liu G, Ru K, Liu X, Yu Y, Liu J, Wu Y, Quan F, Guo Z (2016) Generation of transgenic cattle expressing human β-defensin 3 as an approach to reducing susceptibility to Mycobacterium bovis infection. FEBS J 283:776–790
Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, Berrone S, Kleinman C, Wu Z, Abdelwahab S, Lu W, Garzino-Demo A (2005) Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol 79(22):14318–14329. https://doi.org/10.1128/JVI.79.22.14318-14329.2005
Authors’ contributions
SF and ZY were contributed in experiments designed and manuscripts writing; CX and LX operated the experiments. LGH were analyzed the datas. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All the data and materials referred in this manuscript were all availability.
Consent for publication
All the authors are all agree for publication in this journal.
Ethics approval and consent to participate
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies. The experimental procedure was approved by the Animal Care and Use Committee of the Northwest A&F University and performed in accordance with animal welfare and ethics guidelines.
Funding
This study was funded by Grant from the National Key Project for Production of Transgenic Livestock, PR China (No. 2013ZX-08007-004).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Su, F., Chen, X., Liu, X. et al. Expression of recombinant HBD3 protein that reduces Mycobacterial infection capacity. AMB Expr 8, 42 (2018). https://doi.org/10.1186/s13568-018-0573-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-018-0573-8