Abstract
Bacterial communities of biofilms growing on artificial substrates were examined at two time periods (7 and 14 days) and two locations (lentic and lotic areas) in a hypereutrophic urban river of eastern China. Previous studies in this river network indicated that variations of microbial communities were the major factor affecting the distribution of antibiotic resistant genes highlighting the importance of understanding controls of microbial communities. Bacterial communities associated with biofilms were determined using epifluorescence microscopy and high-throughput sequencing. Results showed that sampling time and site had significant effects on the abundances of surface-associated bacteria. No significant differences were found in the number of surface-associated bacteria between two substrate types (filament vs. slide). Sequencing revealed microbial communities attached to artificial substrates in a hypereutrophic urban river were composed of 80,375 OTUs, and distributed in 47 phyla. Proteobacteria and Cyanobacteria/Chloroplast were the two dominant phyla, followed by Planctomycetes, Actinobacteria, Verrucomicrobia, Firmicutes and Bacteroidetes. Taxonomic composition showed ammonia-oxidizing microorganisms, fecal indicator bacteria and pathogens enriched in attached microbial communities, especially the ammonia-oxidizing Nitrosomonas bacteria. These results indicated that there were significant temporal and intra-river heterogeneity of attached microbial community structure, but no significant difference in community composition was detected between the two substrate types.
Similar content being viewed by others
Introduction
Rivers are an important resource for human society and ecosystems by providing water for consumption, agriculture, industry and replenishing other freshwater ecosystems (Araya et al. 2003; Vörösmarty et al. 2010). Compared to wildland rivers, rivers that runthrough urban centers are highly impacted by human activities, such as wastewater discharge, channelization, and bank reinforcement. Consequently, urban rivers tend to be among the most degraded aquatic ecosystems in the world. In China, rapid economic growth and urbanization within the past several decades has resulted in dramatically degraded water quality and decline in ecosystem function of urban rivers. About 80% of Chinese urban rivers were reported to be heavily polluted and degraded, especially in the densely populated eastern coastal plains (Cai et al. 2016; Qiu 2011; Zhang and Xu 2011). Hence, improved knowledge of the ecological characteristics of urban rivers in China is essential to assess local public health concerns and support river ecological restoration.
Aquatic biofilms colonize various surfaces in riverine systems and play very important roles in aquatic ecology (e.g., food web and habitat) and water quality dynamics (e.g., degradation and transformation). The surfaces of artificial or natural substrates immersed in the aquatic environments are rapidly colonized by microorganisms, primarily bacteria, forming many unique microbial ecosystems, such as epiphyton, epipelon, epixylon, epilithon, epipsammon, and so on (Azim et al. 2005; Li et al. 2014; Pohlon et al. 2010; Qian et al. 2007; Salta et al. 2013). The bacterial communities within these microecosystems are highly sensitive to environmental fluctuations (Araya et al. 2003; Paerl et al. 2003). They are also hot spots for the turnover of allochthonous organic matter and mineral nutrients (Augspurger et al. 2008; Geesey et al. 1978; Pohlon et al. 2010) including anthropogenic pollutants, particularly in hypereutrophic urban rivers lacking macroorganisms. In addition, the surface-associated microbial communities have been widely used in the remediation of contaminated waters (Gil-Allué et al. 2015; Wu et al. 2012, 2014). Artificial substrates have been widely used to collect and study surface-associated microorganisms (Brummer et al. 2000; Jones et al. 2007; Wang et al. 2013), such as in examining water treatment processes, because they are stable and easily obtained (Dong et al. 2003; Guzzon et al. 2008; Wan et al. 2016). Considering the important significance of surface-associated bacterial communities in urban rivers, especially for their possible applications in river restorations, there is a critical need to better understand the composition and functions of bacterial biofilm communities in urban rivers.
The identification of bacterial communities at higher taxonomic resolution is a powerful tool to better understand the intricacies of microbial ecological processes. Rapid advances in high-throughput sequencing enable low cost detection of bacterial communities at high-resolution, even rare taxa with low relative abundance (Lemos et al. 2011; Logares et al. 2012). A previous report from the river network examined in this study indicated that variations in microbial communities was the major factor affecting the distribution of antibiotic resistant genes highlighting the importance of understanding controls of microbial communities (Zhou et al. 2017). Therefore, in order to better understand surface-associated bacterial communities in hypereutrophic urban rivers, we utilized high-throughput sequencing to explore the bacterial community composition. The primary objective of this work was to investigate the properties of bacterial communities attached to artificial substrates in a hypereutrophic urban river for the purpose of enhancing river ecological restoration activities and assessing human health concerns.
Materials and methods
Study site and experimental design
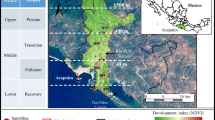
The study was conducted in the Wen-Rui Tang River, a typical coastal plain river network, located in Wenzhou, eastern China. This river flows through a densely populated area (~ 3.0 million city and 9.1 million regional population) where garbage and untreated industrial/municipal wastes are often dumped indiscriminately into the river network. Due to rapid economic development and urbanization, water quality in the Wen-Rui Tang River has degraded dramatically (Lu et al. 2011; Yang et al. 2013). According to China surface water quality standards (GB3838-2002), Wen-Rui Tang River water quality is highly degraded, V grade or less, due to the high contents of total nitrogen, total phosphorus and ammonium (Mei et al. 2014). The Shunao and Hengjiang Rivers are tributaries of the Wen-Rui Tang River and their water quality is representative of the impacts resulting from rapid urbanization that has occurred over the past two decades. A lentic-dominated site along the Shunao River (Site A: 27.929854°N, 120.705249°E), and a lotic-dominated site located at the confluence of the Shunao and Hengjiang Rivers (Site B: 27.929687°N, 120.708203°E) were selected for investigation (Fig. 1). Dissolved oxygen (DO) was recorded at the time of water collection using a multi parameter probe (YSI 650MDS, YSI Incorporated, Yellow Springs, Ohio). Water samples were collected at the mid-point of the 14 day experiment for analysis of total nitrogen (TN), ammonium (NH4+–N), nitrate (NO3−–N), nitrite (NO2−–N), total phosphorus (TP), orthophosphate (PO43−–P) and total organic carbon (TOC) were analyzed according to Jin and Tu (1990).
Polyethylene slides are widely used for the study of biofilms in natural aquatic environments while polyethylene filaments are a standard material used in biofilm studies for water treatment technologies. Therefore, we deployed both of these substrates in this study to examine the development of bacterial communities under hypereutrophic conditions in this urban river network. The substrate materials were thoroughly washed with tap water, rinsed with distilled water, and then air dried before deployment. After measuring their surface area, these materials (slide: 5 × 1.5 × 0.16 cm; filaments: length 10 cm, diameter 0.06 cm, 9 filaments per replicate) were anchored at a 30-cm depth below the water surface. Artificial substrates were harvested 7 and 14 days after deployment in April 2016. Triplicate artificial substrates were randomly collected at each time point. Collected samples were placed in sterile bags and transported to the laboratory (~ 0.5 h) for immediate processing.
Biofilms attached to artificial substrates were detached with a sterile soft toothbrush in 400 mL sterile distilled water, and then treated with probe sonication as follows: ultrasonic time 3 s, interval time 8 s, repetition 75 times, power 600 W (Cai et al. 2013, 2014). After detachment, 10 mL of sample was immediately fixed with 2% formaldehyde (final concentration) and cooled at 4 °C for later direct counting of cells. Simultaneously, 150 mL of sample was filtered onto a 0.2 μm polycarbonate membrane filter (47 mm diameter, Millipore), and stored at − 20 °C for subsequent molecular analyses.
Cell counts
The number of bacterial cells was determined microscopically after staining with DAPI (4′,6-diamidino-2-phenylindole; Porter and Feig 1980). DAPI was added to the samples at a final concentration of 1 μg/mL, and samples were allowed to incubate at room temperature for 10 min. Then samples were filtered onto black polycarbonate filters (0.2 μm pore size, 25 mm diameter; Millipore) with a < 10 mmHg vacuum. Cells were enumerated using a Leica fluorescent microscope (DM4000B, Germany). A minimum of 20 randomly selected fields of view were counted per sample.
DNA extraction, 16S rDNA amplification and sequencing
Total DNA was extracted from frozen filters with the E.Z.N.A.® Water DNA Kit (Omega, USA) according to manufacturer’s protocols. The region-specific bacterial/archaeal primer pairs for DNA amplification were S-D-Bact-0341-b-S-17, 5′-CCTACGGGNGGCWGCAG-3′, and S-D-Bact-0785-a-A-21, 5′-GACTACHVGGGTATCTAATCC-3′ (Klindworth et al. 2013), with Illumina adapters added. PCR reactions were performed using KAPA HiFi HotStart ReadyMix PCR Kit (2×) (Kapa Biosystems, US) in a 25 μL reaction volume with an initial denaturation step at 95 °C for 3 min, followed by 25 cycles each of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, followed by a final extension at 72 °C for 5 min. Sequencing was performed on the Illumina platform at Shanghai Xiangyin Biotechnology Co., Ltd. Denoised sequences were aligned and sorted into operational taxonomic units (OTUs) at the 97% similarity level, which corresponds approximately to the species level. Taxonomy was assigned using the Ribosomal Database Project (RDP) classifier (Cole et al. 2014). Good’s coverage, abundance-based coverage estimator (ACE), Chao1 richness estimator, Shannon index, and Simpson index were calculated based on the OTU data. All sequence data from this study were submitted to the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under Accession Number SRP119548.
Statistical analysis
Univariate data were expressed as means and standard errors, and considered statistically significant at p values < 0.05. Multivariate statistical analysis was carried out using Canoco for Windows 5.0 (Ter Braak and Šmilauer 2012) and PAST (PAleontological STatistics v1.81) (Hammer et al. 2001). Multivariate analyses of community structure were carried out on data for genus relative abundance. Principal component analysis (PCA) was used to investigate how community composition varied between sites, artificial substrates, and sampling times. Analysis of similarity (ANOSIM) was used to statistically test the effects of sampling time, substrate type, and site on bacterial community structure. ANOSIM is a nonparametric method to test for differences between two or more groups, based on any distance measure (Clarke 1993). In the present study, the distance indices of genus relative abundance were calculated using Bray–Curtis indices. The significance was computed by permutation of group membership, with 10,000 replicates. ANOSIM generates a test statistic, R, and the magnitude of R is indicative of the degree of separation between groups, with a score of 1 indicating complete separation, and 0 indicating no separation.
Results
Water quality characterization
The physicochemical water quality characteristics at the two study sites in the Wen-Rui Tang River are displayed in Table 1. Both sites had high nitrogen, phosphorus, and total organic carbon concentrations. Dissolved inorganic nitrogen (NH4+–N and NO3−–N) was the main form of nitrogen while particulate forms of P were higher than dissolved PO43−. Total phosphorus concentration was higher at Site B (lotic) compared to Site A (lentic), although both sites had similar orthophosphate concentrations. The higher energy lotic conditions of Site B may enhance resuspension of sediment particles along with their associated P fraction. Total organic carbon, a possible energy source for the microbial community, was similar between the two sites. Dissolved oxygen concentrations (4.3–5.5 mg/L) were considerable below saturation levels of ~ 9.0 mg/L reflective of an aquatic system with high concentrations of oxygen demanding substances (e.g., organic matter, NH4+–N).
Bacterial abundance
The effects of sampling time (7 vs. 14 days), site (lentic vs. lotic) and substrate type (slide vs. filament) on the number of surface-associated bacteria are shown in Fig. 2. Both sampling time and site had significant effects on the number of surface-associated bacteria and there was also a significant interaction between sampling time and site. At Site A (lentic), the number of surface-associated bacteria showed a marked increase with time, especially for the filament substrate. In contrast, the number of surface-associated bacteria at Site B (lotic) slightly declined with time. No significant difference was found in the number of surface-associated bacteria between the two substrate types, but the number of bacteria attached to the filament was markedly higher than that attached to the slide on day 14 at Site A.
Community composition, species richness and diversity
A number of microbial diversity indices for the 16S rDNA Illumina reads are shown in Table 2. The mean Good’s coverage ranged from 92.4 to 95.8%, indicating that the vast majority of phylotypes were detected. Results from high-throughput sequencing showed that microbial assemblages colonizing the artificial substrates were species-rich (maximum mean 9165 OTUs). In general, there were more OTUs on the filament than slide. Species richness and diversity of the surface-attached communities decreased from 7 to day 14.
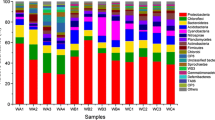
Across all samples, 80,375 OTUs were identified from the data of high-throughput sequencing, and distributed among 47 phyla. The average relative abundance of dominant phyla (sum of the values > 99%) are shown in Fig. 3. The two predominant phyla were Proteobacteria and Cyanobacteria/Chloroplast; the sum of their abundance exceeded 50%. There were marked temporal variations in the relative abundance of dominant phyla. For example, the relative abundance of Proteobacteria was higher on day 7 than on day 14, whereas the relative abundance of Cyanobacteria/Chloroplast was lower on day 7 than day 14.
Due to the high ammonia nitrogen concentrations associated with the studied river system, the microbial community related with ammonia transformations is of great interest. Therefore, we especially focused on the ammonia-oxidizing microorganisms in the community attached to artificial substrates. Nitrosomonas was the predominant genus among ammonia-oxidizers identified (Fig. 4). The temporal variation in the sum of ammonia-oxidizer abundance showed an increasing trend with time, regardless of the attachment substrate. Additionally, considering that domestic sewage pollution is an important contributor to degraded water quality in the Wen-Rui Tang River, we focused on the genera of fecal indicator bacteria and pathogens. There were many fecal indicator bacteria and pathogens in the microbial community attached to artificial substrates (Fig. 5). The relative abundance of fecal indicator bacteria and pathogens decreased gradually with time.
The effects of sampling time, site and substrate type on microbial communities attached to artificial substrates were examined using PCA and ANOSIM analyses. Although one sample failed to sequence, it had little influence on the results. PCA analysis showed that bacterial communities were largely overlapping for the two substrates, regardless of the sampling time. In contrast, Site A bacterial communities were distinct from the bacterial communities at Site B. Throughout the 14-day time course, the PCA analysis illustrated that differences in bacterial community structures between the two sites were diminished (Fig. 6). ANOSIM analyses further showed significant differences in bacterial community structure both between sites and between sampling times (Table 3). No significant difference was detected between the two substrates.
Discussion
Identification of microbes attached to artificial substrates in a hypereutrophic urban river revealed a complex microbial community structure. The results indicated that the microbial communities were composed of 80,375 OTUs distributed among 47 phyla. Attached microbial communities were dominated by the phyla Proteobacteria, Cyanobacteria/Chloroplast, Planctomycetes, Actinobacteria, Verrucomicrobia, Firmicutes and Bacteroidetes, with generally little variation in relative abundance between the two substrate types (Fig. 3). Our results are consistent with those of previous studies showing that Proteobacteria was the most abundant phylogenetic group in most river studies (Bricheux et al. 2013; Carney et al. 2015; Kochling et al. 2017), and biofilms in eutrophic waters were dominated by Cyanobacteria (Danilov and Ekelund 2002).
Sufficiently illuminated aquatic biofilms containing mixed communities are actively involved in the ammonia-oxidizing process (Anderson 2016; Coci et al. 2008, 2010). Hence, this study focused on the composition of ammonia-oxidizing microorganisms due to the high ammonium concentrations found in this hypereutrophic urban river. These complex attached microbial communities contained three genera of ammonia-oxidizing bacteria (Nitrosomonas, Nitrosospira and Nitrosococcus) and two genera of ammonia-oxidizing archaea (Nitrososphaera and Nitrosopumilus), of which the genus Nitrosomonas was the dominant in each sample, similar to a study from 10 wastewater treatment systems by Gao et al. (2014). Interestingly, over time, the relative abundance of the genus Nitrosomonas increased sharply, regardless of the attachment substrate and sampling site (Fig. 4). Compared with the water column, solid surfaces were more densely populated with ammonia-oxidizing bacteria in aquatic environments (Coci et al. 2008; Matulewich and Finstein 1978). The reasons may be that the extracellular polymeric materials produced by the microbes during the formation of biofilms in aquatic environments provide benefits for ammonia-oxidizing bacteria, and also the surface-attached growth has been proposed to offer resilience against environmental constraints (Powell and Prosser 1992). Obviously, the significant increase in the relative abundance of ammonia-oxidizing bacteria was linked to their ability to adapt to surface-attached growth. These results are consistent with the idea that nitrogen transformation processes in small-river ecosystems are primarily associated with the attached microbial communities (Herrmann et al. 2011; Lock 1993).
Microbial communities within aquatic biofilms have been proposed as potential bioindicator for changes in water quality due to their rapid response to environmental conditions and their great diversity (Witt et al. 2011). Therefore, we focus on whether the attached microbial communities can identify human-health concerns in urban river water quality. Considering polluted river waters may contain a large variety of pathogenic microorganisms (Servais et al. 2007), such human health risks are assessed by enumerating fecal indicator bacteria and pathogens. In the present experiment, the results of high-throughput sequencing documented the presence of fecal indicator bacteria and pathogens in microbial communities attached to artificial substrates in the Wen-Rui Tang River, although the relative abundance of fecal indicator bacteria and pathogens decreased gradually with time (Fig. 5). Unexpectedly, the genus Legionella, which is one of the main causative agents of severe atypical pneumonias (Yanez et al. 2005), was found in each sample. Our results support the premise that the Wen-Rui Tang River receives appreciable inputs of untreated sewage, which poses a serious risk to humans and environmental health.
The microbial communities attached to artificial substrates in this hypereutrophic urban river showed variable responses to sampling time, site and substrate type. Araya et al. (2003) reported that microbial community structures (determined by denaturing gradient gel electrophoresis, DGGE) in biofilms from an urban river matured within 3–7 days of their formation and did not change appreciably over longer time periods. However, results of cell counts from our study documented significant differences in the number of microbes attached on artificial substrates between sampling days 7 and 14 (Fig. 2). The analysis of the species richness and diversity of the surface-attached communities showed a regular decrease from 7 to 14 day (Table 2), suggesting that there were further changes in the surface-attached microbial communities after 7 days. The ANOSIM also indicated that significant differences in attached microbial community structures existed between the two sampling times (Table 3). One possible reason for the difference between our results and those of Araya et al. (2003) may result from the method used for studying the microbial community. In contrast to DGGE analysis, high-throughput sequencing provides more information on microbial community structure (Lemos et al. 2011; Logares et al. 2012).
In addition to temporal heterogeneity, the intra-river heterogeneity (lentic vs. lotic) in attached microbial community structure was significant. Although PCA analysis showed that attached microbial communities from the two sites became more similar over time, the attached microbial communities were clearly separated between the two sites (Fig. 6). Moreover, significant differences in the number and community composition of attached microbes between the two study sites were examined (Fig. 2 and Table 3). There were no marked differences in nutrient status between the two sites separated by about 300 m (Table 1), but the two sites had different hydrodynamic conditions. This may be a primary reason for the dissimilarity in attached microbial communities between the two sites.
Generally, a substrate is necessary for the attachment and the development of attached microbes in biofilms within aquatic environments. The physicochemical property of substrate may influence the attached microbial community structure (Dang and Lovell 2000). The two substrates used in the present study were both plastics and had similar surface properties. No significant differences in attached microbial community composition were detected between the two substrates in our study (Fig. 2, Table 3). However, compared with the slide, the number of bacteria attached on the filament showed a marked increase with time at Site A (Fig. 2). This difference in attached microbial number might be attributed to differences in the space structure and specific surface area characteristics of the two substrates.
In conclusion, our results revealed that microbial communities attached on artificial substrates in a hypereutrophic urban river were composed of 80,375 OTUs that distributed among 47 phyla. Proteobacteria and Cyanobacteria/Chloroplast were the two dominant phyla, followed by Planctomycetes, Actinobacteria, Verrucomicrobia, Firmicutes and Bacteroidetes. Further analysis of taxonomic composition showed that ammonia-oxidizing microorganisms, fecal indicator bacteria and pathogens were enriched in attached microbial communities, especially the ammonia-oxidizing bacteria of the genus Nitrosomonas. Furthermore, our results demonstrated that there were significant temporal and intra-river heterogeneity in the attached microbial community structure, but no significant difference was detected between the two substrate types. These results support the hypothesis that ammonium oxidation is a major source of oxygen demand in these hypereutrophic waterways. Providing instream artificial substrates (e.g., roots of floating wetlands, suspended woody or plastic materials) to enhance ammonium oxidation to nitrate with subsequent denitrification in the anoxic sediments maybe a mechanism to promote attenuation of excess nitrogen in these hypereutrophic waterways.
Abbreviations
- ACE:
-
abundance-based coverage estimator
- ANOSIM:
-
analysis of similarity
- DO:
-
dissolved oxygen
- NH4 +–N:
-
ammonium
- NO2 −–N:
-
nitrite
- NO3 −–N:
-
nitrate
- OTUs:
-
operational taxonomic units
- PCA:
-
principal component analysis
- PO4 3−–P:
-
orthophosphate
- TN:
-
total nitrogen
- TOC:
-
total organic carbon
- TP:
-
total phosphorus
References
Anderson OR (2016) Marine and estuarine natural microbial biofilms: ecological and biogeochemical dimensions. AIMS Microbiol 2(3):304–331
Araya R, Tani K, Takagi T, Yamaguchi N, Nasu M (2003) Bacterial activity and community composition in stream water and biofilm from an urban river determined by fluorescent in situ hybridization and DGGE analysis. FEMS Microbiol Ecol 43(1):111–119
Augspurger C, Gleixner G, Kramer C, Küsel K (2008) Tracking carbon flow in a 2-week-old and 6-week-old stream biofilm food web. Limnol Oceanogr 53(2):642–650
Azim ME, Beveridge MCM, van Dam AA, Verdegem MCJ (2005) Periphyton and aquatic production: an introduction. In: Azim ME, Verdegem MCJ, van Dam AA, Beveridge MCM (eds) Periphyton: ecology, exploitation and management. CABI Publishing, Wallingford, pp 1–13
Bricheux G, Morin L, Le Moal G, Coffe G, Balestrino D, Charbonnel N, Bohatier J, Forestier C (2013) Pyrosequencing assessment of prokaryotic and eukaryotic diversity in biofilm communities from a French river. Microbiologyopen 2(3):402–414
Brummer IHM, Fehr W, Wagner-Dobler I (2000) Biofilm community structure in polluted rivers: abundance of dominant phylogenetic groups over a complete annual cycle. Appl Environ Microbiol 66(7):3078–3082
Cai XL, Gao G, Tang XM, Dong BL, Dai JY, Chen D, Song YZ (2013) The response of epiphytic microbes to habitat and growth status of Potamogeton malaianus Miq. Lake Taihu. J Basic Microbiol 53(10):828–837
Cai XL, Gao G, Yang J, Tang XM, Dai JY, Chen D, Song YZ (2014) An ultrasonic method for separation of epiphytic microbes from freshwater submerged macrophytes. J Basic Microbiol 54(7):758–761
Cai W, Li Y, Wang PF, Niu LH, Zhang WL, Wang C (2016) Effect of the pollution level on the functional bacterial groups aiming at degrading bisphenol A and nonylphenol in natural biofilms of an urban river. Environ Sci Pollut Res 23(15):15727–15738
Carney RL, Mitrovic SM, Jeffries T, Westhorpe D, Curlevski N, Sey-mour JR (2015) River bacterioplankton community responses to a high inflow event. Aquat Microb Ecol 75(3):187–205
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18(1):117–143
Coci M, Bodelier PLE, Laanbroek HJ (2008) Epiphyton as a niche for ammonia-oxidizing bacteria: detailed comparison with benthic and pelagic compartments in shallow freshwater lakes. Appl Environ Microbiol 74(7):1963–1971
Coci M, Nicol GW, Schmid M, Kamst-van Agterveld MP, Bodelier PLE, Laanbroek HJ (2010) Quantitative assessment of ammonia-oxidizing bacterial communities in the epiphyton of submerged macrophytes in shallow lakes. Appl Environ Microbiol 76(6):1813–1821
Cole JR, Wang Q, Fish JA, Chai BL, McGarrell DM, Sun YN, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM (2014) Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42(D1):D633–D642
Dang HY, Lovell CR (2000) Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl Environ Microbiol 66(2):467–475
Danilov RA, Ekelund NGA (2002) Periphyton communities on natural substrata in eu-, meso- and oligotrophic lakes at higher latitude. Biologia 57(4):433–436
Dong DM, Li Y, Zhang JJ, Hua XY (2003) Comparison of the adsorption of lead, cadmium, copper, zinc and barium to freshwater surface coatings. Chemosphere 51(5):369–373
Gao JF, Luo X, Wu GX, Li T, Peng YZ (2014) Abundance and diversity based on amoA genes of ammonia-oxidizing archaea and bacteria in ten wastewater treatment systems. Appl Microbiol Biotechnol 98(7):3339–3354
Geesey GG, Mutch R, Costerton JW, Green RB (1978) Sessile bacteria: an important component of microbial population in small mountain streams. Limnol Oceanogr 23(6):1214–1223
Gil-Allué C, Schirmer K, Tlili A, Gessner MO, Behra R (2015) Silver nanoparticle effects on stream periphyton during short-term exposures. Environ Sci Technol 49(2):1165–1172
Guzzon A, Bohn A, Diociaiuti M, Albertano P (2008) Cultured phototrophic biofilms for phosphorus removal in wastewater treatment. Water Res 42(16):4357–4367
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):1–9
Herrmann M, Scheibe A, Avrahami S, Küsel K (2011) Ammonium availability affects the ratio of ammonia-oxidizing bacteria to ammonia-oxidizing archaea in simulated creek ecosystems. Appl Environ Microbiol 77(5):1896–1899
Jin XC, Tu QY (1990) Investigation handbook of lake eutrophication, 2nd edn. China Environmental Science Press, Beijing
Jones PR, Cottrell MT, Kirchman DL, Dexter SC (2007) Bacterial community structure of biofilms on artificial surfaces in an estuary. Microb Ecol 53(1):153–162
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41(1):e1
Kochling T, Sanz JL, Galdino L, Florencio L, Kato MT (2017) Impact of pollution on the microbial diversity of a tropical river in an urbanized region of northeastern Brazil. Int Microbiol 20(1):11–24
Lemos LN, Fulthorpe RR, Triplett EW, Roesch LFW (2011) Rethinking microbial diversity analysis in the high throughput sequencing era. J Microbiol Methods 86(1):42–51
Li YF, Chen YR, Yang JL, Bao WY, Guo XP, Liang X, Shi HY, Li JL, Ding DW (2014) Effects of substratum type on bacterial community structure in biofilms in relation to settlement of plantigrades of the mussel Mytilus coruscus. Int Biodeterior Biodegr 96:41–49
Lock MA (1993) Attached microbial communities in rivers. In: Ford TE (ed) Aquatic microbiology—an ecological approach. Blackwell Scientific Publications, Oxford, pp 113–138
Logares R, Haverkamp THA, Kumar S, Lanzen A, Nederbragt AJ, Quince C, Kauserud H (2012) Environmental microbiology through the lens of high-throughput DNA sequencing: synopsis of current platforms and bioinformatics approaches. J Microbiol Methods 91(1):106–113
Lu P, Mei K, Zhang YJ, Liao LL, Long BB, Dahlgren RA, Zhang MH (2011) Spatial and temporal variations of nitrogen pollution in Wen-Rui Tang River watershed, Zhejiang, China. Environ Monit Assess 180:501–520
Matulewich VA, Finstein MS (1978) Distribution of autotrophic nitrifying bacteria in a polluted river (the Passaic). Appl Environ Microbiol 35(1):67–71
Mei K, Liao LL, Zhu YL, Lu P, Wang ZF, Dahlgren RA, Zhang MH (2014) Evaluation of spatial-temporal variations and trends in surface water quality across a rural–suburban–urban interface. Environ Sci Pollut Res 21(13):8036–8051
Paerl HW, Dyble J, Moisander PH, Noble RT, Piehler MF, Pinckney JL, Steppe TF, Twomey L, Valdes LM (2003) Microbial indicators of aquatic ecosystem change: current applications to eutrophication studies. FEMS Microbiol Ecol 46(3):233–246
Pohlon E, Marxsen J, Küsel K (2010) Pioneering bacterial and algal communities and potential extracellular enzyme activities of stream biofilms. FEMS Microbiol Ecol 71(3):364–373
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25(5):943–948
Powell SJ, Prosser JI (1992) Inhibition of biofilm populations of Nitrosomonas europaea. Microb Ecol 24(1):43–50
Qian PY, Lau SCK, Dahms HU, Dobretsov S, Harder T (2007) Marine biofilms as mediators of colonization by marine macroorganisms: implications for antifouling and aquaculture. Mar Biotechnol 9(4):399–410
Qiu J (2011) China to spend billions cleaning up groundwater. Science 334(6057):745
Salta M, Wharton JA, Blache Y, Stokes KR, Briand JF (2013) Marine biofilms on artificial surfaces: structure and dynamics. Environ Microbiol 15(11):2879–2893
Servais P, Billen G, Goncalves A, Garcia-Armisen T (2007) Modelling microbiological water quality in the Seine river drainage network: past, present and future situations. Hydrol Earth Syst Sci 11(5):1581–1592
Ter Braak CJF, Šmilauer P (2012) CANOCO reference manual and user’s guide: software for ordination, version 5.0. Biometris, Wageningen
Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Liermann CR, Davies PM (2010) Global threats to human water security and river biodiversity. Nature 467(7315):555–561
Wan JJ, Liu XM, Kerr PG, Wu CX, Wu YH (2016) Comparison of the properties of periphyton attached to modified agro-waste carriers. Environ Sci Pollut Res 23(4):3718–3726
Wang XM, Ma MY, Liu JL (2013) Biological characteristics of biofilms formed on different substrata in a shallow lake in Haihe basin (China). Bull Environ Contam Toxicol 90(4):414–420
Witt V, Wild C, Uthicke S (2011) Effect of substrate type on bacterial community composition in biofilms from the Great Barrier Reef. FEMS Microbiol Lett 323(2):188–195
Wu YH, Li TL, Yang LZ (2012) Mechanisms of removing pollutants from aqueous solutions by microorganisms and their aggregates: a review. Bioresour Technol 107:10–18
Wu Y, Xia L, Liu N, Gou S, Nath B (2014) Cleaning and regeneration of periphyton biofilm in surface water treatment systems. Water Sci Technol 69(2):235–243
Yanez MA, Carrasco-Serrano C, Barbera VM, Catalan V (2005) Quantitative detection of Legionella pneumophila in water samples by immunomagnetic purification and real-time PCR amplification of the dotA gene. Appl Environ Microbiol 71(7):3433–3441
Yang LP, Mei K, Liu XM, Wu LS, Zhang MH, Xu JM, Wang F (2013) Spatial distribution and source apportionment of water pollution in different administrative zones of Wen-Rui-Tang (WRT) river watershed, China. Environ Sci Pollut Res 20(8):5341–5352
Zhang M, Xu J (2011) Nonpoint source pollution, environmental quality, and ecosystem health in China: introduction to the special section. J Environ Qual 40(6):1685–1694
Zhou ZC, Zheng J, Wei YY, Chen T, Dahlgren RA, Shang X, Chen H (2017) Antibiotic resistance genes in an urban river as impacted by bacterial community and physicochemical parameters. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-0032-0
Authors’ contributions
XLC designed the experiments. XLC, LY, QYS, LYJ and TW performed the experiments. XLC, LY and QYS analyzed the data. XLC wrote the manuscript. RAD revised the paper. All authors read and approved the final manuscript.
Acknowledgements
We thank all the colleagues at Key Laboratory of Watershed Science and Health of Zhejiang Province, Wenzhou Medical University for their technical assistance throughout this research.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This research was supported by the Zhejiang Provincial Natural Science Foundation of China (LQ16C030005), the National Natural Science Foundation of China (41601528), and the Scientific Research Project of Wenzhou Medical University (QTJ13014).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cai, X., Yao, L., Sheng, Q. et al. Properties of bacterial communities attached to artificial substrates in a hypereutrophic urban river. AMB Expr 8, 22 (2018). https://doi.org/10.1186/s13568-018-0545-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-018-0545-z